Biomass Accumulation and Net Primary Production during the Early Stage of Secondary Succession after a Severe Forest Disturbance in Northern Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Measurements
2.2.1. Species and Biomass
2.2.2. Leaf Area Index
2.2.3. Net Primary Production
2.3. Statistical Analyses
3. Results
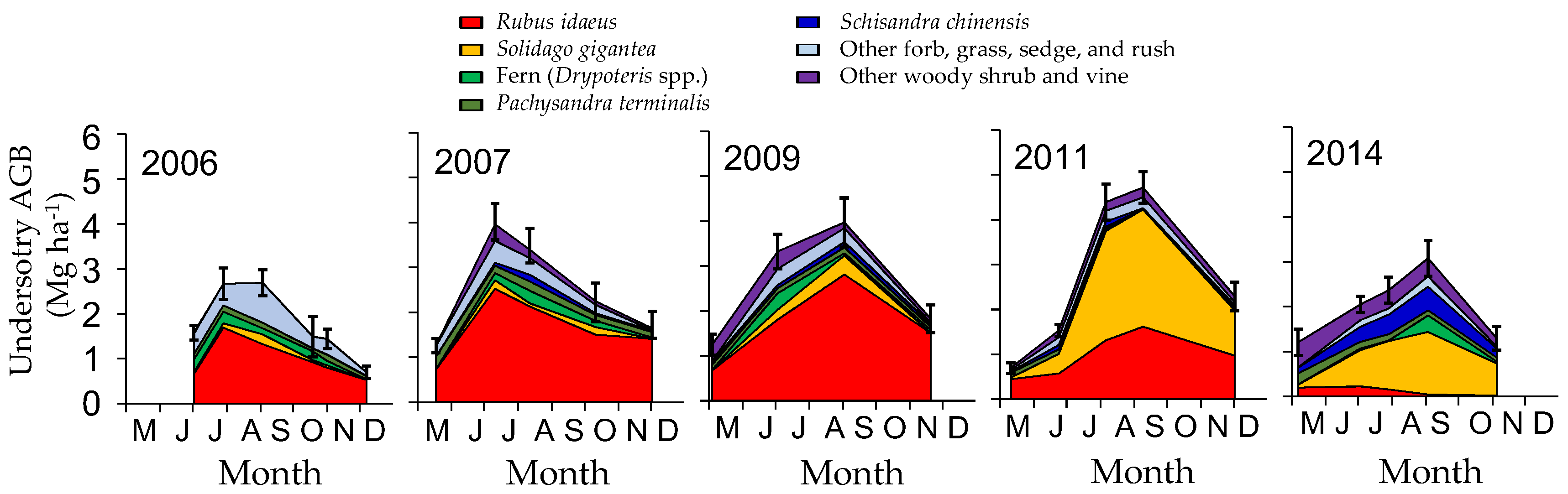
3.1. Change in Aboveground Biomass and Species Composition
3.2. Regeneration of Deciduous Trees
3.3. Leaf Area Index
3.4. Belowground Biomass
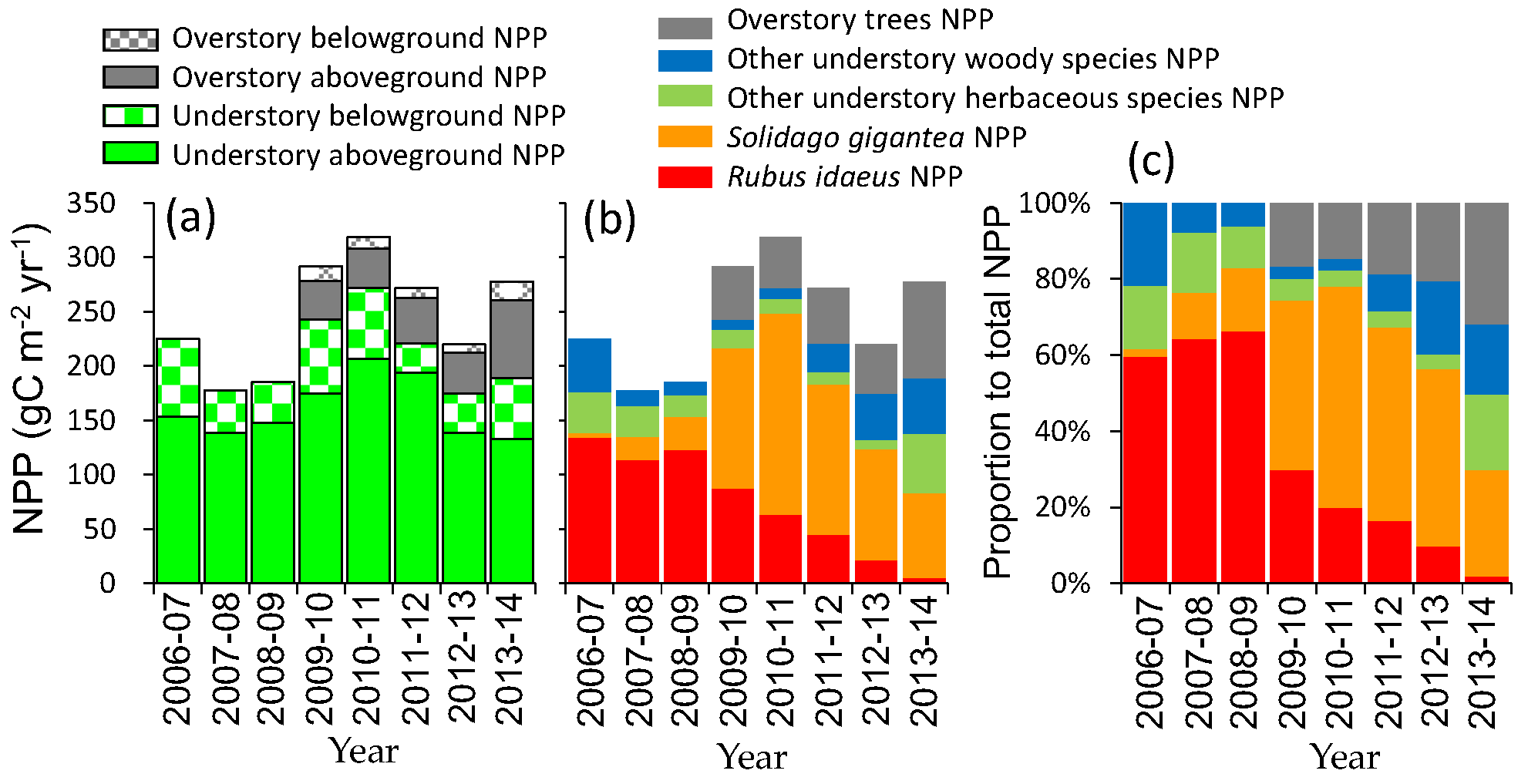
3.5. Net Primary Production
4. Discussion
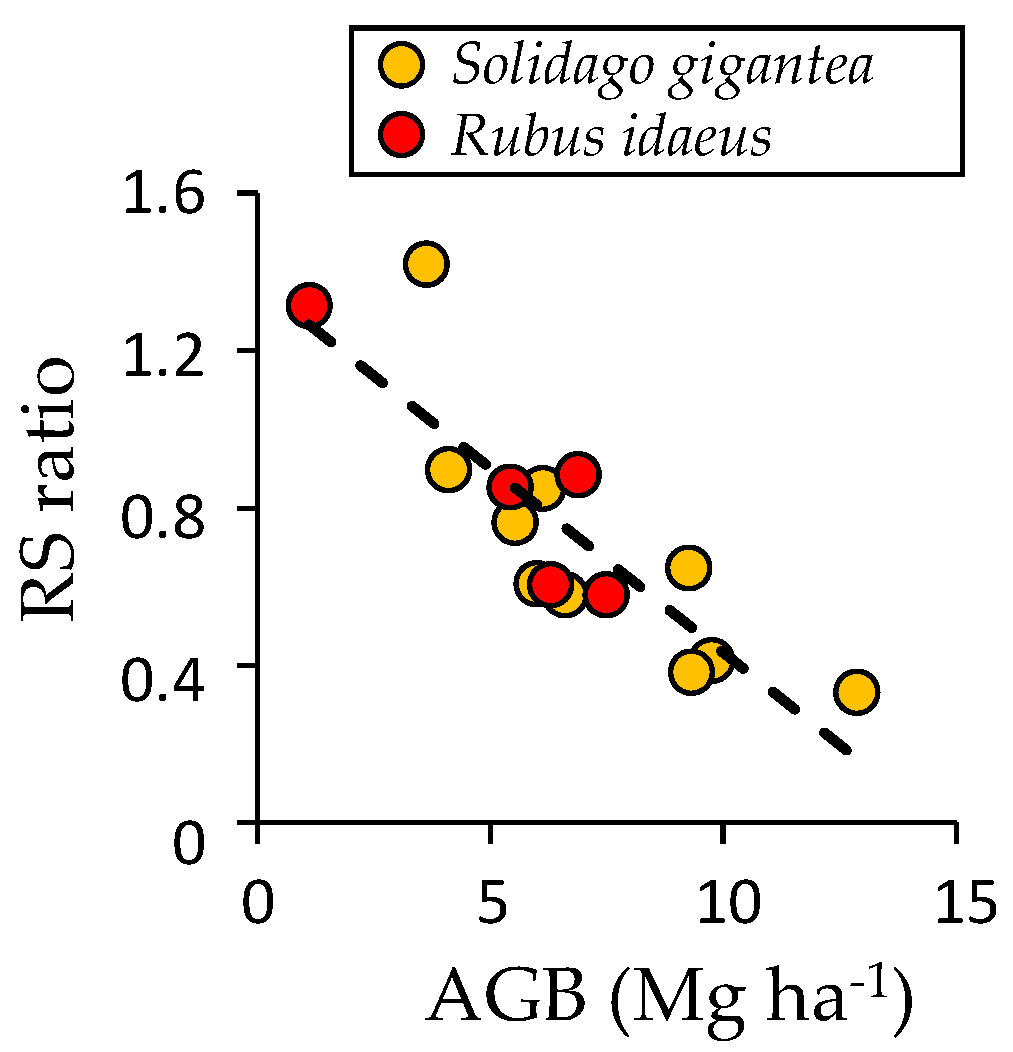
4.1. Understory Species after Disturbance
4.2. Secondary Forest Recovery and Biomass Accumulation
4.3. Change in Productivity during Secondary Succession
4.4. Possible Effects of Invasive Species on Succession and Carbon Accumulation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Species | Stand Density | Aboveground Biomass | ||||||
|---|---|---|---|---|---|---|---|---|
| Taller Than 0.5 m | Taller Than 2 m | Taller Than 0.5 m | Taller Than 2 m | |||||
| (trees ha−1) | (%) | (trees ha−1) | (%) | (Mg·ha−1) | (%) | (Mg·ha−1) | (%) | |
| Betula platyphylla Sukaczev var. japonica (Miq.) H.Hara | 1775 | 40% | 1133 | 67% | 1.732 | 67% | 1.671 | 72% |
| Betula ermanii Cham. | 483 | 11% | 117 | 7% | 0.289 | 11% | 0.255 | 11% |
| Magnolia kobus DC. var. borealis Sarg. | 358 | 8% | 100 | 6% | 0.175 | 7% | 0.148 | 6% |
| Maackia amurensis Rupr. et Maxim. | 275 | 6% | 133 | 8% | 0.090 | 4% | 0.064 | 3% |
| Cerasus maximowiczii (Rupr.) Kom. | 350 | 8% | 67 | 4% | 0.055 | 2% | 0.036 | 2% |
| Quercus crispula Blume | 67 | 2% | 17 | 1% | 0.045 | 2% | 0.033 | 1% |
| Hydrangea paniculata Siebold | 217 | 5% | 17 | 1% | 0.039 | 2% | 0.021 | 1% |
| Magnolia obovata Thunb. | 125 | 3% | 17 | 1% | 0.038 | 1% | 0.032 | 1% |
| Syringa reticulata (Blume) H.Hara | 50 | 1% | 25 | 1% | 0.021 | 1% | 0.016 | 1% |
| Tilia japonica (Miq.) Simonk. | 8 | 0% | 8 | 0% | 0.021 | 1% | 0.021 | 1% |
| Aria alnifolia (Siebold et Zucc.) Decne. | 75 | 2% | 8 | 0% | 0.015 | 1% | 0.013 | 1% |
| Larix kaempferi (Lamb.) Carriere | 67 | 2% | 8 | 0% | 0.013 | 1% | 0.004 | 0% |
| Betula maximowicziana Regel | 58 | 1% | 17 | 1% | 0.013 | 0% | 0.009 | 0% |
| Fraxinus lanuginosa Koidz. f. serrata (Nakai) Murata | 92 | 2% | 25 | 1% | 0.009 | 0% | 0.006 | 0% |
| Ligustrum tschonoskii Decne. | 233 | 5% | 0 | 0% | 0.007 | 0% | 0.000 | 0% |
| Crataegus chlorosarca Maxim. | 42 | 1% | 0 | 0% | 0.003 | 0% | 0.000 | 0% |
| Ulmus davidiana Planch var. japonica Nakai | 83 | 2% | 0 | 0% | 0.003 | 0% | 0.000 | 0% |
| Sorbaria sorbifolia (L.) A.Braun var. stellipila Maxim. | 17 | 0% | 0 | 0% | 0.001 | 0% | 0.000 | 0% |
| Aralia elata (Miq.) Seem. | 8 | 0% | 0 | 0% | 0.000 | 0% | 0.000 | 0% |
| Total | 4383 | 100% | 1692 | 100% | 2.570 | 100% | 2.328 | 100% |
References
- Amiro, B.; Barr, A.; Barr, J.; Black, T.; Bracho, R.; Brown, M.; Chen, J.; Clark, K.; Davis, K.; Desai, A.; et al. Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J. Geophys. Res. Biogeosci. 2010, 115. [Google Scholar] [CrossRef]
- Sano, T.; Hirano, T.; Liang, N.; Hirata, R.; Fujinuma, Y. Carbon dioxide exchange of a larch forest after a typhoon disturbance. For. Ecol. Manag. 2010, 260, 2214–2223. [Google Scholar] [CrossRef]
- Takagi, K.; Fukuzawa, K.; Liang, N.; Kayama, M.; Nomura, M.; Hojyo, H.; Sugata, S.; Shibata, H.; Fukazawa, T.; Takahashi, Y.; et al. Change in CO2 balance under a series of forestry activities in a cool-temperate mixed forest with dense undergrowth. Glob. Chang. Biol. 2009, 15, 1275–1288. [Google Scholar] [CrossRef]
- Goulden, M.; McMillan, A.; Winston, G.; Rocha, A.; Manies, K.; Harden, J.; Bond-Lamberty, B. Patterns of NPP, GPP, respiration, and NEP during boreal forest succession. Glob. Chang. Biol. 2011, 17, 855–871. [Google Scholar] [CrossRef]
- Myneni, R.; Dong, J.; Tucker, C.; Kaufmann, R.; Kauppi, P.; Liski, J.; Zhou, L.; Alexeyev, V.; Hughes, M. A large carbon sink in the woody biomass of northern forests. Proc. Natl. Acad. Sci. USA 2001, 98, 14784–14789. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.; Fang, J.; Houghton, R.; Kauppi, P.; Kurz, W.; Phillips, O.; Shvidenko, A.; Lewis, S.; Canadell, J.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.; Likens, G. Carbon in the biota. In Carbon and the Biosphere, Proceedings of the 24th Brookhaven Symposium in Biology, Upton, NY, USA, 16–18 May 1972; Technical Information Center, US Atomic Energy Commission: Washington, DC, USA, 1973. [Google Scholar]
- Ooba, M.; Hayashi, K.; Machimura, T.; Matsui, T. Assessments of regional carbon circulation from multiple aspects by a biogeochemical model: A case study of forests in Toyota, Japan. J. Agric. Meteorol. 2014, 70, 41–54. [Google Scholar] [CrossRef]
- Dale, V.; Joyce, L.; McNulty, S.; Neilson, R.; Ayres, M.; Flannigan, M.; Hanson, P.; Irland, L.; Lugo, A.; Peterson, C.; et al. Climate change and forest disturbances. Bioscience 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Running, S. Is global warming causing more, larger wildfires? Science 2006, 313, 927–928. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.; Fisher, J.; Zeng, H.; Chapman, E.; Baker, D.; Hurtt, G. Hurricane Katrina’s carbon footprint on U.S. Gulf Coast forests. Science 2007, 318, 1107. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2007: The AR4 Synthesis Report; IPCC: Geneva, Switzerland, 2007; p. 104. [Google Scholar]
- Ayres, M.; Lombardero, M. Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Sci. Total Environ. 2000, 262, 263–286. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.; Miller, A.; Mohan, J.; Hudiburg, T.; Duval, B.; DeLucia, E. Altered dynamics of forest recovery under a changing climate. Glob. Chang. Biol. 2013, 19, 2001–2021. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, J.; Futuyma, D.; Shafer, H.; Simberloff, D. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Liao, C.; Peng, R.; Luo, Y.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol. 2008, 177, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Hirata, R.; Takagi, K.; Ito, A.; Hirano, T.; Saigusa, N. The impact of climate variation and disturbances on the carbon balance of forests in Hokkaido, Japan. Biogeosciences 2014, 11, 5139–5154. [Google Scholar] [CrossRef]
- MacLean, D.; Wein, R. Changes in understory vegetation with increasing stand age in New Brunswick forests: Species composition, cover, biomass, and nutrients. Can. J. Bot. 1977, 55, 2818–2831. [Google Scholar] [CrossRef]
- Nakashizuka, T.; Numata, M. Regeneration process of climax beech forest 1: Structure of a beech forest with the undergrowth of Sasa. Jpn. J. Ecol. 1982, 32, 57–67. [Google Scholar]
- Messier, C.; Kimmins, J. Above- and below-ground vegetation recovery in recently clearcut and burned sites dominated by Gaultheria shallon in coastal British Columbia. For. Ecol. Manag. 1991, 46, 275–294. [Google Scholar] [CrossRef]
- Wilson, A.; Shure, D. Plant competition and nutrient limitation during early succession in the southern Appalachian Mountains. Am. Midl. Nat. 1993, 129, 1–9. [Google Scholar] [CrossRef]
- Norby, R.; Kozlowski, T. Allelopathic potential of ground cover species on Pinus resinosa seedlings. Plant Soil 1980, 57, 363–374. [Google Scholar] [CrossRef]
- Alaback, P. Dynamics of understory biomass in Sitka spruce western hemlock forests of Southeast Alaska. Ecology 1982, 63, 1932–1948. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S. Net primary production and net ecosystem production of a boreal black spruce wildfire chronosequence. Glob. Chang. Biol. 2004, 10, 473–487. [Google Scholar] [CrossRef]
- Elliott, K.; Boring, L.; Swank, W. Aboveground biomass and nutrient accumulation 20 years after clear-cutting a southern Appalachian watershed. Can. J. For. Res. 2002, 32, 667–683. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, A.; Comeau, P.; Tsze, M.; Kimmins, J. Aboveground biomass and nutrient accumulation in an age sequence of aspen (Populus tremuloides) stands in the boreal white and black spruce zone, British-Columbia. For. Ecol. Manag. 1995, 78, 127–138. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, A.; Simard, S.; Kimmins, J. Aboveground biomass and nutrient accumulation in an age sequence of paper birch (Betula papyrifera) in the interior cedar hemlock zone, British Columbia. For. Ecol. Manag. 1996, 83, 27–38. [Google Scholar] [CrossRef]
- Hiura, T.; Sano, J.; Konno, Y. Age structure and response to fine scale disturbances of Abies sachalinensis, Picea jezoensis, Picea glehnii, and Betula ermanii growing under the influence of a dwarf bamboo understory in northern Japan. Can. J. For. Res. 1996, 26, 289–297. [Google Scholar] [CrossRef]
- Mou, P.; Fahey, T.; Hughes, J. Effects of soil disturbance on vegetation recovery and nutrient accumulation following whole-tree harvest of a northern hardwood ecosystem. J. Appl. Ecol. 1993, 30, 661–675. [Google Scholar] [CrossRef]
- Hokkaido Forestry Research Institute. Flash report on wind damage caused by typhoon No. 18 in 2004. Kohshunai Seas. Rep. 2004, 137, 1–12. [Google Scholar]
- Hirano, T.; Hirata, R.; Fujinuma, Y.; Saigusa, N.; Yamamoto, S.; Harazono, Y.; Takada, M.; Inukai, K.; Inoue, G. CO2 and water vapor exchange of a larch forest in northern Japan. Tellus Ser. B. 2003, 55, 244–257. [Google Scholar] [CrossRef]
- Hirata, R.; Hirano, T.; Saigusa, N.; Fujinuma, Y.; Inukai, K.; Kitamori, Y.; Takahashi, Y.; Yamamoto, S. Seasonal and interannual variations in carbon dioxide exchange of a temperate larch forest. Agric. For. Meteorol. 2007, 147, 110–124. [Google Scholar] [CrossRef]
- Hirano, T.; Suzuki, K.; Hirata, R. Energy balance and evapotranspiration changes in a larch forest caused by severe disturbance during an early secondary succession. Agric. For. Meteorol. 2017, 232, 457–468. [Google Scholar] [CrossRef]
- Sakai, Y.; Takahashi, M.; Tanaka, N. Root biomass and distribution of a Picea-Abies stand and a Larix-Betula stand in pumiceous Entisols in Japan. J. For. Res. 2007, 12, 120–125. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Meteorological Data. 2016. Available online: http://www.data.jma.go.jp/obd/stats/etrn/index.php (accessed on 1 May 2016). (In Japanese) [Google Scholar]
- Yonekura, K.; Kajita, T. BG Plants YList: An Online Service of Japanese Plant Names, Including a Nomenclature Index. 2003. Available online: http://ylist.info (accessed on 5 September 2016).
- Takeda, T.; Oguma, H.; Sano, T.; Yone, Y.; Fujinuma, Y. Estimating the plant area density of a Japanese larch (Larix kaempferi Sarg.) plantation using a ground-based laser scanner. Agric. For. Meteorol. 2008, 148, 428–438. [Google Scholar] [CrossRef]
- Sano, T. Effect of Typhoon Disturbance on the Exchange and Accumulation of Carbon in a Northern Forest Ecosystem. Ph.D. Thesis, Hokkaido University, Hokkaido, Japan, 2011; p. 107. [Google Scholar]
- Sano, T.; Hirano, T.; Takeda, T.; Fujinuma, Y. Performance of optical indirect methods to assess the change in leaf area index of a larch plantation through thinning. J. Agric. Meteorol. 2012, 68, 35–43. [Google Scholar] [CrossRef]
- Sprugel, D. Correcting for bias in log-transformed allometric equations. Ecology 1983, 64, 209–210. [Google Scholar] [CrossRef]
- Yamamoto, K. LIA32. 2003. Available online: http://www.agr.nagoya-u.ac.jp/~shinkan/lia32/index.html (accessed on 1 May 2016). (In Japanese)
- Johnson, C.; Zarin, D.; Johnson, A. Post-disturbance aboveground biomass accumulation in global secondary forests. Ecology 2000, 81, 1395–1401. [Google Scholar] [CrossRef]
- Clark, D.; Brown, S.; Kicklighter, D.; Chambers, J.; Thomlinson, J.; Ni, J. Measuring net primary production in forests: Concepts and field methods. Ecol. Appl. 2001, 11, 356–370. [Google Scholar] [CrossRef]
- Gill, R.; Jackson, R. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- Palviainen, M.; Finer, L.; Mannerkoski, H.; Piirainen, S.; Starr, M. Responses of ground vegetation species to clear-cutting in a boreal forest: Aboveground biomass and nutrient contents during the first 7 years. Ecol. Res. 2005, 20, 652–660. [Google Scholar] [CrossRef]
- Yashiro, Y.; Shizu, Y.; Adachi, T.; Ohtsuka, T.; Lee, N.; Iimura, Y.; Koizumi, H. The effect of dense understory dwarf bamboo (Sasa senanensis) on soil respiration before and after clearcutting of cool temperate deciduous broad-leaved forest. Ecol. Res. 2012, 27, 577–586. [Google Scholar] [CrossRef]
- Marks, P. The role of pin cherry (Prunus pensylvanica L.) in the maintenance of stability in northern hardwood ecosystems. Ecol. Monogr. 1974, 44, 73–88. [Google Scholar] [CrossRef]
- Boring, L.; Swank, W. The role of black locust (Robinia pseudo-acacia) in forest succession. J. Ecol. 1984, 72, 749–766. [Google Scholar] [CrossRef]
- Hendrickson, O. Biomass and nutrients in regenerating woody vegetation following whole-tree and conventional harvest in a northern mixed forest. Can. J. For. Res. 1988, 18, 1427–1436. [Google Scholar] [CrossRef]
- Hughes, J.; Fahey, T. Colonization dynamics of herbs and shrubs in a disturbed northern hardwood forest. J. Ecol. 1991, 79, 605–616. [Google Scholar] [CrossRef]
- Toyooka, H.; Ishizuka, M.; Osawa, A.; Kushima, H.; Kanazawa, Y.; Sato, A. Forest succession over a thirty-four year period following a catastrophic windstorm in the headwaters of the river Ishikari, Hokkaido. Bull. For. For. Prod. Res. Inst. 1992, 363, 59–151. [Google Scholar]
- Ricard, J.; Messier, C. Abundance, growth and allometry of red raspberry (Rubus idaeus L.) along a natural light gradient in a northern hardwood forest. For. Ecol. Manag. 1996, 81, 153–160. [Google Scholar] [CrossRef]
- Jobidon, R. Short-term effect of three mechanical site preparation methods on species diversity. Tree Plant. Notes 1990, 41, 39–42. [Google Scholar]
- Noguchi, M.; Yoshida, T. Factors influencing the distribution of two co-occurring dwarf bamboo species (Sasa kurilensis and S. senanensis) in a conifer-broad leaved mixed stand in northern Hokkaido. Ecol. Res. 2005, 20, 25–30. [Google Scholar] [CrossRef]
- Weber, E.; Jakobs, G. Biological flora of central Europe: Solidago gigantea Aiton. Flora 2005, 200, 109–118. [Google Scholar] [CrossRef]
- Igarashi, H. Handbook of Naturalized Plant Species in Hokkaido 2000; Hokkaido Wildlife Laboratory: Sapporo, Japan, 2001. [Google Scholar]
- Morimoto, J.; Morimoto, M.; Nakamura, F. Initial vegetation recovery following a blowdown of a conifer plantation in monsoonal East Asia: Impacts of legacy retention, salvaging, site preparation, and weeding. For. Ecol. Manag. 2011, 261, 1353–1361. [Google Scholar] [CrossRef]
- Bartha, S.; Szentes, S.; Horvath, A.; Hazi, J.; Zimmermann, Z.; Molnar, C.; Dancza, I.; Margoczi, K.; Pal, R.; Purger, D.; et al. Impact of mid-successional dominant species on the diversity and progress of succession in regenerating temperate grasslands. Appl. Veg. Sci. 2014, 17, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Tsuyuzaki, S. Vegetation development patterns on skislopes in lowland Hokkaido, northern Japan. Biol. Cons. 2002, 108, 239–246. [Google Scholar] [CrossRef]
- Archambault, L.; Morissette, J.; Bernier-Cardou, M. Forest succession over a 20-year period following clearcutting in balsam fir yellow birch ecosystems of eastern Quebec, Canada. For. Ecol. Manag. 1998, 102, 61–74. [Google Scholar] [CrossRef]
- Whitney, G. The productivity and carbohydrate economy of a developing stand of Rubus idaeus. Can. J. Bot. 1982, 60, 2697–2703. [Google Scholar] [CrossRef]
- Nord, E.; Lynch, J. Plant phenology: A critical controller of soil resource acquisition. J. Exp. Bot. 2009, 60, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Hirano, T.; Zheng, Z.; Tang, J.; Fujinuma, Y. Soil CO2 efflux of a larch forest in northern Japan. Biogeosciences 2010, 7, 3447–3457. [Google Scholar] [CrossRef]
- Del Fabbro, C.; Gusewell, S.; Prati, D. Allelopathic effects of three plant invaders on germination of native species: A field study. Biol. Invasions 2014, 16, 1035–1042. [Google Scholar] [CrossRef]


| Variables | Unit | 2003 a | 2010 | 2014 |
|---|---|---|---|---|
| Elapsed years after disturbance | years | –1 b | +5 | +9 |
| Population | trees ha−1 | 1179 c | 3190 ± 1210 d | 4380 ± 2580 d |
| Mean height | m | 12.2 c | 1.49 | 2.02 |
| Maximum height | m | No data | 4.10 | 6.10 |
| Basal area | m2·ha−1 | 29.7 | 0.19 ± 0.06 d | 1.12 ± 0.26 d |
| Dominant species | Larix kaempferi | Betula platyphylla | ||
| Ulmus davidiana | Betula ermanii | |||
| Year | Understory (Mg·ha−1) | Overstory (Mg·ha−1) | Total BGB (Mg·ha−1) | Contribution to Total Biomass % |
|---|---|---|---|---|
| 2006 | 3.01 ± 0.32 | 3.01 ± 0.36 | 53 | |
| 2007 | 3.57 ± 0.51 | 3.57 ± 0.54 | 51 | |
| 2009 | 3.97 ± 0.54 | 3.97 ± 0.54 | 50 | |
| 2011 | 4.38 ± 0.33 | 0.45 ± 0.15 | 4.83 ± 0.34 | 45 |
| 2012 | 4.20 ± 0.39 | 0.59 ± 0.16 | 4.79 ± 0.41 | 44 |
| 2013 | 3.25 ± 0.34 | 0.68 ± 0.19 | 3.94 ± 0.42 | 44 |
| 2014 | 3.33 ± 0.43 | 0.96 ± 0.25 | 4.29 ± 0.53 | 42 |
| Site | Years Since Disturbance | AGB | BGB | Total Biomass | Disturbance 1 | Dominant Species | Dominant Species Type 2 | Reference |
|---|---|---|---|---|---|---|---|---|
| (years) | (Mg·ha−1) | (Mg·ha−1) | (Mg·ha−1) | |||||
| Hokkaido, Japan | 2 | 2.69 | 3.01 | 5.70 | WSL | Rubus idaeus | S | This study |
| 5 | 3.97 | 3.97 | 7.94 | Rubus idaeus | S | |||
| 7 | 5.94 | 4.83 | 10.77 | Solidago gigantea | H | |||
| 10 | 5.89 | 4.29 | 10.17 | Solidago gigantea and Betula spp. | HT | |||
| British Columbia, Canada | 2 | 1.4 | 1.9 | 3.3 | CB | Gauletheria shallon | S | [20] |
| 4 | 4.2 | 4.1 | 8.3 | Gauletheria shallon | S | |||
| 8 | 5.6 | 11.4 | 17.0 | Gauletheria shallon | S | |||
| Eastern Finland | 7 | 2.3 | NA | NA | C | Vaccinium spp. and moss | SM | [45] |
| Gifu, central Japan | 9–12 | 5.9 | 14.0 | 19.9 | C | Sasa senanensis | S | [46] |
| New Hampshire, USA | 1 | 1.4 | NA | NA | C | Prunus pensylvanica | T | [47] |
| 4 | 17.5 | NA | NA | Prunus pensylvanica | T | |||
| 6 | 32.6 | NA | NA | Prunus pensylvanica | T | |||
| New Hampshire, USA | 1 | NA | NA | 1.5 | C | Betula spp. and Prunus pensylvanica | T | [24] |
| 2 | NA | NA | 5.8 | Prunus pensylvanica | T | |||
| 3 | NA | NA | 14.2 | Prunus pensylvanica | T | |||
| 4 | NA | NA | 19.5 | Prunus pensylvanica | T | |||
| 5 | NA | NA | 31.3 | Prunus pensylvanica | T | |||
| 6 | NA | NA | 34.6 | Prunus pensylvanica | T | |||
| North Carolina, USA | 4 | 21.9 | 10.7 | 32.6 | C | Robinia pseudo-acacia | T | [48] |
| Ontario, Canada | 4 | 7.9 | NA | NA | C | Populus spp. | T | [49] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazaki, T.; Hirano, T.; Sano, T. Biomass Accumulation and Net Primary Production during the Early Stage of Secondary Succession after a Severe Forest Disturbance in Northern Japan. Forests 2016, 7, 287. https://doi.org/10.3390/f7110287
Yazaki T, Hirano T, Sano T. Biomass Accumulation and Net Primary Production during the Early Stage of Secondary Succession after a Severe Forest Disturbance in Northern Japan. Forests. 2016; 7(11):287. https://doi.org/10.3390/f7110287
Chicago/Turabian StyleYazaki, Tomotsugu, Takashi Hirano, and Tomohito Sano. 2016. "Biomass Accumulation and Net Primary Production during the Early Stage of Secondary Succession after a Severe Forest Disturbance in Northern Japan" Forests 7, no. 11: 287. https://doi.org/10.3390/f7110287






