Monitoring Changes in Water Use Efficiency to Understand Drought Induced Tree Mortality
Abstract
1. Introduction
2. Materials and Methods
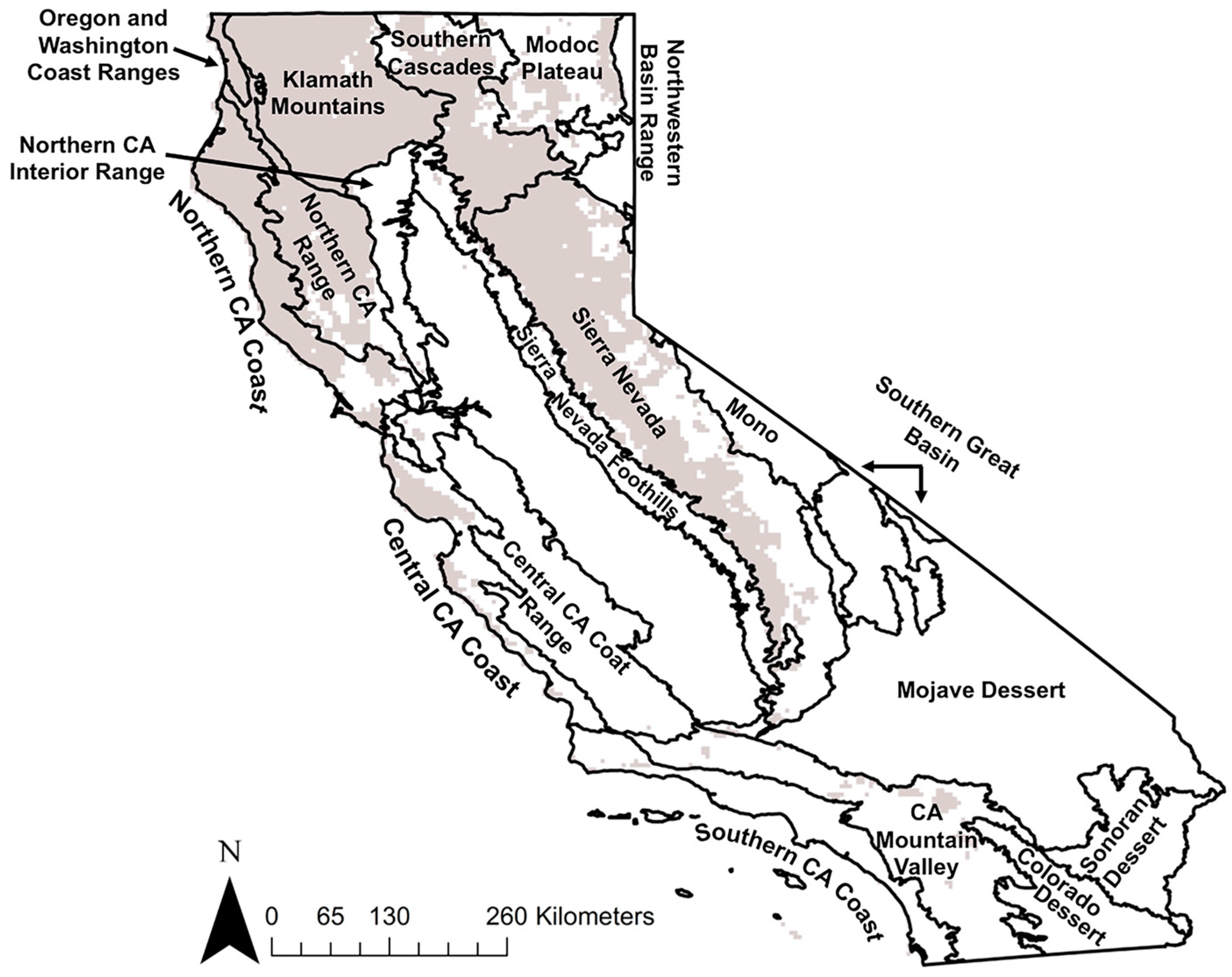
2.1. California Forests
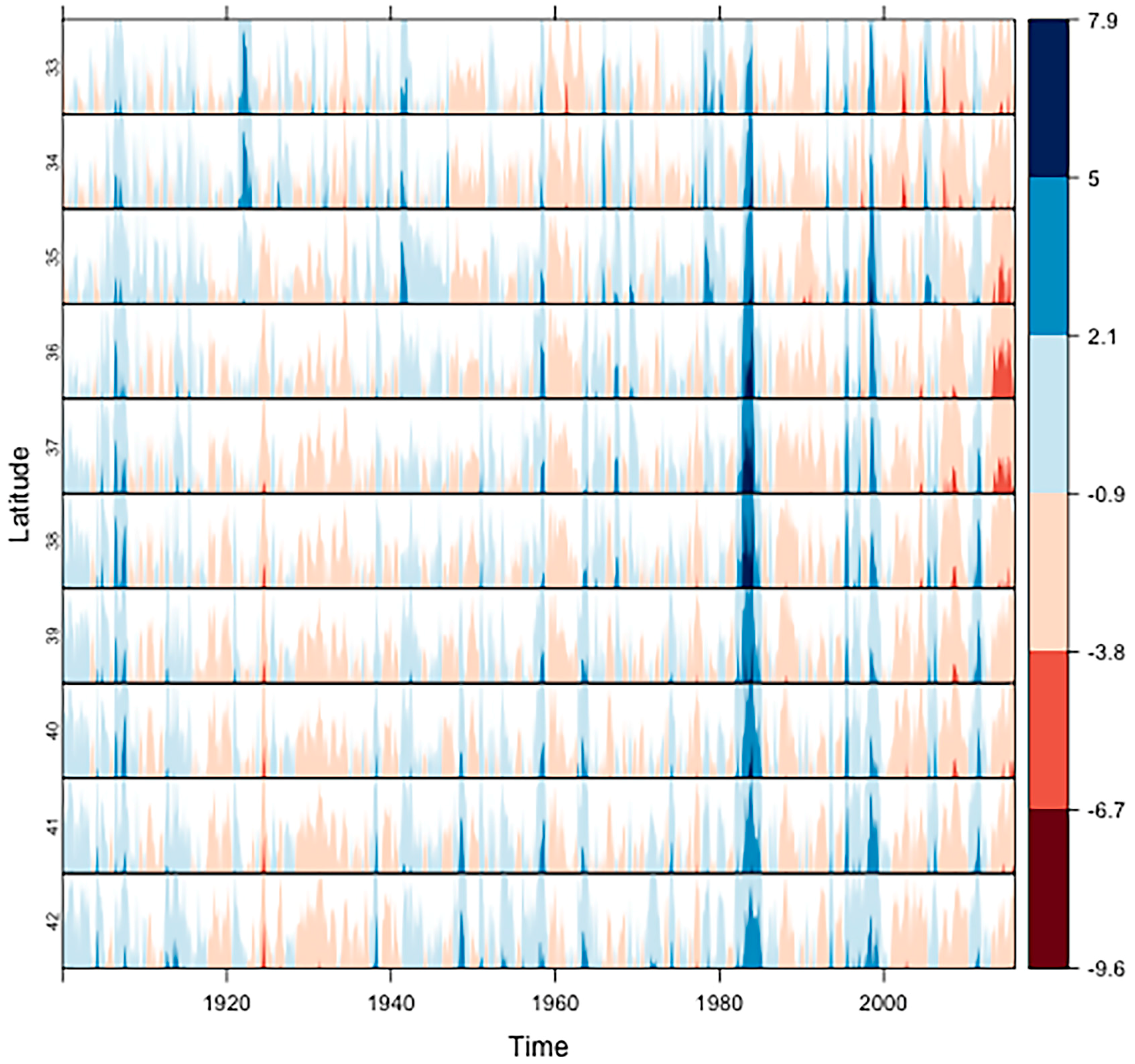
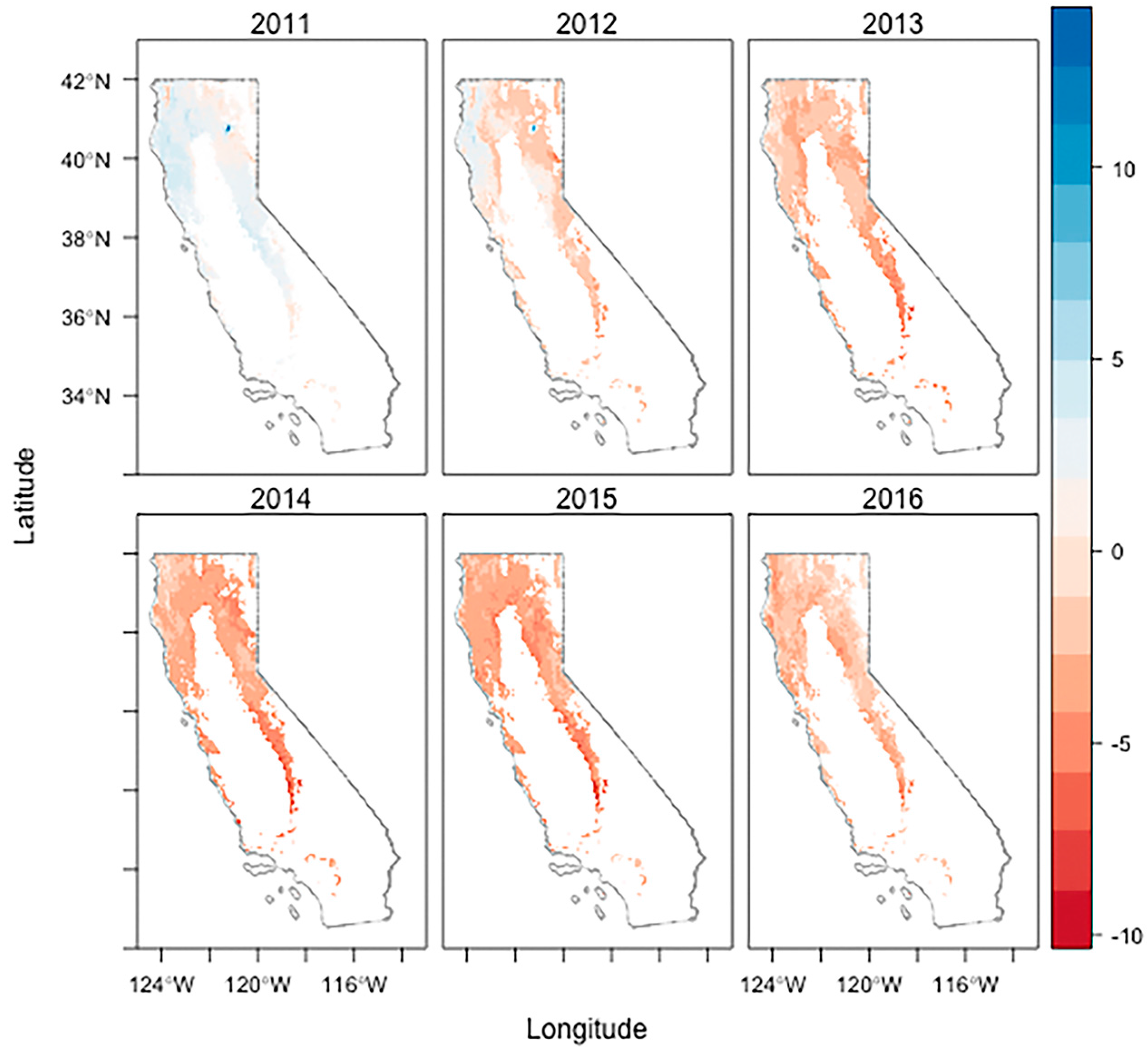
2.2. The Most Severe Drought on Record
2.3. Ecosystem Water Use Efficiency
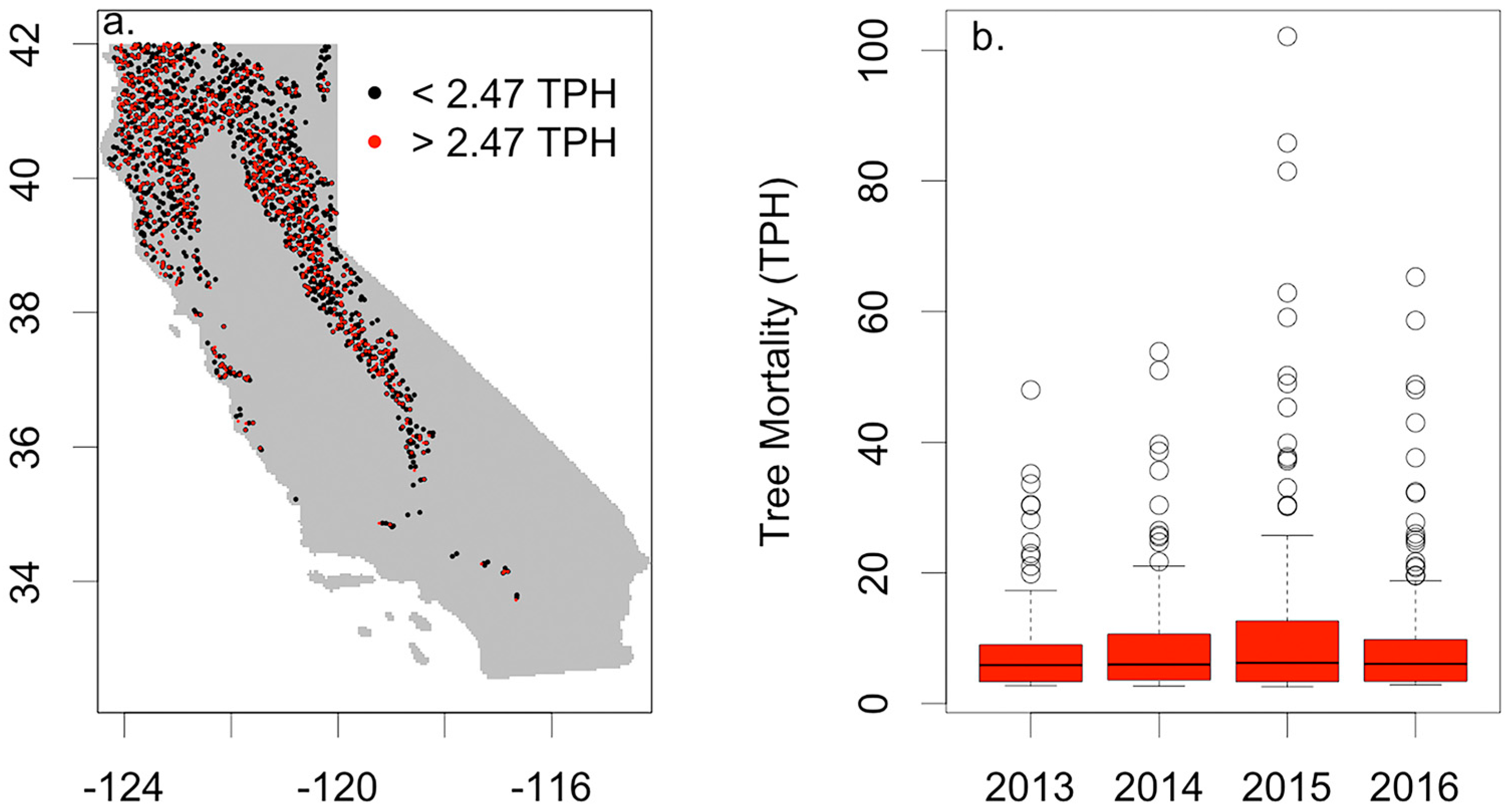
2.4. Tree Mortality
2.5. Statistical Analysis
3. Results
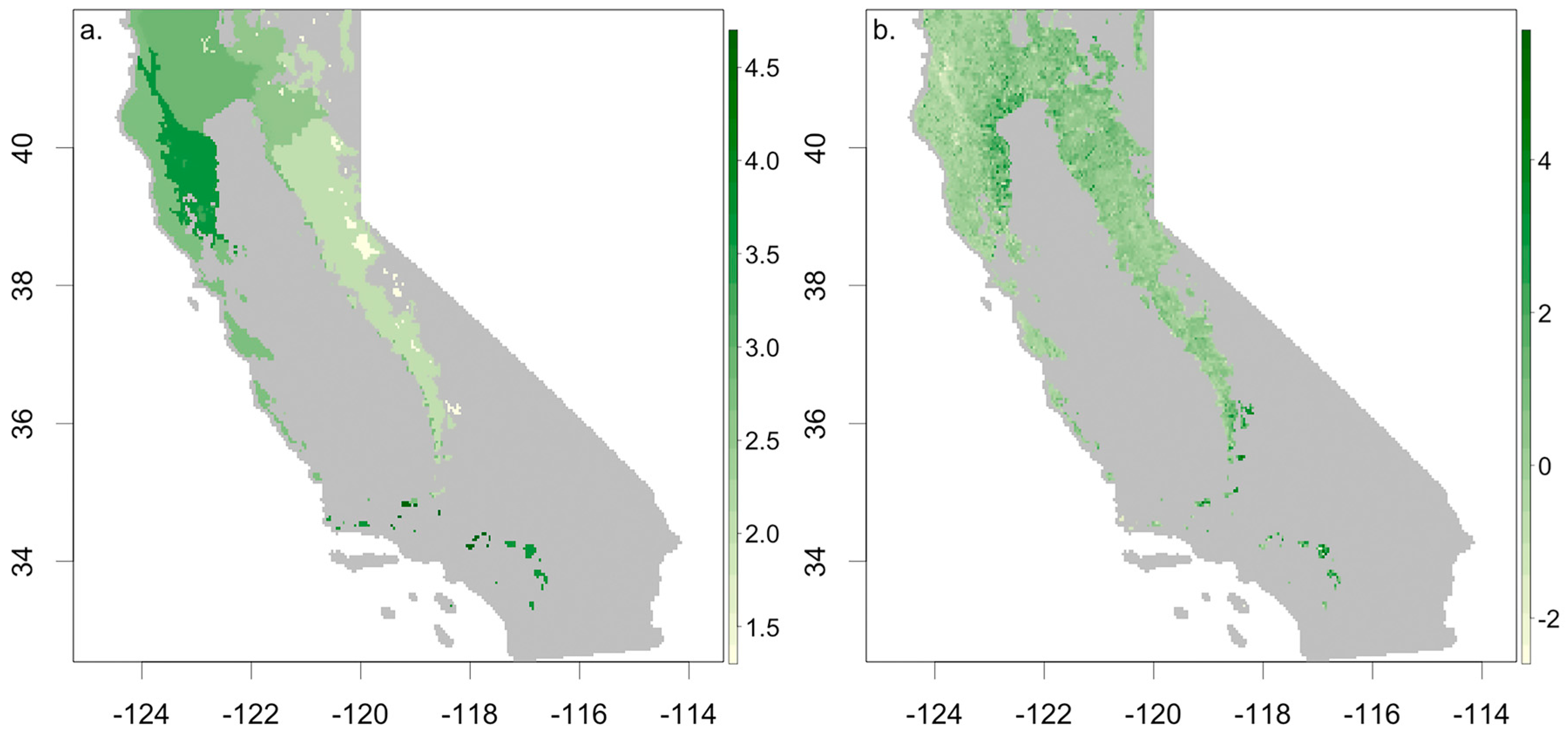
3.1. Ecosystem Water Use Efficiency
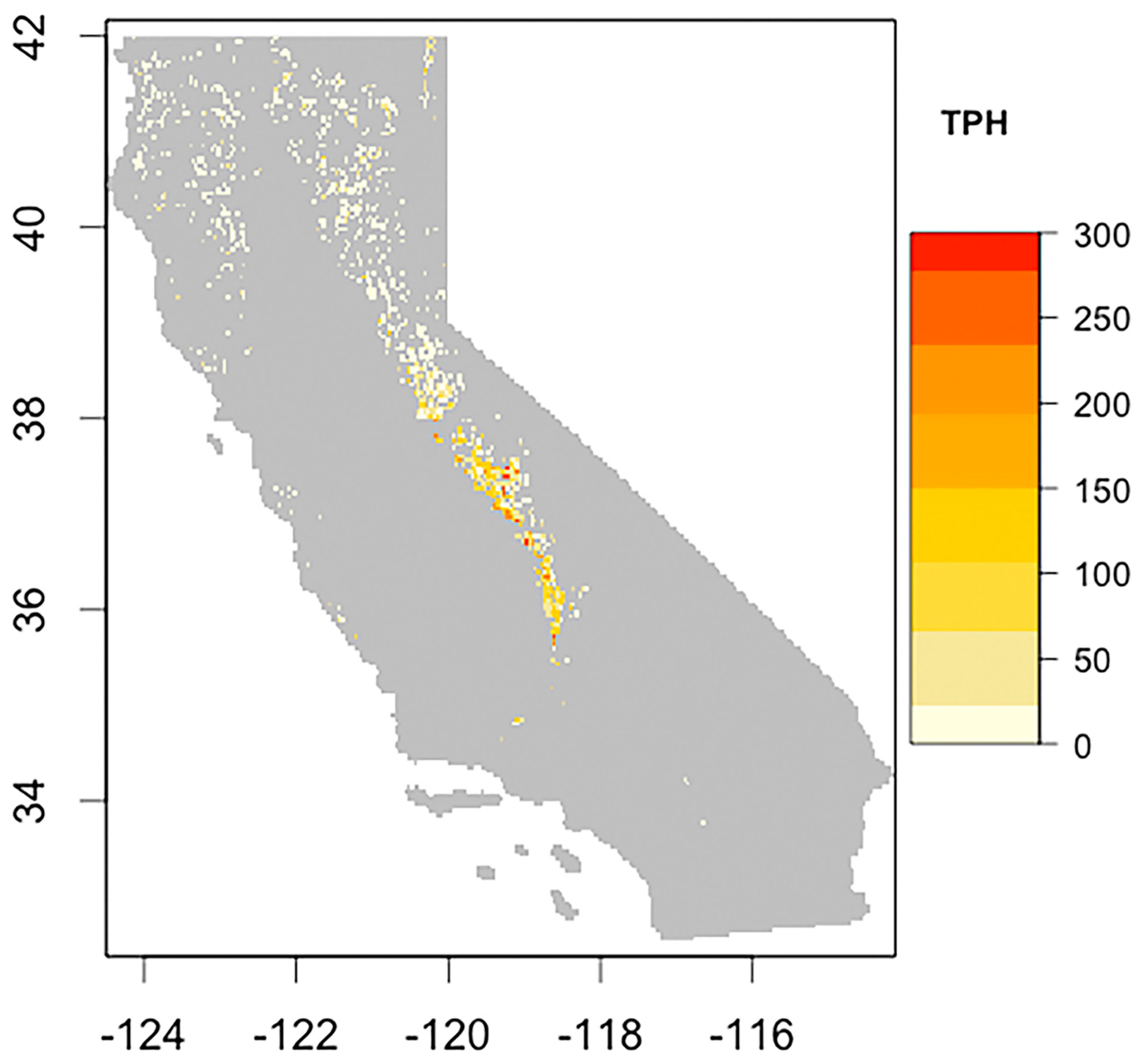
3.2. Drought Induced Tree Mortality
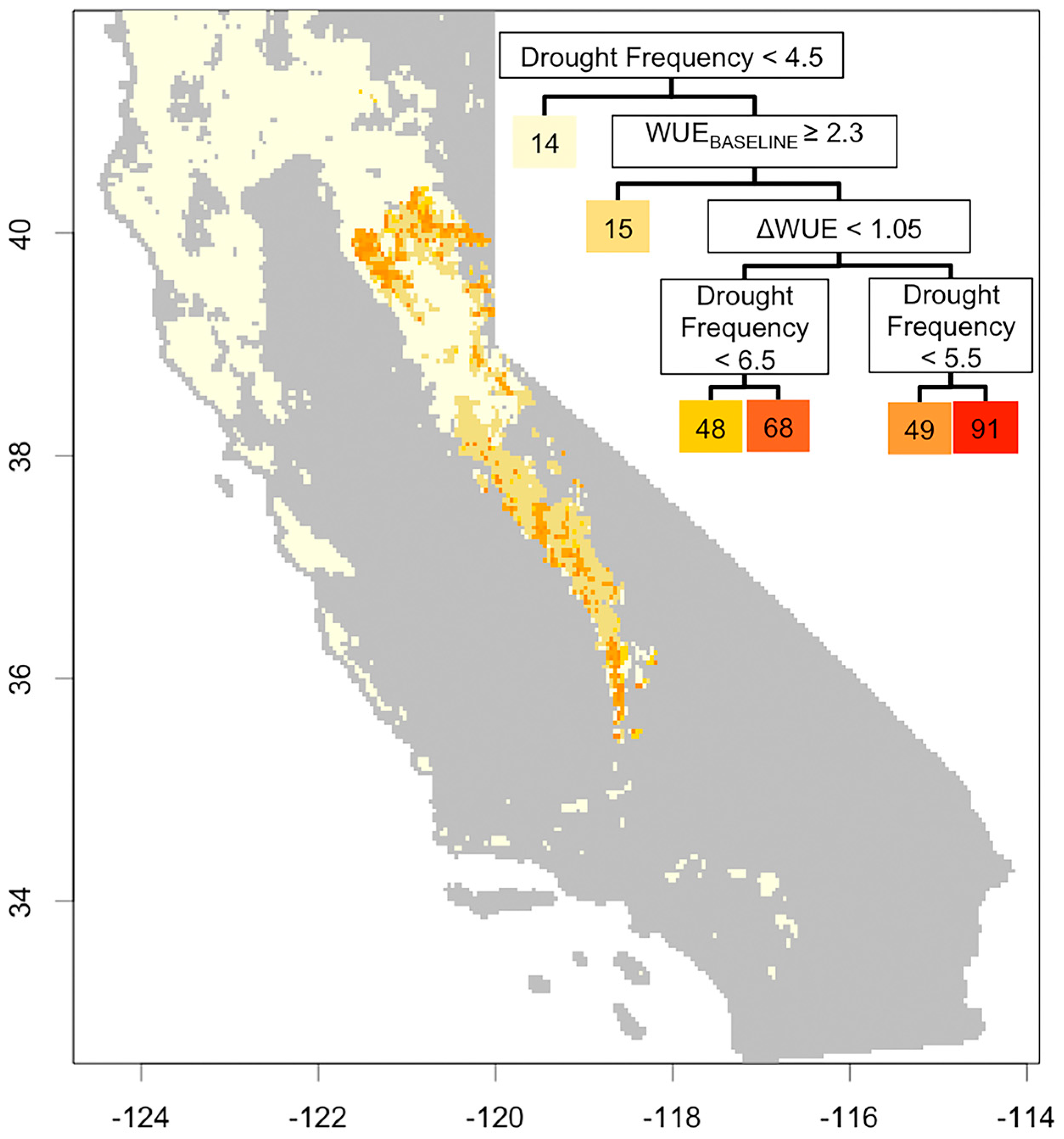
3.3. Understanding Drought Induced Tree Mortality
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Allen, C.D.; Breshears, D.D. Drought-induced shift of a forest–woodland ecotone: Rapid landscape response to climate variation. Proc. Natl. Acad. Sci. USA 1998, 95, 14839–14842. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Boisvert-Marsh, L.; Perie, C.; de Blois, S. Shifting with climate? Evidence for recent changes in tree species distribution at high latitudes. Ecosphere 2014, 5, 1–33. [Google Scholar] [CrossRef]
- Guarín, A.; Taylor, A.H. Drought triggered tree mortality in mixed conifer forests in Yosemite National Park, California, USA. For. Ecol. Manag. 2005, 218, 229–244. [Google Scholar] [CrossRef]
- Asner, G.P.; Brodrick, P.G.; Anderson, C.B.; Vaughn, N.; Knapp, D.E.; Martin, R.E. Progressive forest canopy water loss during the 2012–2015 California drought. Proc. Natl. Acad. Sci. USA 2016, 113, E249–E255. [Google Scholar] [CrossRef] [PubMed]
- Diffenbaugh, N.S.; Pal, J.S.; Trapp, R.J.; Giorgi, F. Fine-scale processes regulate the response of extreme events to global climate change. Proc. Natl. Acad. Sci. USA 2005, 102, 15774–15778. [Google Scholar] [CrossRef] [PubMed]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Malone, S.L.; Tulbure, M.G.; Pérez-Luque, A.J.; Assal, T.J.; Bremer, L.L.; Drucker, D.P.; Hillis, V.; Varela, S.; Goulden, M.L. Drought resistance across California ecosystems: Evaluating changes in carbon dynamics using satellite imagery. Ecosphere 2016. [Google Scholar] [CrossRef]
- Emmerich, W.E. Ecosystem Water Use Efficiency in a Semiarid Shrubland and Grassland Community. Rangel. Ecol. Manag. 2007, 60, 464–470. [Google Scholar] [CrossRef]
- Angeler, D.G.; Allen, C.R. Quantifying resilience. J. Appl. Ecol. 2016, 53, 617–624. [Google Scholar] [CrossRef]
- Huxman, T.E.; Smith, M.D.; Fay, P.A.; Knapp, A.K.; Shaw, M.R.; Loik, M.E.; Smith, S.D.; Tissue, D.T.; Zak, J.C.; Weltzin, J.F.; et al. Convergence across biomes to a common rain-use efficiency. Nature 2004, 429, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, M.; Ciais, P.; Papale, D.; Valentini, R.; Running, S.; Viovy, N.; Cramer, W.; Granier, A.; Ogée, J.; Allard, V.; et al. Reduction of ecosystem productivity and respiration during the European summer 2003 climate anomaly: A joint flux tower, remote sensing and modelling analysis. Glob. Chang. Biol. 2007, 13, 634–651. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.; Xing, Q.; Pan, Q.; Huang, J.; Yang, D.; Han, X. Primary production and rain use efficiency across a precipitation gradient on the Mongolia Plateau. Ecology 2008, 89, 2140–2153. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhuang, Q. Evaluating evapotranspiration and water-use efficiency of terrestrial ecosystems in the conterminous United States using MODIS and AmeriFlux data. Remote Sens. Environ. 2010, 114, 1924–1939. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, H.; Peng, C.; Liu, J.; Wei, X.; Fang, X.; Liu, S.; Zhou, G.; Yu, S. Evaluating the effects of future climate change and elevated CO2 on the water use efficiency in terrestrial ecosystems of China. Ecol. Model. 2011, 222, 2414–2429. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, J.; Ju, W.; Zhou, Y.; Wang, S.; Wu, X. Water use efficiency of China’s terrestrial ecosystems and responses to drought. Sci. Rep. 2015, 5, 13799. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guan, H.; Batelaan, O.; McVicar, T.R.; Long, D.; Piao, S.; Liang, W.; Liu, B.; Jin, Z.; Simmons, C.T. Contrasting responses of water use efficiency to drought across global terrestrial ecosystems. Sci. Rep. 2016, 6, 23284. [Google Scholar] [CrossRef] [PubMed]
- Ponce Campos, G.E.; Moran, M.S.; Huete, A.; Zhang, Y.; Bresloff, C.; Huxman, T.E.; Eamus, D.; Bosch, D.D.; Buda, A.R.; Gunter, S.A.; et al. Ecosystem resilience despite large-scale altered hydroclimatic conditions. Nature 2013, 494, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, M.; Tenhunen, J.D.; Roupsard, O.; Ourcival, J.-M.; Rambal, S.; Miglietta, F.; Peressotti, A.; Pecchiari, M.; Tirone, G.; Valentini, R. Severe drought effects on ecosystem CO2 and H2O fluxes at three Mediterranean evergreen sites: Revision of current hypotheses? Glob. Chang. Biol. 2002, 8, 999–1017. [Google Scholar] [CrossRef]
- Villalba, R.; Veblen, T.T. Influence of Large-scale Climatic Variability on Episodic Tree Mortality in Northern Patagonia. Ecology 1998, 79, 2624–2640. [Google Scholar] [CrossRef]
- Fensham, R.J.; Holman, J.E. Temporal and spatial patterns in drought-related tree dieback in Australian savanna. J. Appl. Ecol. 1999, 36, 1035–1050. [Google Scholar] [CrossRef]
- Williamson, G.B.; Laurance, W.F.; Oliveira, A.A.; Delamonica, P.; Gascon, C.; Lovejoy, T.E.; Pohl, L. Amazonian tree mortality during the 1997 El Nino drought. Conserv. Biol. 2000, 14, 1538–1542. [Google Scholar] [CrossRef]
- Ferrell, G.T.; Otrosina, W.J.; Demars, C.J., Jr. Predicting susceptibility of white fir during a drought-associated outbreak of the fir engraver, Scolytus ventralis, in California. Can. J. For. Res. 1994, 24, 302–305. [Google Scholar] [CrossRef]
- Speer, J.H.; Swetnam, T.W.; Wickman, B.E.; Youngblood, A. Changes in pandora moth outbreak dynamics during the past 622 Years. Ecology 2001, 82, 679–697. [Google Scholar] [CrossRef]
- Macomber, S.A.; Woodcock, C.E. Mapping and monitoring conifer mortality using remote sensing in the Lake Tahoe Basin. Remote Sens. Environ. 1994, 50, 255–266. [Google Scholar] [CrossRef]
- Savage, M. Anthropogenic and natural disturbance and patterns of mortality in a mixed conifer forest in California. Can. J. For. Res. 1994, 24, 1149–1159. [Google Scholar] [CrossRef]
- Savage, M. The role of anthropogenic influences in a mixed-conifer forest mortality episode. J. Veg. Sci. 1997, 8, 95–104. [Google Scholar] [CrossRef]
- Mattson, W.J.; Haack, R.A. The Role of Drought in Outbreaks of Plant-Eating Insects. Bioscience 1987, 37, 110–118. [Google Scholar] [CrossRef]
- Kobe, R.K.; Pacala, S.W.; Silander, J.A.; Canham, C.D. Juvenile Tree Survivorship as a Component of Shade Tolerance. Ecol. Appl. 1995, 5, 517–532. [Google Scholar] [CrossRef]
- Pacala, S.W.; Canham, C.D.; Saponara, J.; Silander, J.A.; Kobe, R.K.; Ribbens, E. Forest Models Defined by Field Measurements: Estimation, Error Analysis and Dynamics. Ecol. Monogr. 1996, 66, 1–43. [Google Scholar] [CrossRef]
- Wyckoff, P.H.; Clark, J.S. The relationship between growth and mortality for seven co-occurring tree species in the southern Appalachian Mountains. J. Ecol. 2002, 90, 604–615. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Bailey, R.G. Description of the Ecoregions of the United States; No. 1391; USDA Forest Service: Ogden, Utah, 1995.
- Christensen, G.A.; Waddell, K.L.; Stanton, S.M.; Kuegler, O. California’s Forest Resources: Forest Inventory and Analysis, 2001–2010; U.S. Forest Service: Portland, Oregon, 2016.
- Friedl, M.A.; Sulla-Menashe, D.; Tan, B.; Schneider, A.; Ramankutty, N.; Sibley, A.; Huang, X. MODIS Collection 5 global land cover: Algorithm refinements and characterization of new datasets. Remote Sens. Environ. 2010, 114, 168–182. [Google Scholar] [CrossRef]
- Piechota, T.C.; Dracup, J.A. Drought and Regional Hydrologic Variation in the United States: Associations with the El Niño-Southern Oscillation. Water Resour. Res. 1996, 32, 1359–1373. [Google Scholar] [CrossRef]
- Robeson, S.M. Revisiting the recent California drought as an extreme value. Geophys. Res. Lett. 2015, 42, 2015GL064593. [Google Scholar] [CrossRef]
- Wells, N.; Goddard, S.; Hayes, M.J. A Self-Calibrating Palmer Drought Severity Index. J. Clim. 2004, 17, 2335–2351. [Google Scholar] [CrossRef]
- Hessburg, P.F.; Kuhlmann, E.E.; Swetnam, T.W. Examining the Recent Climate through the Lens of Ecology: Inferences from Temporal Pattern Analysis. Ecol. Appl. 2005, 15, 440–457. [Google Scholar] [CrossRef]
- Klos, R.J.; Wang, G.G.; Bauerle, W.L.; Rieck, J.R. Drought impact on forest growth and mortality in the southeast USA: An analysis using Forest Health and Monitoring data. Ecol. Appl. 2009, 19, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Littell, J.S.; McKenzie, D.; Peterson, D.L.; Westerling, A.L. Climate and wildfire area burned in western U.S. ecoprovinces, 1916–2003. Ecol. Appl. 2009, 19, 1003–1021. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.J.; Veblen, T.T.; Eisenhart, K.S.; Jarvis, D.; Kulakowski, D. Drought induces spruce beetle (Dendroctonus rufipennis) outbreaks across northwestern Colorado. Ecology 2014, 95, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, L.; Guo, H.; Leinenkugel, P.; Zhou, Y.; Li, L.; Shen, Q. Drought impact on vegetation productivity in the Lower Mekong Basin. Int. J. Remote Sens. 2014, 35, 2835–2856. [Google Scholar] [CrossRef]
- Schrier, G.; Barichivich, J.; Briffa, K.R.; Jones, P.D. A scPDSI-based global data set of dry and wet spells for 1901–2009. J. Geophys. Res. D Atmos. 2013, 118, 4025–4048. [Google Scholar] [CrossRef]
- Busby, S.J. Simulating multiyear drought events in North America with the HadCM3 climate model. Weather 2008, 63, 240–243. [Google Scholar] [CrossRef]
- Yu, M.; Liu, X.; Wei, L.; Li, Q.; Zhang, J.; Wang, G. Drought Assessment by a Short-/Long-Term Composited Drought Index in the Upper Huaihe River Basin, China. Adv. Meteorol. 2015, 2016. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, G.; Fu, Y.; Sun, X.; Li, Y.; Shi, P.; Wang, Y.; Zheng, Z. Effects of vegetation control on ecosystem water use efficiency within and among four grassland ecosystems in China. Glob. Chang. Biol. 2008, 14, 1609–1619. [Google Scholar] [CrossRef]
- Kuglitsch, F.G.; Reichstein, M.; Beer, C.; Carrara, A.; Ceulemans, R.; Granier, A.; Janssens, I.A.; Koestner, B.; Lindroth, A.; Loustau, D.; et al. Characterisation of ecosystem water-use efficiency of european forests from eddy covariance measurements. Biogeosci. Discuss 2008, 5, 4481–4519. [Google Scholar] [CrossRef]
- Monson, R.K.; Prater, M.R.; Hu, J.; Burns, S.P.; Sparks, J.P.; Sparks, K.L.; Scott-Denton, L.E. Tree species effects on ecosystem water-use efficiency in a high-elevation, subalpine forest. Oecologia 2010, 162, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Xing, X.; Zhang, Z.; Xia, J.; Zhou, X.; Song, B.; Li, L.; Wan, S. Water-use efficiency in response to climate change: From leaf to ecosystem in a temperate steppe. Glob. Chang. Biol. 2011, 17, 1073–1082. [Google Scholar] [CrossRef]
- Tang, X.; Li, H.; Desai, A.R.; Nagy, Z.; Luo, J.; Kolb, T.E.; Olioso, A.; Xu, X.; Yao, L.; Kutsch, W.; et al. How is water-use efficiency of terrestrial ecosystems distributed and changing on Earth? Sci. Rep. 2014, 4, 7483. [Google Scholar] [CrossRef] [PubMed]
- Roupsard, O.; le Maire, G.; Nouvellon, Y.; Dauzat, J.; Jourdan, C.; Navarro, M.; Bonnefond, J.-M.; Saint-André, L.; Mialet-Serra, I.; Hamel, O.; et al. Scaling-up productivity (NPP) using light or water use efficiencies (LUE, WUE) from a two-layer tropical plantation. Agrofor. Syst. 2009, 76, 409–422. [Google Scholar] [CrossRef]
- Law, B.E.; Falge, E.; Gu, L.; Baldocchi, D.D.; Bakwin, P.; Berbigier, P.; Davis, K.; Dolman, A.J.; Falk, M.; Fuentes, J.D.; et al. Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agric. For. Meteorol. 2002, 113, 97–120. [Google Scholar] [CrossRef]
- Zhao, M.; Heinsch, F.A.; Nemani, R.R.; Running, S.W. Improvements of the MODIS terrestrial gross and net primary production global data set. Remote Sens. Environ. 2005, 95, 164–176. [Google Scholar] [CrossRef]
- Running, S.W.; Zhao, M. Daily GPP and Annual NPP (MOD17A2/A3) Products NASA Earth Observing System MODIS Land Algorithm; Numerical Terradynamic Simulation Group: Missoula, MT, USA, 2015. [Google Scholar]
- Mu, Q.; Heinsch, F.A.; Zhao, M.; Running, S.W. Development of a global evapotranspiration algorithm based on MODIS and global meteorology data. Remote Sens. Environ. 2007, 111, 519–536. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Loh, W.-Y. Classification and Regression Tree Methods. In Encyclopedia of Statistics in Quality and Reliability; John Wiley & Sons, Ltd.: Hoboken, New Jersey, 2008; ISBN 9780470061572. [Google Scholar]
- Therneau, T.; Atkinson, B.; Ripley, B. Rpart: Recursive Partitioning and Regression Trees. Available online: cran.r-project.org (accessed on 3 August 2017).
- De’ath, G.; Fabricius, K.E. Classification and Regression Trees: A Powerful Yet Simple Technique for Ecological Data Analysis. Ecology 2000, 81, 3178–3192. [Google Scholar] [CrossRef]
- Prasad, A.M.; Iverson, L.R.; Liaw, A. Newer Classification and Regression Tree Techniques: Bagging and Random Forests for Ecological Prediction. Ecosystems 2006, 9, 181–199. [Google Scholar] [CrossRef]
- Bivand, R.; Keitt, T.; Rowlingson, B.; Pebesma, E.; Sumner, M.; Hijmans, R.; Rouault, E. Rgdal: Bindings for the Geospatial Data Abstraction Library. Available online: cran.r-project.org (accessed on 3 August 2017).
- Hijmans, R.J.; van Etten, J.; Cheng, J.; Mattiuzzi, M.; Sumner, M.; Greenberg, J.A.; Lamigueiro, P.O.; Bevan, A.; Racine, E.B.; Shortridge, A. Raster: Geographic Data Analysis and Modeling. Available online: cran.r-project.org (accessed on 3 August 2017).
- Baldocchi, D. A comparative study of mass and energy exchange rates over a closed C3 (wheat) and an open C4 (corn) crop: II. CO2 exchange and water use efficiency. Agric. For. Meteorol. 1994, 67, 291–321. [Google Scholar] [CrossRef]
- Beer, C.; Ciais, P.; Reichstein, M.; Baldocchi, D.; Law, B.E.; Papale, D.; Soussana, J.-F.; Ammann, C.; Buchmann, N.; Frank, D.; et al. Temporal and among-site variability of inherent water use efficiency at the ecosystem level. Glob. Biogeochem. Cycles 2009, 23, GB2018. [Google Scholar] [CrossRef]
- Van Mantgem, P.J.; Stephenson, N.L. Apparent climatically induced increase of tree mortality rates in a temperate forest. Ecol. Lett. 2007, 10, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.J. Water Relations of Plants; Academic Press: Orlando, FL, USA, 1983. [Google Scholar]
- Sharpe, P.J.H.; Wu, H.I.; Cates, R.G.; Coeschl, J.D. Energetics of Pine Defense Systems to Bark Beetle Attack; Forest Service General Technical Report SO.S6—United States; Southern Forest Experiment Station (USA): Beltsville, MD, USA; Washington, DC, USA, 1985.
- Leung, L.R.; Ghan, S.J. Pacific Northwest climate sensitivity simulated by a regional climate model driven by a GCM. Part II: 2 × CO2 simulations. J. Clim. 1999, 12, 2031–2053. [Google Scholar] [CrossRef]
- Dettinger, M.D.; Cayan, D.R.; Meyer, M.K.; Jeton, A.E. Simulated Hydrologic Responses to Climate Variations and Change in the Merced, Carson, and American River Basins, Sierra Nevada, California, 1900–2099. Clim. Chang. 2004, 62, 283–317. [Google Scholar] [CrossRef]
- Cayan, D.R.; Maurer, E.P.; Dettinger, M.D.; Tyree, M.; Hayhoe, K. Climate change scenarios for the California region. Clim. Chang. 2008, 87, 21–42. [Google Scholar] [CrossRef]
| Variables | Description | Study Period | Resolution | Source |
|---|---|---|---|---|
| Forest Cover | MODIS land cover type (MCD12Q1) | 2012 | 500 m | NASA |
| scPDSI | Self-calibrated Palmer drought severity index | 1900–2016 | 4 km | USDA |
| GPP | MODIS gross primary productivity (MOD17; g C m−2 year−1) | 2002–2014 | 1 km | NASA |
| ET | MODIS evapotranspiration (MOD16; ET mm year−1) | 2002–2014 | 1 km | NASA |
| Tree Mortality | Aerial detection surveys (TPH) | 2013–2016 | 1 km | USFS |
| FIA Plots | FIA stand dynamics for forested ecosystems | 2011–2016 | USFS |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malone, S.L. Monitoring Changes in Water Use Efficiency to Understand Drought Induced Tree Mortality. Forests 2017, 8, 365. https://doi.org/10.3390/f8100365
Malone SL. Monitoring Changes in Water Use Efficiency to Understand Drought Induced Tree Mortality. Forests. 2017; 8(10):365. https://doi.org/10.3390/f8100365
Chicago/Turabian StyleMalone, Sparkle L. 2017. "Monitoring Changes in Water Use Efficiency to Understand Drought Induced Tree Mortality" Forests 8, no. 10: 365. https://doi.org/10.3390/f8100365
APA StyleMalone, S. L. (2017). Monitoring Changes in Water Use Efficiency to Understand Drought Induced Tree Mortality. Forests, 8(10), 365. https://doi.org/10.3390/f8100365




