Growth Characteristics of Ectomycorrhizal Seedlings of Quercus glauca, Quercus salicina, Quercus myrsinaefolia, and Castanopsis cuspidata Planted in Calcareous Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants and ECM Fungi
2.2. Preparation of Pot Experiments
2.3. Soil Collection and Analysis
2.4. Establishment of the Pot Experiment
2.5. Measurement of Seedling Growth
2.6. Measurement of the Rate of Colonization of ECM Fungi
2.7. Photosynthetic Capacity
2.8. Analysis of Element Concentrations in Plants
2.9. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
3.2. Growth Characteristics
3.3. The Percentage of ECM Colonization
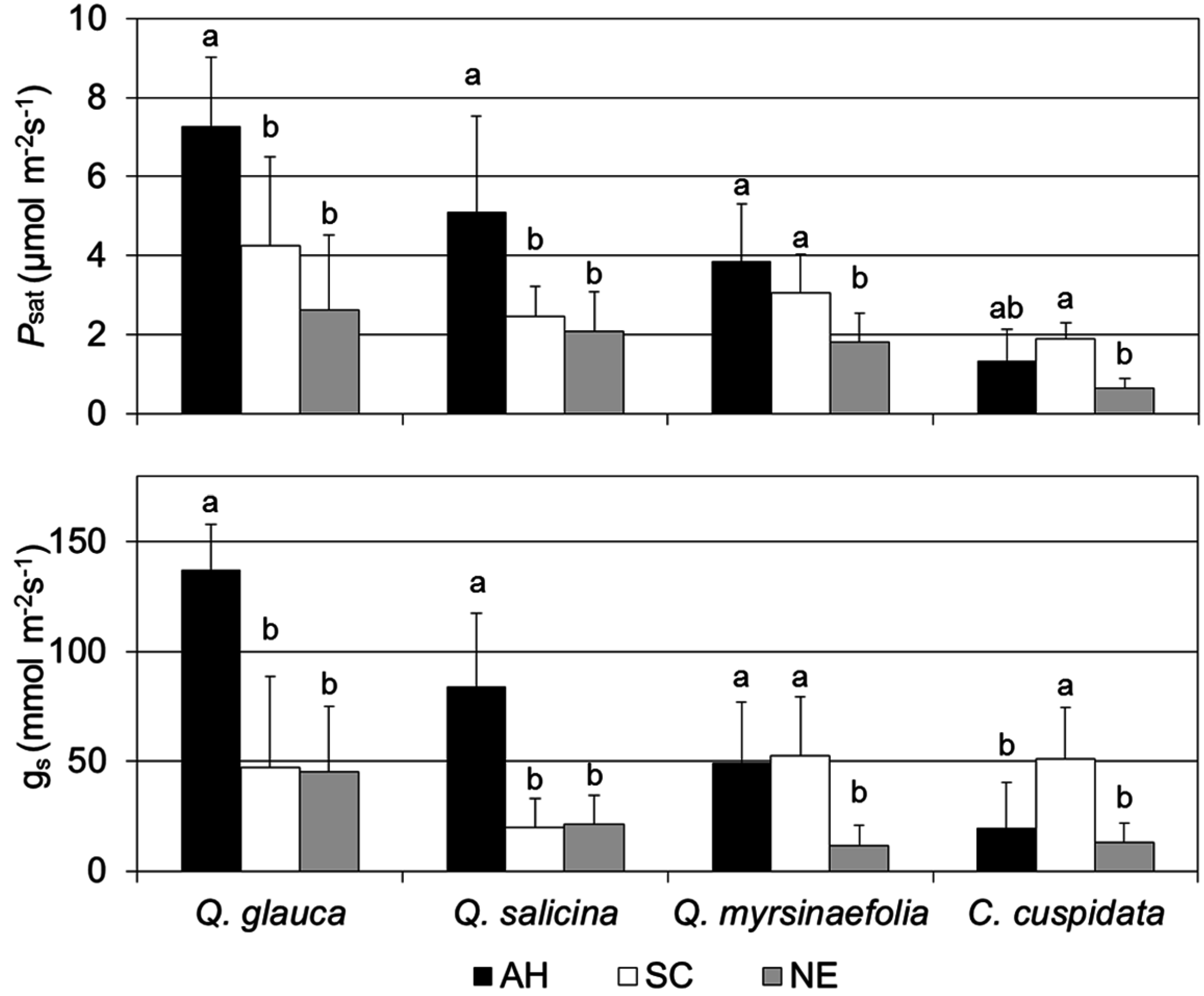
3.4. Photosynthetic Capacity
3.5. Element Concentrations in Leaves
3.6. Element Concentrations and Contents in Roots
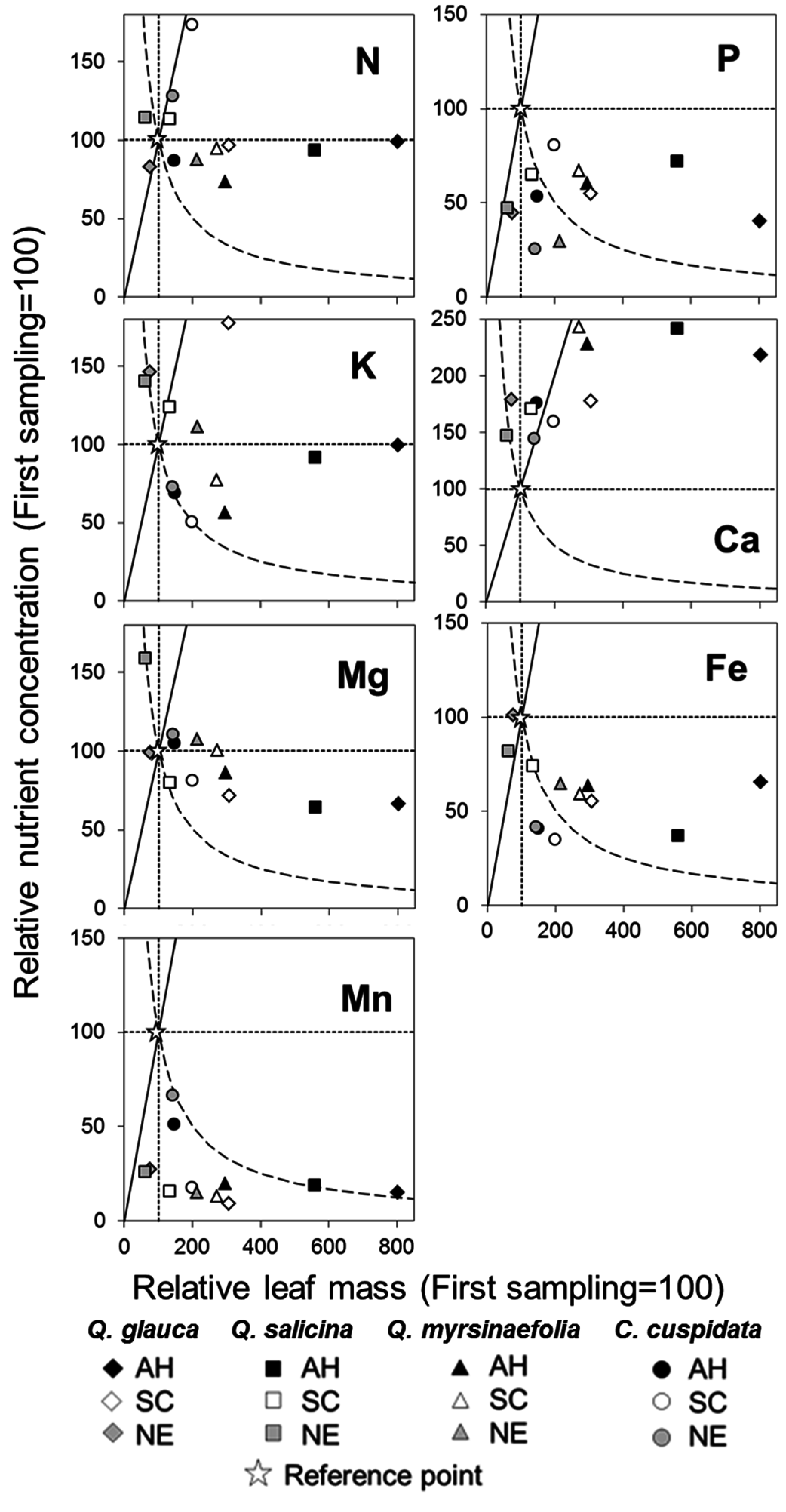
3.7. Vector Analysis between Relative Dry Mass and Nutrient Concentration
4. Discussion
4.1. Growth and Photosynthesis
4.2. Nutrient Relationships
4.3. Relationships among Variables
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ford, D.C.; Williams, P.W. Karst Geomorphology and Hydrology; Chapman & Hall: London, UK, 1989; p. 620. [Google Scholar]
- Haleem, A.A.; Loeppert, R.H.; Anderson, W.B. Role of soil carbonate and iron oxide in iron nutrition of soybean in calcareous soils of Egypt and the United States. In Iron Nutrition in Soils and Plants; Abadía, J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherland, 1995; pp. 307–314. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: New York, NY, USA, 1995; p. 889. [Google Scholar]
- Chen, Y.; Barak, P. Iron nutrition of plants in calcareous soils. Adv. Agron. 1982, 35, 217–240. [Google Scholar]
- Tyler, G. Mineral nutrient limitation of calcifuge plants in phosphate sufficient limestone soil. Ann. Bot. 1996, 77, 649–656. [Google Scholar] [CrossRef]
- Jefferies, R.L.; Willis, A.J. Studies on the calcicole-calcifuge habit: II. The influence of calcium on the growth and establishment of four species in soil and sand cultures. J. Ecol. 1964, 52, 691–707. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology, 4th ed.; Springer: Berlin, Germany, 2003; p. 513. [Google Scholar]
- Zohlen, A.; Tyler, G. Soluble inorganic tissue phosphorus and calcicole-calcifuge behaviour of plants. Ann. Bot. 2004, 94, 427–432. [Google Scholar] [CrossRef]
- Oates, T. Lime and limestone. In Kirk-Othmer Encyclopedia of Chemical Technology; Kirk-Othmer, Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2010; pp. 1–53. [Google Scholar]
- Ursic, K.A.; Kenkel, N.C.; Larson, D.W. Revegetation dynamics of cliff faces in abandoned limestone quarries. J. Appl. Ecol. 1997, 34, 289–303. [Google Scholar] [CrossRef]
- Duan, W.J.; Ren, H.; Fu, S.L.; Wang, J.; Yang, L.; Zhang, J. Natural recovery of different areas of a deserted quarry in south China. J. Environ. Sci. 2008, 20, 476–481. [Google Scholar] [CrossRef]
- Kayama, M. Growth characteristics of Lauraceae tree species planted on limestone quarry. Ann. Rep. Kyushu Res. Cen. FFPRI 2011, 23, 16–17. (In Japanese) [Google Scholar]
- Kayama, M. Growth characteristics of four Lauraceae tree species planted on calcareous and brown forest soils. Kyushu J. For. Res. 2013, 66, 63–66. (In Japanese) [Google Scholar]
- Kira, T. Forest ecosystems of east and Southeast Asia in a global perspective. Ecol. Res. 1991, 6, 185–200. [Google Scholar] [CrossRef]
- Fang, J.Y.; Song, Y.C.; Liu, H.Y.; Piao, S.L. Vegetation-climate relationship and its application in the division of vegetation zone in China. Acta Bot. Sin. 2002, 44, 1105–1122. [Google Scholar]
- Menitsky, Y.L. Oaks of Asia; Science Publishers: Enfield, CT, USA, 2005; p. 549. [Google Scholar]
- Yamanaka, T. The limestone vegetation in middle Kyushu. Res. Rep. Kochi Univ. Nat. Sci. 1966, 15, 1–9, (In Japanese with English summary). [Google Scholar]
- Yamanaka, T. The forest and scrub vegetation in limestone areas of Shikoku, Japan. Vegetatio 1969, 19, 286–307. [Google Scholar] [CrossRef]
- Miyawaki, A. Vegetation of Japan, Chugoku; Shibundo: Tokyo, Japan, 1983; p. 540, (In Japanese with English summary). [Google Scholar]
- Xu, Z.R. A study of the vegetation and floristic affinity of the limestone forest in southern and southwestern China. Ann. Mo. Bot. Gard. 1995, 82, 570–580. [Google Scholar]
- Miyawaki, A. Vegetation of Japan, Kyushu; Shibundo: Tokyo, Japan, 1981; p. 484, (In Japanese with English summary). [Google Scholar]
- Liu, J.M. The reproductive and regenerative counter measures of the main woody species in Maolan karst forest. Sci. Silvae Sin. 2000, 36, 115–122, (In Chinese with English summary). [Google Scholar]
- Yao, Y.Q.; Zhang, Z.H.; Liang, S.C.; Bi, X.L.; Li, G.R.; Hu, G. Structure of Cyclobalanopsis glauca population on karst hills of Guilin. J. Zhejiang For. Sci. Technol. 2008, 28, 8–11, (In Chinese with English summary). [Google Scholar]
- Feroz, S.M.; Hagihara, A. Comparative studies on community ecology of two types of subtropical forest grown in silicate and limestone habitats in the northern part of Okinawa Island, Japan. Taiwania 2008, 53, 139–149. [Google Scholar]
- Newman, E.I.; Reddell, P. The distribution of mycorrhizas among families of vascular plants. New Phytol. 1987, 106, 745–751. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, Y.-L. Phylogenic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- Wallander, H. Uptake of P from apatite by Pinus sylvestris seedlings colonised by different ectomycorrhizal fungi. Plant Soil 2000, 218, 249–256. [Google Scholar] [CrossRef]
- Ström, L.; Owen, A.G.; Godbold, D.L.; Jones, D.L. Organic acid behaviour in a calcareous soil: Sorption reactions and biodegradation rates. Soil Biol. Biochem. 2001, 33, 2125–2133. [Google Scholar] [CrossRef]
- Tyler, G. Some ecophysiological and historical approaches to species richness and calcicole/calcifuge behavior—Contribution to a debate. Folia Geobot. 2003, 38, 419–428. [Google Scholar] [CrossRef]
- Van der Heijden, E.W.; Vosatka, M. Mycorrhizal association of Salix repens L. communities in succession of dune ecosystems. II. Mycorrhizal dynamics and interactions of ectomycorrhizal and arbuscular mycorrhizal fungi. Can. J. Bot. 1999, 77, 1833–1841. [Google Scholar] [CrossRef]
- Wiemken, V.; Laczko, E.; Ineichen, K.; Boller, T. Effects of elevated carbon dioxide and nitrogen fertilization on mycorrhizal fine roots and the soil microbial community in beech-spruce ecosystems on siliceous and calcareous soil. Microb. Ecol. 2001, 42, 126–135. [Google Scholar]
- Kennedy, K.H.; Maxwell, J.F.; Lumyong, S. Fire and the production of Astraeus odoratus (Basidiomycetes) sporocarps in deciduous dipterocarp-oak forests in northern Thailand. Maejo Int. J. Sci. Technol. 2012, 6, 483–504. [Google Scholar]
- Lapeyrie, F. The role of ectomycorrhizal fungi in calcareous soil tolerance by “symbiocalcicole” woody plants. Ann. Sci. For. 1990, 21, 579–589. [Google Scholar] [CrossRef]
- Taste, F.P.; Schmidt, M.G.; Berch, S.M.; Bulmer, C.; Egger, K.N. Effects of ectomycorrhizal inoculants on survival and growth of interior Douglas-fir seedlings on reforestation sites and partially rehabilitated landings. Can. J. For. Res. 2004, 34, 2074–2088. [Google Scholar] [CrossRef]
- Marx, D.H.; Maul, S.B.; Cordell, C.E. Application of specific ectomycorrhizal fungi in world forestry. In Frontiers in Industrial Mycology; Leatham, G.F., Ed.; Chapman and Hall: New York, NY, USA, 1992; pp. 78–98. [Google Scholar]
- Endo, M. Higher fungi of the Hirao-karst topography. Res. Bull. Cult. Asset Kitakyushu 1973, 13, 53–56. (In Japanese) [Google Scholar]
- Yoshimi, S. Taxonomic study of the Japanese taxa of Scleroderma Pers. Jpn. J. Mycol. 2002, 43, 3–18, (In Japanese with English summary). [Google Scholar]
- Fangfuk, W.; Okada, K.; Petchang, R.; To-anun, C.; Fukuda, M.; Yamada, A. In vitro mycorrhization of edible Astraeus mushrooms and their morphological characterization. Mycoscience 2010, 51, 234–241. [Google Scholar] [CrossRef]
- Makita, N.; Hirano, Y.; Yamanaka, T.; Yoshimura, K.; Kosugi, Y. Ectomycorrhizal-fungal colonization induces physio-morphological changes in Quercus serrata leaves and roots. J. Plant Nutr. Soil Sci. 2012, 17, 900–906. [Google Scholar] [CrossRef]
- Kayama, M.; Yamanaka, T. Growth characteristics of ectomycorrhizal seedlings of Quercus glauca, Quercus salicina, and Castanopsis cuspidata planted on acidic soil. Trees 2014, 28, 569–583. [Google Scholar] [CrossRef]
- Akama, K.; Okabe, H.; Yamanaka, T. Growth of ectomycorrhizal fungi on various culture media. Bull. FFPRI 2008, 7, 165–181, (In Japanese with English summary). [Google Scholar]
- Ohta, A. A new medium for mycelial growth of mycorrhizal fungi. Trans. Mycol. Soc. Jpn. 1990, 31, 323–334. [Google Scholar]
- Oita Prefecture. Fundamental land classification survey, Usuki and Hotojima; Fuji Micro-service Center: Kumamoto, Japan, 1980; p. 51. (In Japanese) [Google Scholar]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; International Soil Reference and Information Centre: Wagningen, The Netherlands, 2002; p. 100. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnson, C.T.; Sumner, M.E. Methods of Soil Analysis, Part 3. Chemical Methods; Soil Science Society of America Inc.: Madison, WI, USA, 1996; p. 1390. [Google Scholar]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998; p. 1220. [Google Scholar]
- Japan Meteorological Agency. Climate Statistics. Available online: http://www.data.jma.go.jp/obd/stats/etrn/index.php (access on 31 October 2010).
- Iwasaki, T.; Okuyama, T.; Sakuratani, T. Climatic estimation of photosynthetically active radiation (PAR) in Kanto and Tokai districts. Bull. Natl. Inst. Agro Environ. Sci. 1986, 1, 1–35, (In Japanese with English summary). [Google Scholar]
- Mohan, V.; Natarajan, K.; Ingleby, K. Anatomical studies of ectomycorrhizas. III. The ectomycorrhizas produced by Rhizopogon luteolus and Scleroderma citrinum on Pinus patula. Mycorrhiza 1993, 3, 51–56. [Google Scholar] [CrossRef]
- Quoreshi, A.M.; Timmer, V.R. Exponential fertilization increases nutrient uptake and ectomycorrhizal development of black spruce seedlings. Can. J. For. Res. 1998, 28, 674–682. [Google Scholar] [CrossRef]
- Goto, S. Digestion method. In Manual of Plant Nutrition; Editorial Committee of Methods for Experiments in Plant Nutrition. Hakuyusha: Tokyo, Japan, 1990; pp. 125–128. (In Japanese) [Google Scholar]
- Valentine, D.W.; Allen, H.L. Foliar responses to fertilization identify nutrient limitation in loblolly pine. Can. J. For. Res. 1990, 20, 144–151. [Google Scholar] [CrossRef]
- Scagel, C.F. Growth and nutrient use of ericaceous plants grown in media amended with sphagnum moss peat or coir dust. HortScience 2003, 38, 46–54. [Google Scholar]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Raaimakers, D.; Boot, R.G.A.; Dijkstra, P.; Pot, S.; Pons, T. Photosynthetic rates in relation to leaf phosphorus content in pioneer versus climax tropical rainforest trees. Oecologia 1995, 102, 120–125. [Google Scholar] [CrossRef]
- Bown, H.E.; Watt, M.S.; Clinton, P.W.; Mason, E.G.; Richardson, B. Partitioning concurrent influences of nitrogen and phosphorus supply on photosynthetic model parameters of Pinus radiata. Tree Physiol. 2007, 27, 335–344. [Google Scholar] [CrossRef]
- Parladé, J.; Peta, J.; Alvarez, I.F. Inoculation of containerized Pseudotsuga menziesii and Pinus pinaster seedlings with spores of five species of ectomycorrhizal fungi. Mycorrhiza 1996, 6, 237–245. [Google Scholar] [CrossRef]
- Chen, Y.L.; Dell, B.; Malajczuk, N. Effects of Scleroderma spore density and age on mycorrhiza formation and growth of containerized Eucalyptus globulus and E. urophylla seedlings. New For. 2006, 31, 453–467. [Google Scholar] [CrossRef]
- Lehto, T.; Zwiazek, J.J. Ectomycorrhizas and water relations of trees: A review. Mycorrhiza 2011, 21, 71–90. [Google Scholar] [CrossRef]
- Morita, S.; Nagata, S. The causes and prevention of the leaf dying of the melon in the calcareous soil. Jpn. J. Soil Sci. Plant Nutr. 1995, 66, 217–222, (In Japanese with English summary). [Google Scholar]
- Terai, A. The effects of organic fertilizer application on limestone-derived soils in Miyakojima Islands, Okinawa. Ryukoku J. Econ. Stud. 2008, 47, 125–138. (In Japanese) [Google Scholar]
- Kayama, M. Concentrations of nutrients for trees grown on limestone region. Ann. Rep. Kyushu Res. Cen. FFPRI 2009, 21, 9–10. (In Japanese) [Google Scholar]
- Kayama, M.; Aoki, N.; Hirayama, D.; Tateno, R.; Kawaji, M.; Yoneda, T. Growth characteristics of Quercus miyagii and Q. glauca var. amamiana seedlings planted on two types of soil. Kyushu J. For. Res. 2012, 65, 64–67. (In Japanese) [Google Scholar]
- Maesako, S.; Yonemaru, S.; Sakai, M. Comparative studies of the physical and chemical properties of surface soils in a sugi plantation and it’s two adjacent broad-leaved forest stands. Bull. Kagoshima Prefect. For. Exp. Stn. 2002, 7, 14–22. (In Japanese) [Google Scholar]
- Sardans, J.; Rodà, F.; Peñuelas, J. Phosphorus limitation and competitive capacities of Pinus halepensis and Quercus ilex subsp. rotundifolia on different soils. Plant Ecol. 2004, 174, 305–317. [Google Scholar]
- Pascual, S.; Olarieta, J.R.; Rodoríguez-Ochoa, R. Development of Queucus ilex is related to soil phosphorus availability on shallow calcareous soils. New For. 2012, 43, 805–814. [Google Scholar] [CrossRef]
- Ito, S.; Ohtsuka, K.; Yamashita, T. Ecological distribution of seven evergreen Quercus species. Veg. Sci. 2007, 24, 53–63. [Google Scholar]
- Miyawaki, A. Vegetation of Japan, Kanto; Shibundo: Tokyo, Japan, 1986; p. 641, (In Japanese with English summary). [Google Scholar]
- Ewald, J. Ecological background of crown condition, growth and nutritional status of Picea abies (L.) Karst. In the Bavarian Alps. Eur. J. For. Res. 2005, 124, 9–18. [Google Scholar] [CrossRef]
- Baier, R.; Ettl, R.; Hahn, C.; Göttlein, A. Early development and nutrition of Norway spruce (Picea abies (L.) Karst.) seedlings on different seedbeds in the Bavarian limestone alps—A bioassay. Ann. For. Sci. 2006, 63, 339–348. [Google Scholar] [CrossRef]
- Volenec, J.J.; Ourry, A.; Joern, B.C. A role for nitrogen reserves in forage regrowth and stress tolerance. Physiol. Plant. 1996, 97, 185–193. [Google Scholar] [CrossRef]
- Gloser, V. The consequences of lower nitrogen availability in autumn for internal nitrogen reserves and spring growth of Calamagrostis epigejos. Plant Ecol. 2005, 179, 119–126. [Google Scholar] [CrossRef]
- Peri, P.L.; Gargaglione, V.; Pastur, G.M. Above- and belowground nutrients storage and biomass accumulation in marginal Nothofagus antarctica forests in southern Patagonia. For. Ecol. Manag. 2008, 255, 2502–2511. [Google Scholar] [CrossRef]
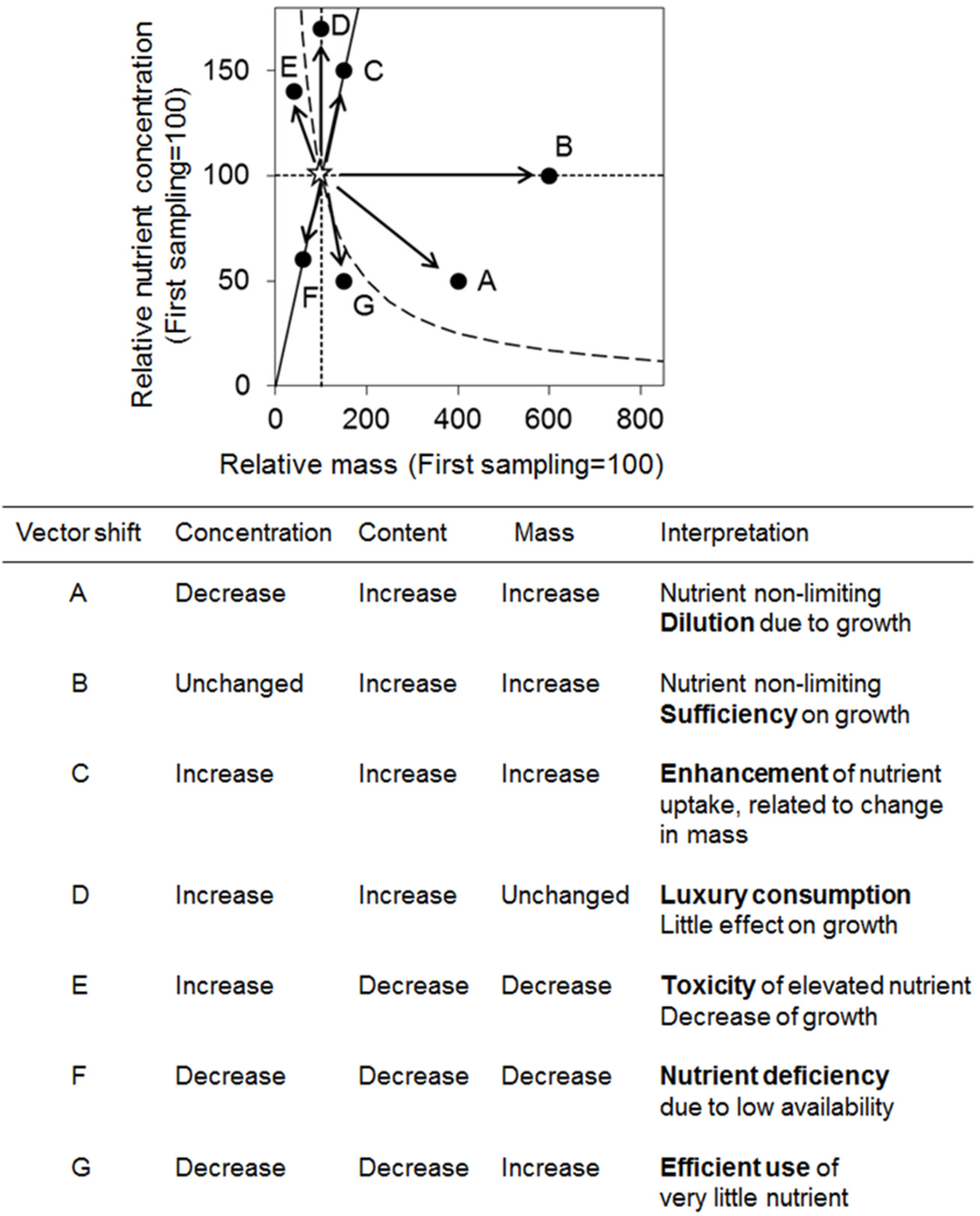
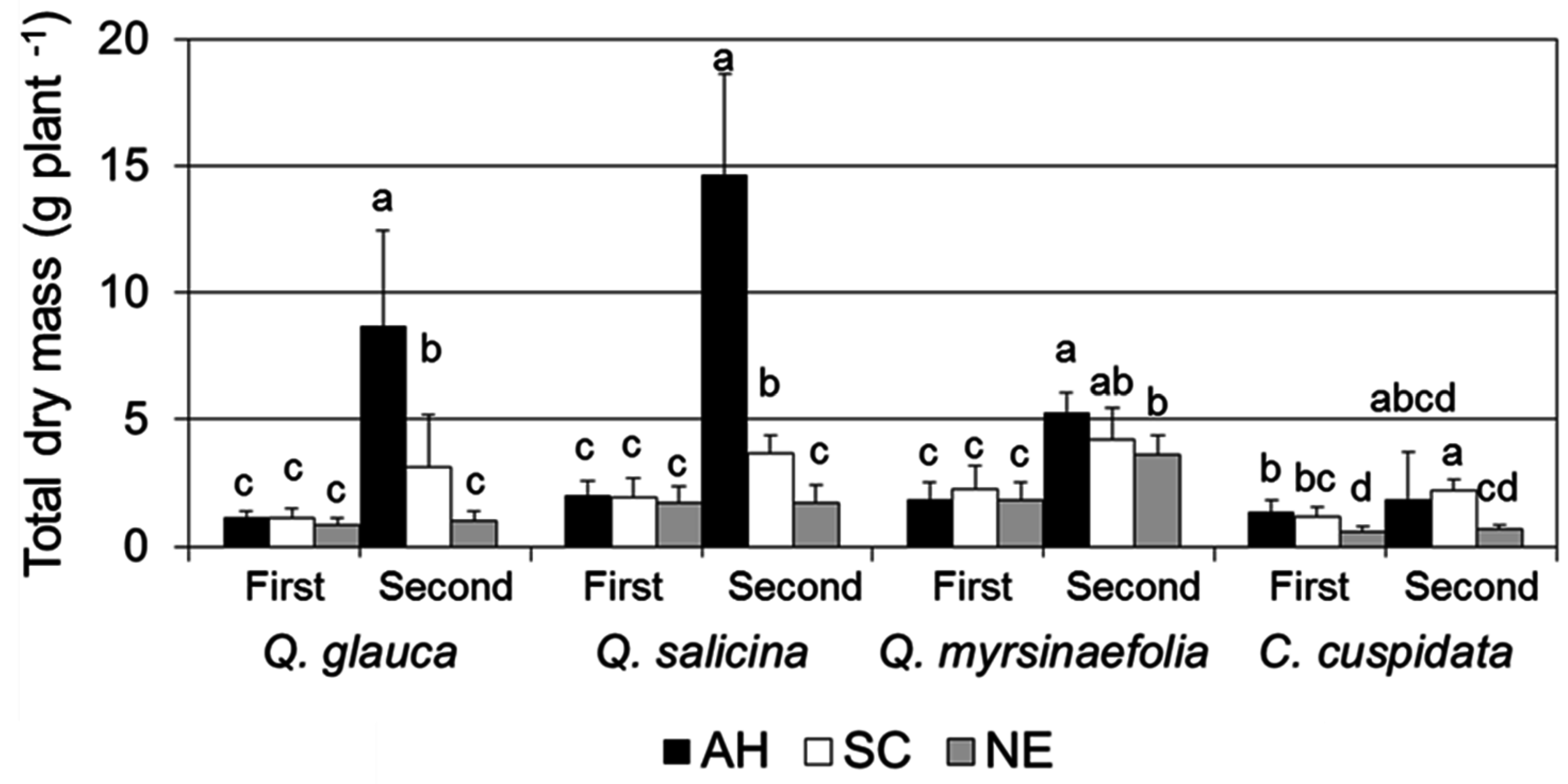
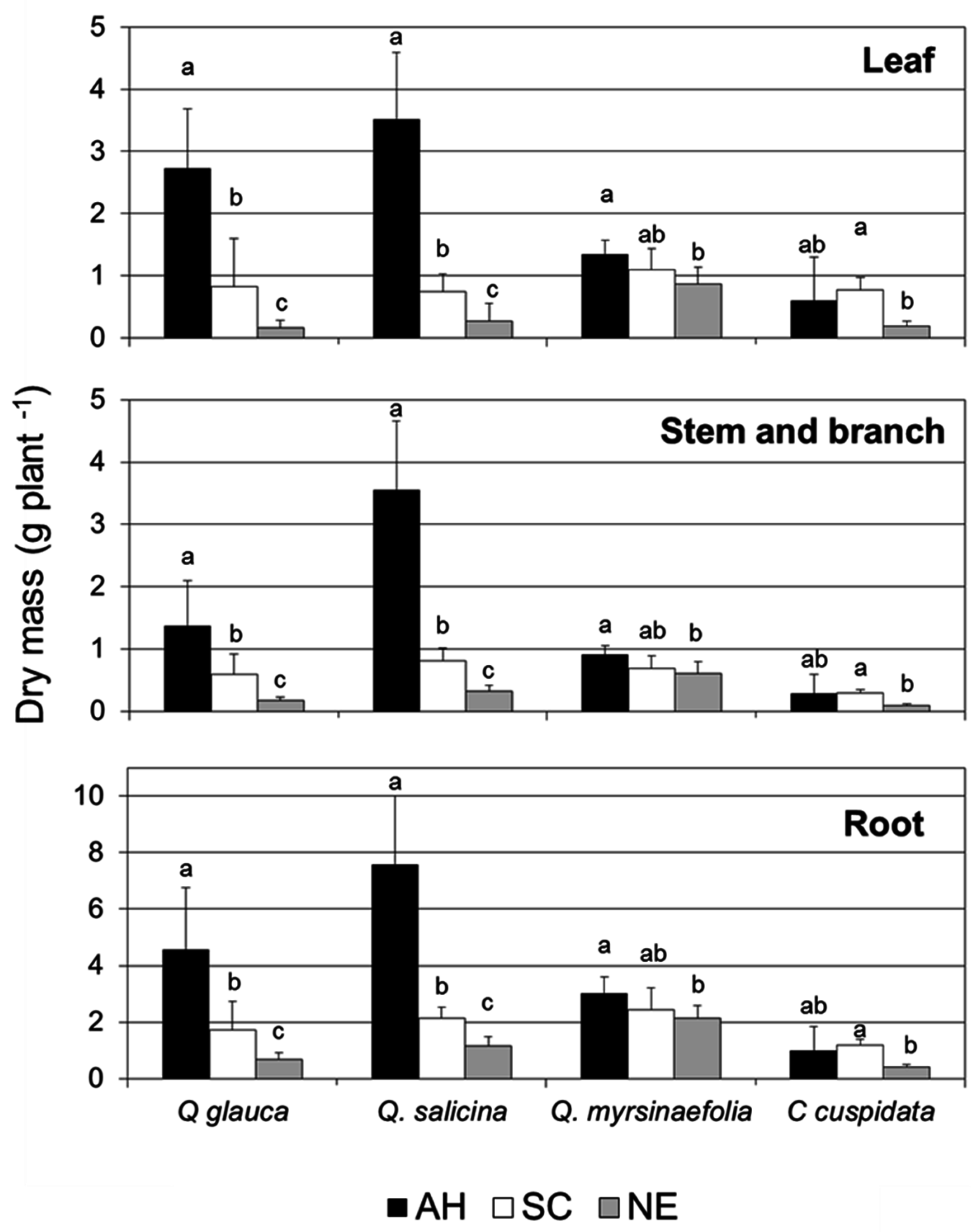
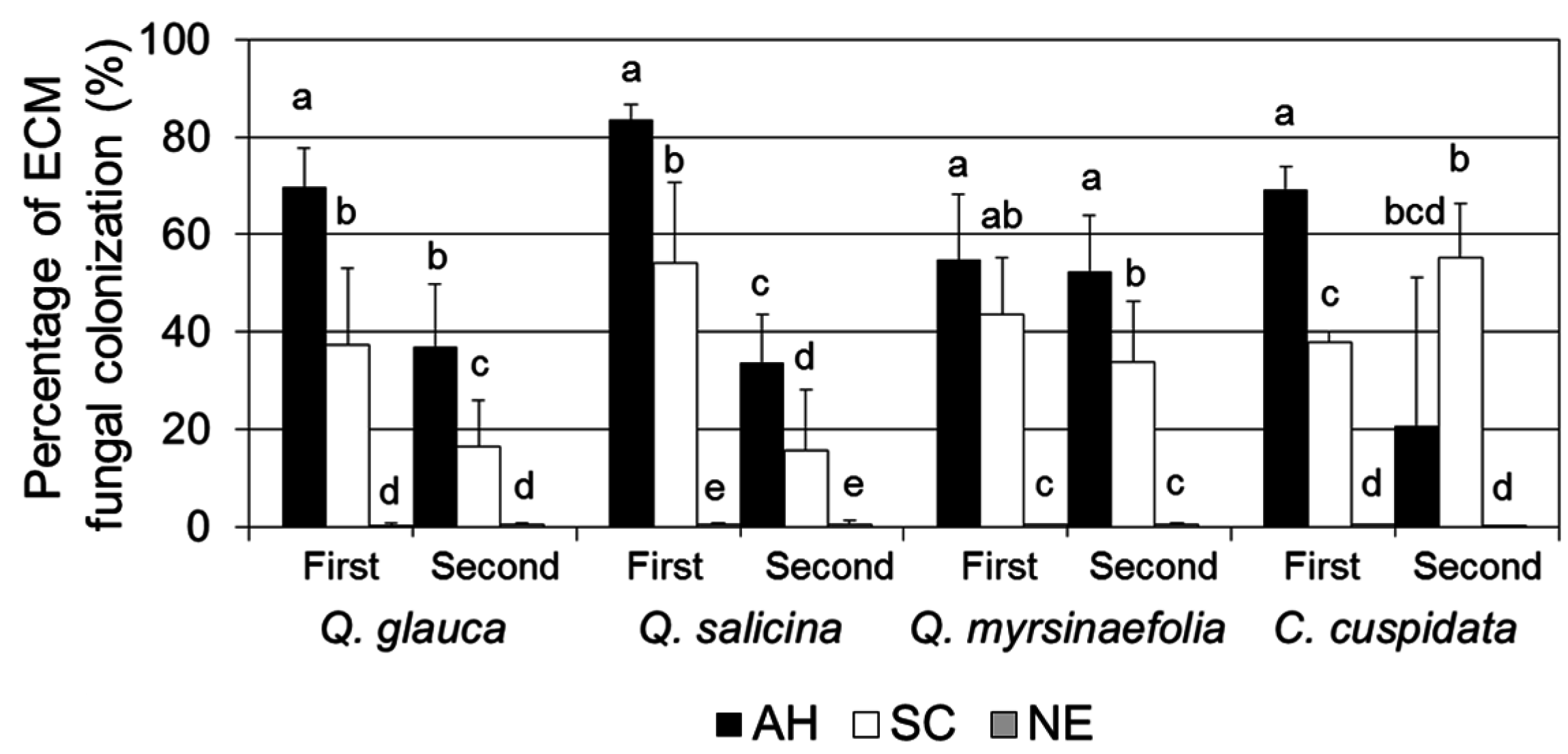
| Year | pH | C (g·kg−1) | N (g·kg−1) | P 1 (mg·kg−1) |
|---|---|---|---|---|
| 2009 | 7.00 ± 0.03 | 26.5 ± 2.6 | 0.962 ± 0.082 | 59.2 ± 11.3 |
| 2010 | 7.02 ± 0.02 | 28.6 ± 1.1 | 0.935 ± 0.089 | 52.1 ± 10.6 |
| Ca2 (g·kg−1) | Mg2 (mg·kg−1) | K2 (mg·kg−1) | Na2 (mg·kg−1) | |
| 2009 | 2.64 ± 0.05 | 62.9 ± 1.3 | 26.0 ± 1.8 | 117 ± 6 |
| 2010 | 2.56 ± 0.05 | 62.1 ± 1.6 | 25.8 ± 0.8 | 115 ± 1 |
| Fe3 (mg·kg−1) | Mn2 (mg·kg−1) | |||
| 2009 | 8.34 ± 0.58 | 4.93 ± 0.59 | ||
| 2010 | 9.52 ± 5.14 | 4.81 ± 0.68 | ||
| Q. glauca | Q. salicina | Q. myrsinaefolia | C. cuspidata | |||||
| R | p | R | p | R | p | R | p | |
| 0.826 | <0.001 * | 0.876 | <0.001 * | 0.638 | <0.001 * | 0.776 | <0.001 * | |
| Elements | Q. glauca | Q. salicina | Q. myrsinaefolia | C. cuspidata | ||||
| R | p | R | p | R | p | R | p | |
| N | 0.217 | 0.199 | –0.350 | 0.038 * | –0.394 | 0.020 * | 0.138 | 0.415 |
| P | 0.030 | 0.859 | 0.435 | 0.010 * | 0.605 | <0.001 * | 0.777 | <0.001 * |
| Element, Inoculation | Q. glauca | Q. salicina | Q. myrsinaefolia | C. cuspidata | |
|---|---|---|---|---|---|
| N (mg·g−1) | AH | 10.7 ± 3.4 a | 9.4 ± 2.2 b | 9.2 ± 2.8 a | 9.7 ± 1.8 b |
| SC | 11.1 ± 1.5 a | 13.2 ± 2.3 a | 11.2 ± 3.8 a | 15.4 ± 3.4 a | |
| NE | 10.5 ± 2.8 a | 15.3 ± 2.0 a | 11.0 ± 2.41 a | 12.2 ± 1.10 a | |
| P (mg·g−1) | AH | 1.43 ± 0.33 a | 1.98 ± 0.30 a | 1.81 ± 0.27 a | 0.84 ± 0.33 a |
| SC | 1.40 ± 0.28 a | 1.34 ± 0.35 b | 1.41 ± 0.12 b | 1.11 ± 0.19 a | |
| NE | 1.35 ± 0.52 a | 1.41 ± 0.62 ab | 0.69 ± 0.29 c | 0.30 ± 0.12 b | |
| K (mg·g−1) | AH | 5.54 ± 0.80 b | 4.52 ± 1.12 a | 3.91 ± 0.50 b | 5.20 ± 2.12 ab |
| SC | 8.58 ± 1.37 a | 5.39 ± 0.83 a | 4.78 ± 1.48 b | 3.96 ± 0.65 b | |
| NE | 8.65 ± 1.15 a | 6.17 ± 2.22 a | 7.15 ± 1.18 a | 5.81 ± 1.18 a | |
| Ca (mg·g−1) | AH | 25.3 ± 7.1 a | 19.6 ± 6.5 a | 26.6 ± 3.3 a | 15.9 ± 2.8 a |
| SC | 19.2 ± 5.8 a | 14.9 ± 3.2 a | 26.8 ± 5.1 a | 16.2 ± 2.7 a | |
| NE | 20.6 ± 4.8 a | 14.6 ± 4.2 a | 23.8 ± 3.30 a | 17.3 ± 5.11 a | |
| Mg (mg·g−1) | AH | 1.40 ± 0.43 a | 1.12 ± 0.35 b | 2.30 ± 0.31 a | 1.86 ± 0.65 ab |
| SC | 1.43 ± 0.40 a | 1.23 ± 0.20 b | 2.47 ± 0.40 a | 1.36 ± 0.25 b | |
| NE | 1.88 ± 0.60 a | 3.50 ± 2.66 a | 2.68 ± 0.41 a | 2.18 ± 0.52 a | |
| Fe (µg·g−1) | AH | 102 ± 20 a | 77 ± 12 b | 85 ± 19 a | 155 ± 139 a |
| SC | 110 ± 44 a | 159 ± 51 a | 79 ± 17 a | 105 ± 26 a | |
| NE | 182 ± 137 a | 215 ± 139 a | 94 ± 21 a | 180 ± 115 a | |
| Mn (µg·g−1) | AH | 204 ± 31 b | 202 ± 76 ab | 273 ± 65 a | 454 ± 239 a |
| SC | 140 ± 67 b | 163 ± 73 b | 200 ± 115 a | 167 ± 58 b | |
| NE | 434 ± 189 a | 363 ± 151 a | 206 ± 67 a | 507 ± 222 a | |
| Element, Inoculation | Q. glauca | Q. salicina | Q. myrsinaefolia | C. cuspidata | |||||
|---|---|---|---|---|---|---|---|---|---|
| N (mg·g−1) | AH | 8.3 ± 1.8 | a | 9.8 ± 1.7 | a | 7.5 ± 1.9 | a | 7.5 ± 1.9 | b |
| SC | 7.3 ± 1.3 | a | 7.6 ± 0.8 | b | 6.3 ± 1.9 | a | 12.2 ± 1.4 | a | |
| NE | 6.8 ± 1.6 | a | 7.7 ± 1.6 | ab | 5.5 ± 1.8 | a | 5.8 ± 1.1 | b | |
| P (mg·g−1) | AH | 2.34 ± 0.49 | a | 3.76 ± 0.70 | a | 2.63 ± 0.74 | a | 1.20 ± 0.92 | ab |
| SC | 1.84 ± 0.76 | ab | 1.21 ± 0.30 | b | 1.95 ± 1.02 | ab | 2.45 ± 0.75 | a | |
| NE | 1.13 ± 0.29 | b | 1.17 ± 0.29 | b | 0.97 ± 0.17 | b | 0.39 ± 0.17 | b | |
| K (mg·g−1) | AH | 2.74 ± 0.42 | a | 2.65 ± 0.42 | a | 2.75 ± 0.36 | a | 2.17 ± 0.50 | a |
| SC | 2.70 ± 1.40 | a | 2.28 ± 0.45 | a | 2.21 ± 0.42 | a | 2.44 ± 0.34 | a | |
| NE | 2.07 ± 0.54 | a | 2.49 ± 0.60 | a | 2.52 ± 0.42 | a | 1.96 ± 0.37 | a | |
| Ca (mg·g−1) | AH | 14.8 ± 2.3 | a | 15.0 ± 2.2 | a | 13.4 ± 1.0 | a | 15.3 ± 2.3 | a |
| SC | 13.8 ± 2.2 | a | 13.8 ± 1.1 | a | 11.7 ± 1.0 | b | 12.5 ± 1.2 | b | |
| NE | 17.0 ± 2.2 | a | 15.7 ± 1.6 | a | 13.5 ± 1.7 | ab | 15.1 ± 1.81 | a | |
| Mg (mg·g−1) | AH | 1.85 ± 0.21 | ab | 1.64 ± 0.23 | a | 1.54 ± 0.18 | b | 1.46 ± 0.18 | a |
| SC | 1.70 ± 0.59 | b | 1.62 ± 0.27 | a | 1.51 ± 0.28 | b | 1.25 ± 0.12 | bc | |
| NE | 2.10 ± 0.32 | a | 1.71 ± 0.26 | a | 1.94 ± 0.19 | a | 1.36 ± 0.16 | ab | |
| Fe (µg·g−1) | AH | 0.99 ± 0.49 | a | 1.45 ± 0.53 | a | 1.38 ± 0.42 | a | 0.76 ± 0.87 | ab |
| SC | 0.82 ± 0.33 | a | 0.76 ± 0.19 | b | 0.64 ± 0.20 | b | 0.69 ± 0.26 | a | |
| NE | 1.49 ± 0.77 | a | 1.32 ± 0.33 | a | 0.98 ± 0.42 | ab | 0.38 ± 0.18 | b | |
| Mn (µg·g−1) | AH | 63 ± 17 | b | 70 ± 20 | a | 39 ± 9 | a | 31 ± 13 | a |
| SC | 73 ± 11 | b | 67 ± 18 | a | 28 ± 6 | b | 33 ± 8 | a | |
| NE | 138 ± 78 | a | 80 ± 20 | a | 34 ± 10 | ab | 24 ± 5 | a | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayama, M.; Yamanaka, T. Growth Characteristics of Ectomycorrhizal Seedlings of Quercus glauca, Quercus salicina, Quercus myrsinaefolia, and Castanopsis cuspidata Planted in Calcareous Soil. Forests 2016, 7, 266. https://doi.org/10.3390/f7110266
Kayama M, Yamanaka T. Growth Characteristics of Ectomycorrhizal Seedlings of Quercus glauca, Quercus salicina, Quercus myrsinaefolia, and Castanopsis cuspidata Planted in Calcareous Soil. Forests. 2016; 7(11):266. https://doi.org/10.3390/f7110266
Chicago/Turabian StyleKayama, Masazumi, and Takashi Yamanaka. 2016. "Growth Characteristics of Ectomycorrhizal Seedlings of Quercus glauca, Quercus salicina, Quercus myrsinaefolia, and Castanopsis cuspidata Planted in Calcareous Soil" Forests 7, no. 11: 266. https://doi.org/10.3390/f7110266






