The Impact of Changes in Species Richness and Species Replacement on Patterns of Taxonomic Homogenization in the Carpathian Forest Ecosystems
Abstract
:1. Introduction
2. Material and Methods
2.1. The Site of Study and Data Collection
2.2. Data Analysis
- (1)
- Sorensen’s dissimilarity index (βsor), which measures dissimilarity in species composition between sites due to both spatial turnover and differences in species richness [33],
- (2)
- Simpson’s dissimilarity index (βsim), which expresses spatial turnover without the influence of richness gradients,
- (3)
- nestedness-resultant dissimilarity index (βnes), which expresses compositional differences in richness caused by nestedness.
3. Results
| Total Pool of Species | No. of Stable Species | No. of Lost Species (% of Total Pool) | No. of Gained Species (% of Total Pool) |
|---|---|---|---|
| S-TM-managed forest | |||
| 133 | 86 | 22 (16.5) | 25 (18.8) |
| WBM-whole forest | |||
| 153 | 91 | 46 (30.1) | 16 (10.5) |
| WBM-managed forest | |||
| 126 | 75 | 38 (30.2) | 13 (10.3) |
| WBM-unmanaged forest | |||
| 128 | 81 | 38 (29.7) | 9 (7.0) |
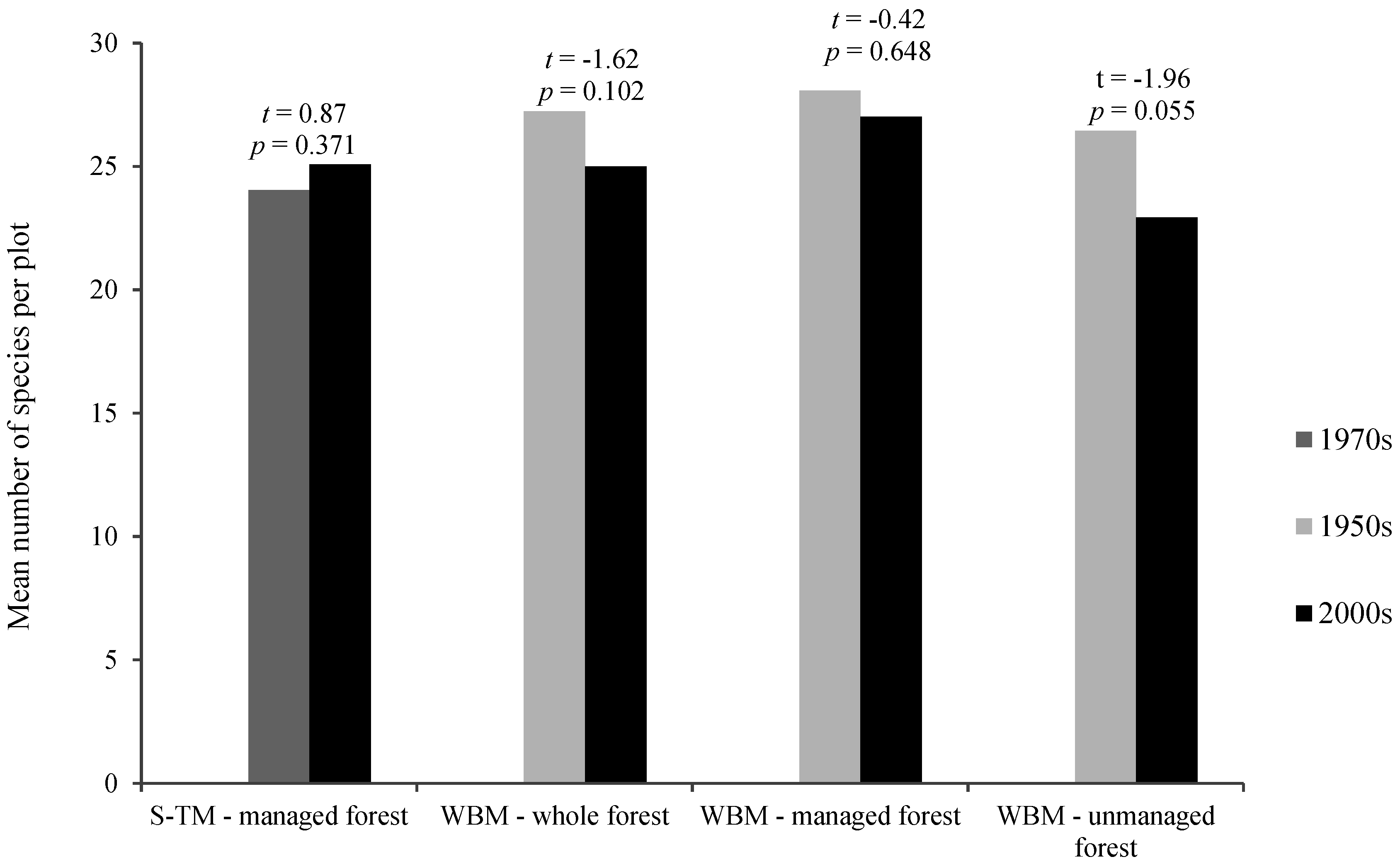
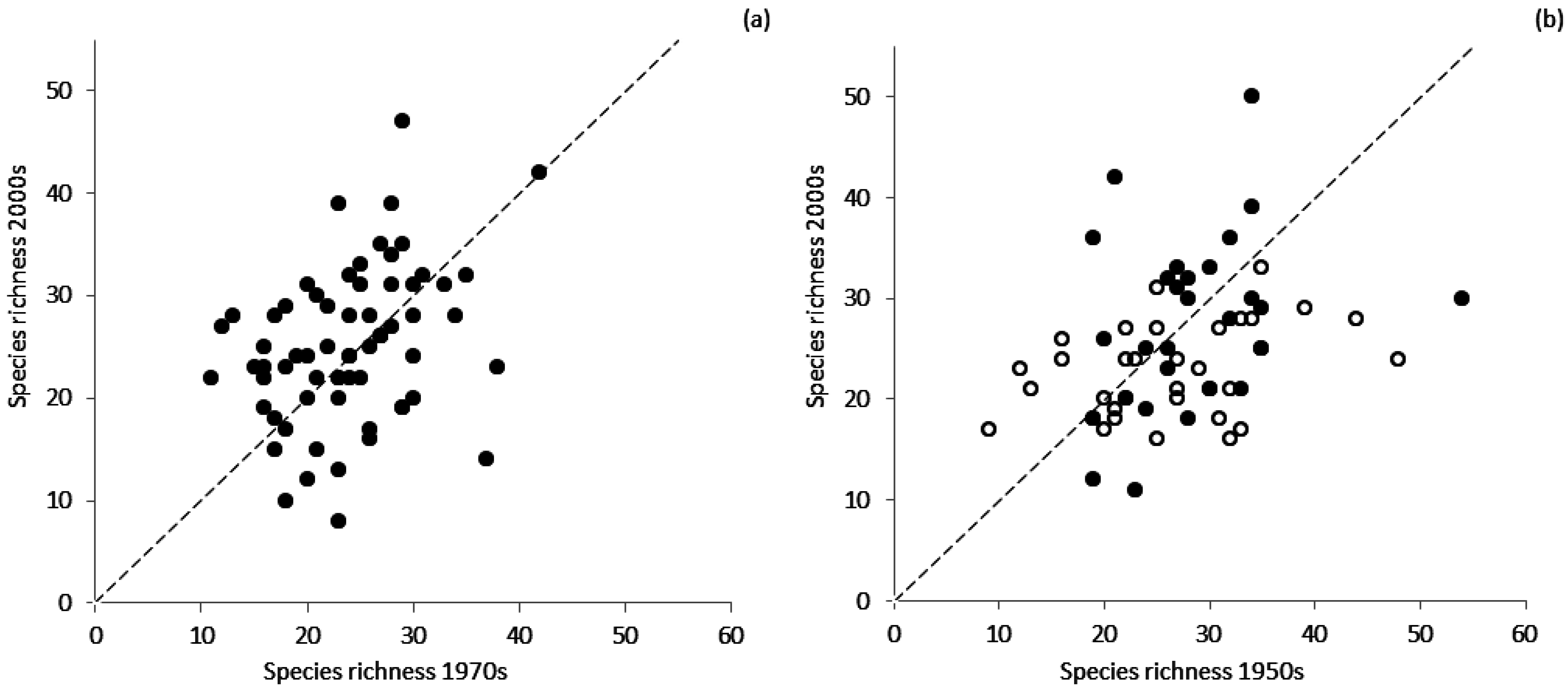
| Mean Value in the Past | Mean Value at Present | Mean Index Change | t | p | |
|---|---|---|---|---|---|
| 1950s/1970s | 2000s | ∆ β | |||
| S-TM-managed forest | |||||
| βsor | 0.51 | 0.48 | −0.03 | −2.4 | 0.0180 |
| βsim | 0.43 | 0.38 | −0.05 | −3.61 | 0.0001 |
| βnes | 0.09 | 0.11 | 0.02 | 3.69 | 0.0002 |
| WBM-whole forest | |||||
| βsor | 0.52 | 0.45 | −0.08 | −15.4 | 0.0001 |
| βsim | 0.44 | 0.34 | −0.09 | −7.69 | 0.0001 |
| βnes | 0.09 | 0.10 | 0.02 | 2.8 | 0.0050 |
| WBM-managed forest | |||||
| βsor | 0.47 | 0.44 | −0.03 | −1.78 | 0.0770 |
| βsim | 0.40 | 0.32 | −0.08 | −4.32 | 0.0001 |
| βnes | 0.08 | 0.12 | 0.05 | 4.24 | 0.0001 |
| WBM-unmanaged forest | |||||
| βsor | 0.55 | 0.42 | −0.12 | −7.12 | 0.0001 |
| βsim | 0.45 | 0.35 | −0.10 | −5.47 | 0.0001 |
| βnes | 0.10 | 0.07 | −0.02 | −3.43 | 0.0010 |
| βsor | βsim | βnes | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| S-TM-managed forest | 8.22 | 0.0001 | 4.09 | 0.0030 | 0.97 | 0.3936 |
| WBM-whole forest | 9.05 | 0.0001 | 9.56 | 0.0001 | 1.76 | 0.1500 |
| WBM-managed forest | 3.47 | 0.0091 | 4.97 | 0.0020 | 1.75 | 0.1680 |
| WBM-unmanaged forest | 7.29 | 0.0001 | 4.93 | 0.0024 | 3.94 | 0.0130 |
4. Discussion
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vellend, M. Conceptual synthesis in community ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.H.; Slatyer, R.O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- Peet, R.K.; Christensen, N.L. Changes in species diversity during secondary forest succession on the North Carolina piedmont. In Diversity and Pattern in Plant Communities; During, H.J., Werger, M.J., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1988; pp. 233–245. [Google Scholar]
- Solar, R.R.D.C.; Barlow, J.; Ferreira, J.; Berenguer, E.; Lees, A.C.; Thomson, J.R.; Louzada, J.; Maués, M.; Moura, N.G.; Oliveira, V.H.F.; et al. How pervasive is biotic homogenization in human-modified tropical forest landscapes? Ecol. Lett. 2015, 18, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L.; Douglas, M.R.; Douglas, M.E.; Fausch, K.D. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 2004, 19, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Olden, J.D.; Rooney, T.P. On defining and quantifying biotic homogenization. Glob. Ecol. Biogeogr. 2006, 15, 113–120. [Google Scholar] [CrossRef]
- Loreau, M. Biodiversity and ecosystem functioning: Recent theoretical advances. Oikos 2000, 91, 3–17. [Google Scholar] [CrossRef]
- Bohn, U.; Gollub, G.; Hettwer, C.; Neuhäuslová, Z.; Raus, Th.; Schlüter, H.; Weber, H. Karte der Natürlichen Vegetation Europas/Map of the Natural Vegetation of Europe, Maßstab/Scale 1:2.500.000, Interaktive/Interactive CD-ROM—Erläuterungstext, Legende, Karten/Explanatory Text, Legend, Maps; Landwirtschaftsverlag: Münster, Germany, 2004. [Google Scholar]
- Brunet, J.; Fritz, Ö.; Richnau, G. Biodiversity in European beech forests—A review with recommendations for sustainable forest management. Ecol. Bull. 2010, 53, 77–94. [Google Scholar]
- Webster, R.; Holt, S.; Avis, C. The Status of the Carpathians. A Report Developed as a Part of The Carpathian Ecoregion Initiative; WWF: Vienna, Austria, 2001; p. 67. [Google Scholar]
- Oszlányi, J.; Grodzińska, K.; Badea, O.; Shparyk, Y. Nature conservation in Central and Eastern Europe with a special emphasis on the Carpathian Mountains. Environ. Pollut. 2004, 130, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Grodzińska, K.; Szarek-Łukaszewska, G. Polish mountain forests: Past, present and future. Environ. Pollut. 1997, 98, 369–374. [Google Scholar] [CrossRef]
- Rackham, O. Ancient woodlands: Modern threats. New Phytol. 2008, 180, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Hannah, L.; Carr, J.L.; Lankerani, A. Human disturbance and natural habitat: A biome level analysis of a global data set. Biodivers. Conserv. 1995, 4, 128–155. [Google Scholar] [CrossRef]
- Bengtsson, J.; Nilsson, S.G.; Franc, A.; Menozzi, P. Biodiversity, disturbances, ecosystem function and management of European forests. For. Ecol. Manag. 2000, 132, 39–50. [Google Scholar] [CrossRef]
- Kaplan, J.O.; Krumhardt, K.M.; Zimmermann, N. The prehistoric and preindustrial deforestation of Europe. Quat. Sci. Rev. 2009, 28, 3016–3034. [Google Scholar] [CrossRef]
- Witkowski, Z.J.; Król, W.; Solarz, W. Carpathian List of Endangered Species; WWF and Institute of Nature Conservation, Polish Academy of Sciences: Vienna, Austria; Krakow, Poland, 2003; p. 64. [Google Scholar]
- Connell, J.H. Diversity in tropical rain forests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Dzwonko, Z. Communities of the Góry Słonne Range (Polish Eastern Carpathians). Fragm. Flor. Geobot. 1977, 23, 161–200. [Google Scholar]
- Skiba, S.; Drewnik, M. Soil Map of the Polish Carpathian Mountains. Rocz. Bieszcz. 2003, 11, 15–20. [Google Scholar]
- Michna, E.; Paczos, S. Zarys klimatu Bieszczadów Zachodnich; Ossolineum: Wrocław, Poland; Warszawa, Poland; Kraków, Poland; Gdańsk, Poland, 1972. [Google Scholar]
- Nowosad, M. Outlines of climate of the Bieszczady National Park and its buffer zone in the light of previous studies. Rocz. Bieszcz. 1995, 4, 163–183. [Google Scholar]
- Environment Protection in the Podkarpackie Voivodship in 2004–2006; Statistical Office in Rzeszów: Rzeszów, Poland, 2007.
- Zarzycki, K. Lasy Bieszczadów Zachodnich. Acta Agrar. Silv. Ser. Silv. 1963, 3, 3–132. [Google Scholar]
- Schramm, W. Lasy i Zwierzyna Gór Sanockich; Państwowe Wydawnictwo Naukowe: Poznań, Poland, 1958. [Google Scholar]
- Glatzel, G. The Impact of historic land use and modern forestry on nutrient relations of Central European forest ecosystems. Fertil. Res. 1991, 27, 1–8. [Google Scholar] [CrossRef]
- Kuemmerle, T.; Hostert, P.; Radeloff, V.C.; Perzanowski, K.; Kruhlov, I. Post-socialist forest disturbance in the Carpathian border region of Poland, Slovakia, and Ukraine. Ecol. Appl. 2007, 17, 1279–1295. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.S.; Bemmerlein, F.A. An outline for data analysis in phytosociology: Past and present. Vegetatio 1989, 81, 17–28. [Google Scholar] [CrossRef]
- Chytrý, M. Phytosociological data give biased estimates of species richness. J. Veg. Sci. 2001, 12, 439–444. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzüge der Vegetationskunde; Springer-Verlag: Wien, Austria; New York, NY, USA, 1964. [Google Scholar]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Koleff, P.; Gaston, K.J.; Lennon, J.J. Measuring beta diversity for presence-absence data. J. Anim. Ecol. 2003, 72, 367–382. [Google Scholar] [CrossRef]
- Lennon, J.J.; Koleff, P.; Greenwood, J.J.D.; Gaston, K.J. The geographical structure of British bird distributions: Diversity, spatial turnover and scale. J. Anim. Ecol. 2001, 70, 966–979. [Google Scholar] [CrossRef]
- Simpson, G.G. Mammals and the nature of continents. Am. J. Sci. 1943, 241, 1–31. [Google Scholar] [CrossRef]
- Baeten, L.; Vangansbeke, P.; Hermy, M.; Peterken, G.; Vanhuyse, K.; Verheyen, K. Distinguishing between turnover and nestedness in the quantification of biotic homogenization. Biodivers. Conserv. 2012, 21, 1399–1409. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Vellend, M. Navigating the multiple meanings of beta diversity: A roadmap for the practicing ecologist. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.P. Use of ordination and other multivariate descriptive methods to study succession. Vegetatio 1977, 35, 165–175. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 2001, 26, 32–46. [Google Scholar]
- Vegan: Community Ecology Package, Version 2.0.; Available online: http://CRAN.R-project.org/package=vegan (accessed on 1 October 2013).
- Zhou, B.; Wong, W.H. A bootstrap-based non-parametric ANOVA method and its application to multi-factorial microarray data. Stat. Sin. 2011, 21, 495–514. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. Available online: http://www.R-project.org/ (accessed on 5 October 2014).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST-Paleontological statistics software package for education and data analysis. Palaeo. Electronica 2001, 4, 1–9. [Google Scholar]
- Schmidt, W. Herb layer species as indicators of biodiversity of managed and unmanaged beech forests. For. Snow Landsc. Res. 2005, 79, 111–125. [Google Scholar]
- Keith, S.A.; Newton, A.C.; Morecroft, M.D.; Bealey, C.E.; Bullock, J.M. Taxonomic homogenization of woodland plant communities over 70 years. Proc. R. Soc. B 2009, 276, 3539–3544. [Google Scholar] [CrossRef] [PubMed]
- Naaf, T.; Wulf, M. Habitat specialists and generalists drive homogenization and differentiation of temperate forest plant communities at the regional scale. Biol. Conserv. 2010, 143, 848–855. [Google Scholar] [CrossRef]
- Smart, S.M.; Thompson, K.; Marrs, R.H.; Le Duc, M.G.; Maskell, L.C.; Firbank, L.G. Biotic homogenization and changes in species diversity across human-modified ecosystems. Proc. R. Soc. B 2006, 273, 2659–2665. [Google Scholar] [CrossRef] [PubMed]
- Durak, T.; Holeksa, J. Biotic homogenisation and differentiation along a habitat gradient resulting from the ageing of managed beech stands. For. Ecol. Manag. 2015, 351, 47–56. [Google Scholar] [CrossRef]
- Durak, T. Changes in vegetation of fertile Carpathian beech forests with perennial honesty Lunaria rediviva based on analysis of the herb layer (Słonne Mountains, Eastern Carpathians). Sylwan 2011, 155, 120–128. [Google Scholar]
- Durak, T. Vegetation changes in East Carpathians piedmont mixed forest under restricted forest management in “Góra Sobień” nature reserve. Sylwan 2009, 153, 627–634. [Google Scholar]
- Turnock, D. Ecoregion-based conservation in the Carpathians and the land-use implications. Land Use Policy 2002, 19, 47–63. [Google Scholar] [CrossRef]
- Forestry 2011; Central Statistical Office of Poland: Warszawa, Poland, 2011.
- Mölder, A.; Bernhardt-Römermann, M.; Schmidt, W. Herb-layer diversity in deciduous forests: Raised by tree richness or beaten by beech? For. Ecol. Manag. 2008, 256, 272–281. [Google Scholar] [CrossRef]
- Durak, T. Changes in diversity of the mountain beech forest herb layer as a function of the forest management method. For. Ecol. Manag. 2012, 276, 154–164. [Google Scholar] [CrossRef]
- Durak, T. Long-term trends in vegetation changes of managed versus unmanaged Eastern Carpathian beech forests. For. Ecol. Manag. 2010, 260, 1333–1344. [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar]
- Stewart, G.H.; Burrows, L.E. Coarse woody debris in oldgrowth temperate beech (Nothofagus) forests of New Zealand. Can. J. For. Res. 1994, 24, 1989–1996. [Google Scholar] [CrossRef]
- Winter, S.; Flade, M.; Schumacher, H.; Kerstan, E.; Möller, G. The importance of near-natural stand structures for the biocoenosis of lowland beech forests. For. Snow Landsc. Res. 2005, 79, 127–144. [Google Scholar]
- Lombardi, F.; Lasserre, B.; Tognetti, R.; Marchetti, M. Deadwood in relation to stand management and forest type in Central Apennines (Molise, Italy). Ecosystems 2008, 11, 882–894. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durak, T.; Durak, R.; Węgrzyn, E.; Leniowski, K. The Impact of Changes in Species Richness and Species Replacement on Patterns of Taxonomic Homogenization in the Carpathian Forest Ecosystems. Forests 2015, 6, 4391-4402. https://doi.org/10.3390/f6124376
Durak T, Durak R, Węgrzyn E, Leniowski K. The Impact of Changes in Species Richness and Species Replacement on Patterns of Taxonomic Homogenization in the Carpathian Forest Ecosystems. Forests. 2015; 6(12):4391-4402. https://doi.org/10.3390/f6124376
Chicago/Turabian StyleDurak, Tomasz, Roma Durak, Ewa Węgrzyn, and Konrad Leniowski. 2015. "The Impact of Changes in Species Richness and Species Replacement on Patterns of Taxonomic Homogenization in the Carpathian Forest Ecosystems" Forests 6, no. 12: 4391-4402. https://doi.org/10.3390/f6124376






