Taxon-Independent and Taxon-Dependent Responses to Drought in Seedlings from Quercus robur L., Q. petraea (Matt.) Liebl. and Their Morphological Intermediates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source Material
2.2. Germination of the Acorns
2.3. Drought Treatment, Measurements, and Scoring
2.4. Data Analysis
3. Results
3.1. Morphological Evaluation of Seedlings
3.2. Survival
3.3. Leaf Senescence
3.4. Bud Burst
3.5. Height and Diameter Growth in the Second Growing Year
3.6. Variance Components
4. Discussion
4.1. Taxon-Independent Responses to Drought
4.2. Taxon-Dependent Responses to Drought
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.J.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and european forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014; Synthesis Report; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2014. [Google Scholar]
- Anderegg, W.R.L.; Berry, J.A.; Field, C.B. Linking definitions, mechanisms, and modeling of drought-induced tree death. Trends Plant Sci. 2012, 17, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic shifts in ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Benigno, S.M.; Dixon, K.W.; Stevens, J.C. Seedling mortality during biphasic drought in sandy mediterranean soils. Funct. Plant Biol. 2014, 41, 1239–1248. [Google Scholar] [CrossRef]
- Niinemets, U. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Psidova, E.; Ditmarova, L.; Jamnicka, G.; Kurjak, D.; Majerova, J.; Czajkowski, T.; Bolte, A. Photosynthetic response of beech seedlings of different origin to water deficit. Photosynthetica 2015, 53, 187–194. [Google Scholar] [CrossRef]
- Leuschner, C.; Backes, K.; Hertel, D.; Schipka, F.; Schmitt, U.; Terborg, O.; Runge, M. Drought responses at leaf, stem and fine root levels of competitive Fagus sylvatica L. and Quercus petraea (Matt.) Liebl. trees in dry and wet years. For. Ecol. Manag. 2001, 149, 33–46. [Google Scholar] [CrossRef]
- Broadmeadow, M.S.J.; Jackson, S.B. Growth responses of Quercus petraea, Fraxinus excelsior and Pinus sylvestris to elevated carbon dioxide, ozone and water supply. New Phytol. 2000, 146, 437–451. [Google Scholar] [CrossRef]
- Thomas, F.M.; Gausling, T. Morphological and physiological responses of oak seedlings (Quercus petraea and Q. robur) to moderate drought. Ann. For. Sci. 2000, 57, 325–333. [Google Scholar] [CrossRef]
- Arend, M.; Kuster, T.; Gunthardt-Goerg, M.S.; Dobbertin, M. Provenance-specific growth responses to drought and air warming in three european oak species (Quercus robur, Q. petraea and Q. pubescens). Tree Physiol. 2011, 31, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Spiess, N.; Oufir, M.; Matusikova, I.; Stierschneider, M.; Kopecky, D.; Homolka, A.; Burg, K.; Fluch, S.; Hausman, J.F.; Wilhelm, E. Ecophysiological and transcriptomic responses of oak (Quercus robur) to long-term drought exposure and rewatering. Environ. Exp. Bot. 2012, 77, 117–126. [Google Scholar] [CrossRef]
- Kuster, T.M.; Arend, M.; Gunthardt-Goerg, M.S.; Schulin, R. Root growth of different oak provenances in two soils under drought stress and air warming conditions. Plant Soil 2013, 369, 61–71. [Google Scholar] [CrossRef]
- Jensen, J.S.; Hansen, J.K. Genetic variation in responses to different soil water treatments in Quercus robur L. Scand. J. For. Res. 2010, 25, 400–411. [Google Scholar] [CrossRef]
- Pflug, E.E.; Siegwolf, R.; Buchmann, N.; Dobbertin, M.; Kuster, T.M.; Gunthardt-Goerg, M.S.; Arend, M. Growth cessation uncouples isotopic signals in leaves and tree rings of drought-exposed oak trees. Tree Physiol. 2015, 35, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Kuster, T.M.; Dobbertin, M.; Gunthardt-Goerg, M.S.; Schaub, M.; Arend, M. A phenological timetable of oak growth under experimental drought and air warming. PLoS ONE 2014, 9, e89724. [Google Scholar] [CrossRef] [PubMed]
- Turcsán, A.; Steppe, K.; Sárközi, E.; Erdélyi, É.; Missoorten, M.; Mees, G.; Vander Mijnsbrugge, K. Early summer drought stress during the first growing year stimulates extra shoot growth in oak seedlings (Quercus petraea). Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Vander Mijnsbrugge, K.; Turcsan, A.; Maes, J.; Duchene, N.; Meeus, S.; Steppe, K.; Steenackers, M. Repeated summer drought and re-watering during the first growing year of oak (Quercus petraea) delay autumn senescence and bud burst in the following spring. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Ducousso, A.; Bordacs, S. Euforgen Technical Guidelines for Genetic Conservation and Use for Pedunculate and Sessile Oaks (Quercus robur and Q. petraea); International Plant Genetic Resources Institute: Rome, Italy, 2004. [Google Scholar]
- Vander Mijnsbrugge, K.; De Cleene, L.; Beeckman, H. A combination of fruit and leaf morphology enables taxonomic classification of the complex Q. robur L.—Q. x rosacea Bechst.—Q. petraea (Matt.) Liebl. in autochthonous stands in flanders. Silvae Genet. 2011, 60, 139–148. [Google Scholar] [CrossRef]
- Bacilieri, R.; Ducousso, A.; Petit, R.J.; Kremer, A. Mating system and asymmetric hybridization in a mixed stand of european oaks. Evolution 1996, 50, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Lepais, O.; Petit, R.J.; Guichoux, E.; Lavabre, J.E.; Alberto, F.; Kremer, A.; Gerber, S. Species relative abundance and direction of introgression in oaks. Mol. Ecol. 2009, 18, 2228–2242. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, G.L. Variation and Evolution in Plants; Columbia University Press: New York, NY, USA, 1950. [Google Scholar]
- Kleinschmit, J.R.G.; Bacilieri, R.; Kremer, A.; Roloff, A. Comparison of morphological and genetic traits of pedunculate oak (Q. robur L.) and sessile oak (Q. petraea (Matt) Liebl). Silvae Genet. 1995, 44, 256–269. [Google Scholar]
- Cochard, H.; Breda, N.; Granier, A.; Aussenac, G. Vulnerability to air-embolism of 3 european oak species (Quercus petraea (Matt) Liebl, Quercus pubescens Willd, Quercus robur L.). Ann. Sci. For. 1992, 49, 225–233. [Google Scholar] [CrossRef]
- Ponton, S.; Dupouey, J.L.; Breda, N.; Feuillat, F.; Bodenes, C.; Dreyer, E. Carbon isotope discrimination and wood anatomy variations in mixed stands of Quercus robur and Quercus petraea. Plant Cell Environ. 2001, 24, 861–868. [Google Scholar] [CrossRef]
- Le Provost, G.; Sulmon, C.; Frigerio, J.M.; Bodenes, C.; Kremer, A.; Plomion, C. Role of waterlogging-responsive genes in shaping interspecific differentiation between two sympatric oak species. Tree Physiol. 2012, 32, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Bacilieri, R.; Ducousso, A.; Kremer, A. Genetic, morphological, ecological and phenological differentiation between Quercus petraea (Matt.) Liebl. and Quercus robur L. in a mixed stand of northwest of France. Sylvae Genet. 1995, 44, 1–10. [Google Scholar]
- Saintagne, C.; Bodénès, C.; Barreneche, T.; Pot, D.; Plomion, C.; Kremer, A. Distribution of genomic regions differentiating oak species assessed by QTL detection. Heredity 2004, 92, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Rellstab, C.; Bühler, A.; Graf, R.; Folly, C.; Gugerli, F. Using joint multivariate analyses of leaf morphology and molecular-genetic markers for taxon identification in three hybridizing European white oak species (Quercus spp.). Ann. For. Sci. 2016, 73, 669–679. [Google Scholar] [CrossRef]
- Curtu, A.; Gailing, O.; Finkeldey, R. Patterns of contemporary hybridization inferred from paternity analysis in a four-oak-species forest. BMC Evolut. Biol. 2007, 9. [Google Scholar] [CrossRef] [PubMed]
- Gugerli, F.; Walser, J.; Dounavi, K.; Holderegger, R.; Finkeldey, R. Coincidence of small-scale spatial discontinuities in leaf morphology and nuclear microsatellite variation of Quercus petraea and Q. robur in a mixed forest. Ann. Bot. 2007, 99, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.; Dupouey, J.L.; Deans, J.D.; Cottrell, J.; Csaikl, U.; Finkeldey, R.; Espinel, S.; Jensen, J.; Kleinschmit, J.; Van Dam, B.; et al. Leaf morphological differentiation between Quercus robur and Quercus petraea is stable across western European mixed oak stands. Ann. For. Sci. 2002, 59, 777–787. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Cox, K.; Van Slycken, J. Conservation approaches for autochthonous woody plants in flanders. Silvae Genet. 2005, 54, 197–206. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Coart, E.; Beeckman, H.; Van Slycken, J. Conservation measures for autochthonous oaks in flanders. For. Genet. 2003, 10, 207–217. [Google Scholar]
- Brouwers, J.; Peeters, B.; Van Steertegem, M.; van Lipzig, N.; Wouters, H.; Beullens, J.; Demuzere, M.; Willems, P.; De Ridder, K.; Maiheu, B.; et al. Mira Climate Report 2015: About Observed and Future Climate Changes in Flanders and Belgium; Flanders Environment Agency: Aalst, Belgium, 2015. [Google Scholar]
- Boratynski, A.; Marcysiak, K.; Lewandowska, A.; Jasinska, A.; Iszkulo, G.; Burczyk, J. Differences in leaf morphology between Quercus petraea and Q. robur adult and young individuals. Silva Fenn. 2008, 42, 115–124. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Christensen, R.H.B. Ordinal: Regression Models for Ordinal Data. R Package Version 2013.10–31. 2013. Available online: http://www.Cran.R-project.Org/package=ordinal/ (accessed on 3 July 2015).
- Vivin, P.; Aussenac, G.; Levy, G. Differences in drought resistance among 3 deciduous oak species grown in large boxes. Ann. Sci. For. 1993, 50, 221–233. [Google Scholar] [CrossRef]
- Neophytou, C.; Aravanopoulos, F.A.; Fink, S.; Dounavi, A. Detecting interspecific and geographic differentiation patterns in two interfertile oak species (Quercus petraea (Matt.) Liebl. and Q. robur L.) using small sets of microsatellite markers. For. Ecol. Manag. 2010, 259, 2026–2035. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Turcsan, A.; Maes, J.; Duchene, N.; Meeus, S.; Steppe, K.; Steenackers, M. Research Institute for Nature and Forest, Geraardsbergen, Belgium. Unpublished work. 2016. [Google Scholar]
- Büsgen, M.; Münch, E.; Thomson, T. The Structure and Life of Forest Trees; Chapman and Hall: London, UK, 1929. [Google Scholar]
- Fonti, P.; Heller, O.; Cherubini, P.; Rigling, A.; Arend, M. Wood anatomical responses of oak saplings exposed to air warming and soil drought. Plant Biol. 2013, 15, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.H. Characteristics of trees predisposed to die. Bioscience 1987, 37, 569–574. [Google Scholar] [CrossRef]
- Dobbertin, M. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: A review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- De Boeck, H.; Dreesen, F.; Janssens, I.; Nijs, I. Climatic characteristics of heat waves and their simulation in plant experiments. Glob. Chang. Biol. 2010, 16, 1992–2000. [Google Scholar] [CrossRef]
- De Boeck, H.; Dreesen, F.; Janssens, I.; Nijs, I. Whole-system responses of experimental plant communities to climate extremes imposed in different seasons. New Phytol. 2011, 189, 806–817. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.W.; Puettmann, K.J. The relative dominance hypothesis explains interaction dynamics in mixed species Alnus rubra/Pseudotsuga menziesii stands. J. Ecol. 2004, 92, 450–463. [Google Scholar]
- Schwinning, S.; Weiner, J. Mechanisms determining the degree of size asymmetry in competition among plants. Oecologia 1998, 113, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Cannell, M.G.R.; Rothery, P.; Ford, E.D. Competition within stands of Picea sitchensis and Pinus contorta. Ann. Bot. (Lond.) 1984, 53, 349–362. [Google Scholar] [CrossRef]
- Van Couwenberghe, R.; Gegout, J.C.; Lacombe, E.; Collet, C. Light and competition gradients fail to explain the coexistence of shade-tolerant Fagus sylvatica and shade-intermediate Quercus petraea seedlings. Ann. Bot. (Lond.) 2013, 112, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Boyden, S.B.; Reich, P.B.; Puettmann, K.J.; Baker, T.R. Effects of density and ontogeny on size and growth ranks of three competing tree species. J. Ecol. 2009, 97, 277–288. [Google Scholar] [CrossRef]
- Lagache, L.; Klein, E.K.; Guichoux, E.; Petit, R.J. Fine-scale environmental control of hybridization in oaks. Mol. Ecol. 2013, 22, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Petit, R.J.; Bodenes, C.; Ducousso, A.; Roussel, G.; Kremer, A. Hybridization as a mechanism of invasion in oaks. New Phytol. 2004, 161, 151–164. [Google Scholar] [CrossRef]
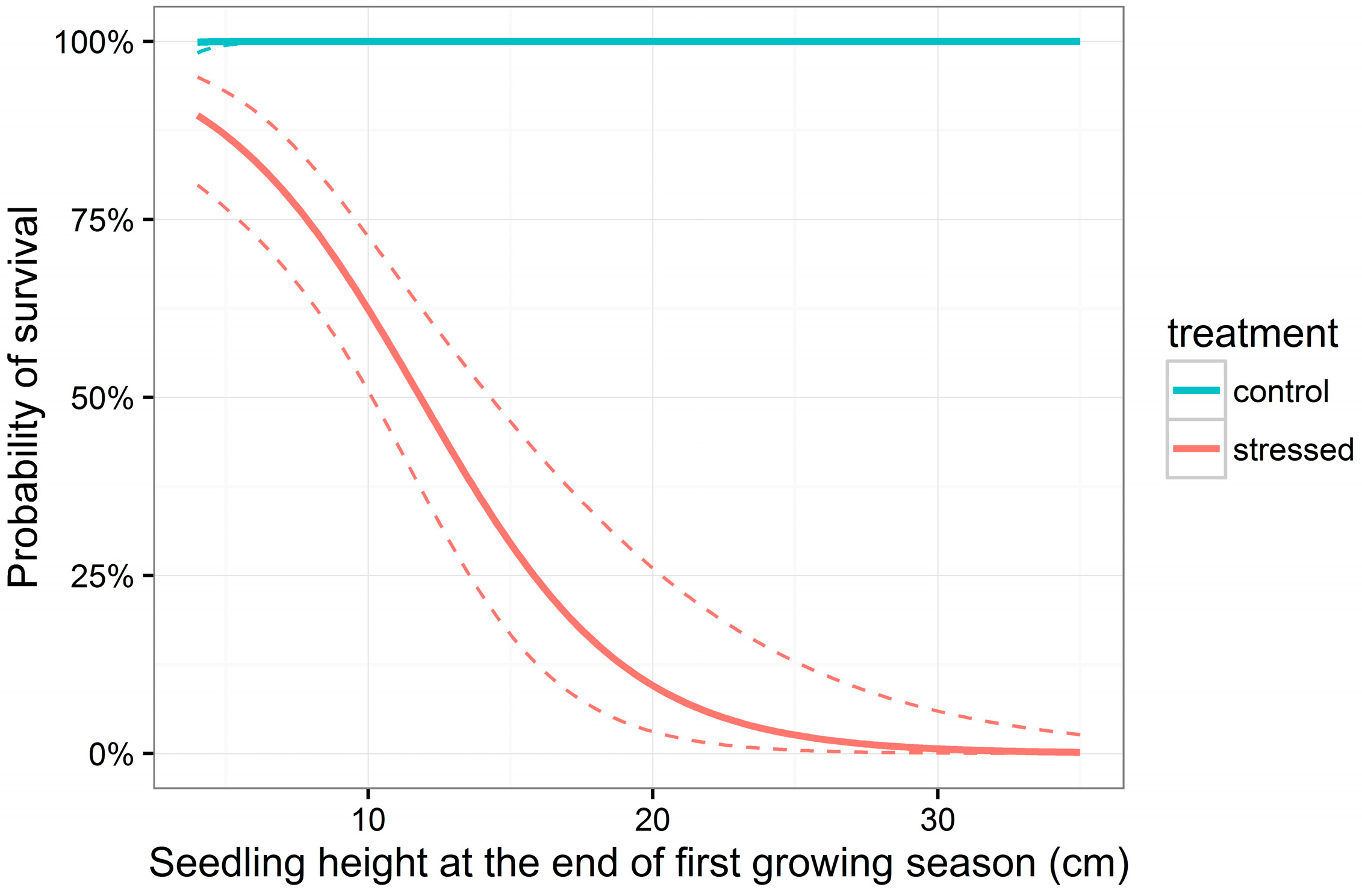
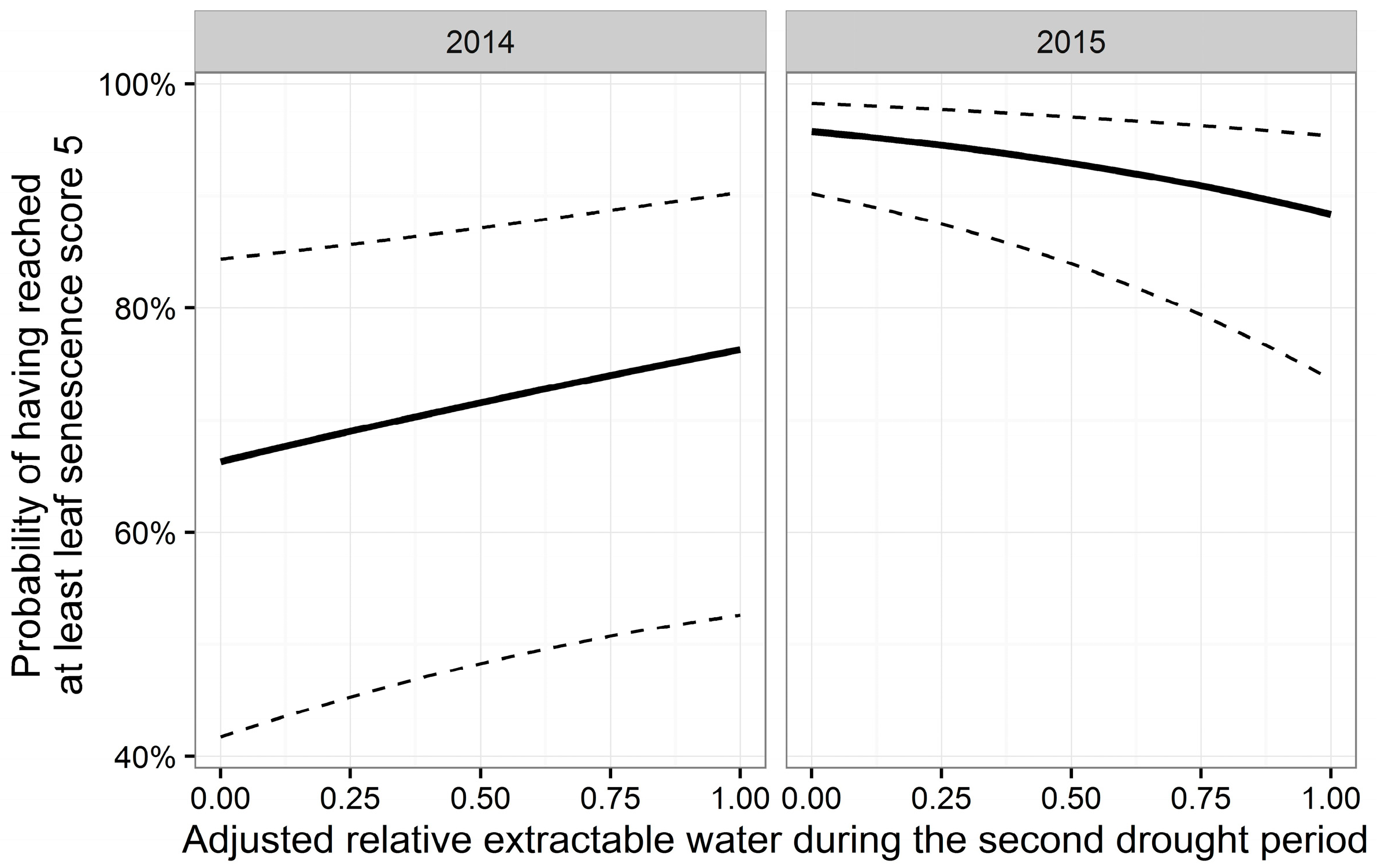
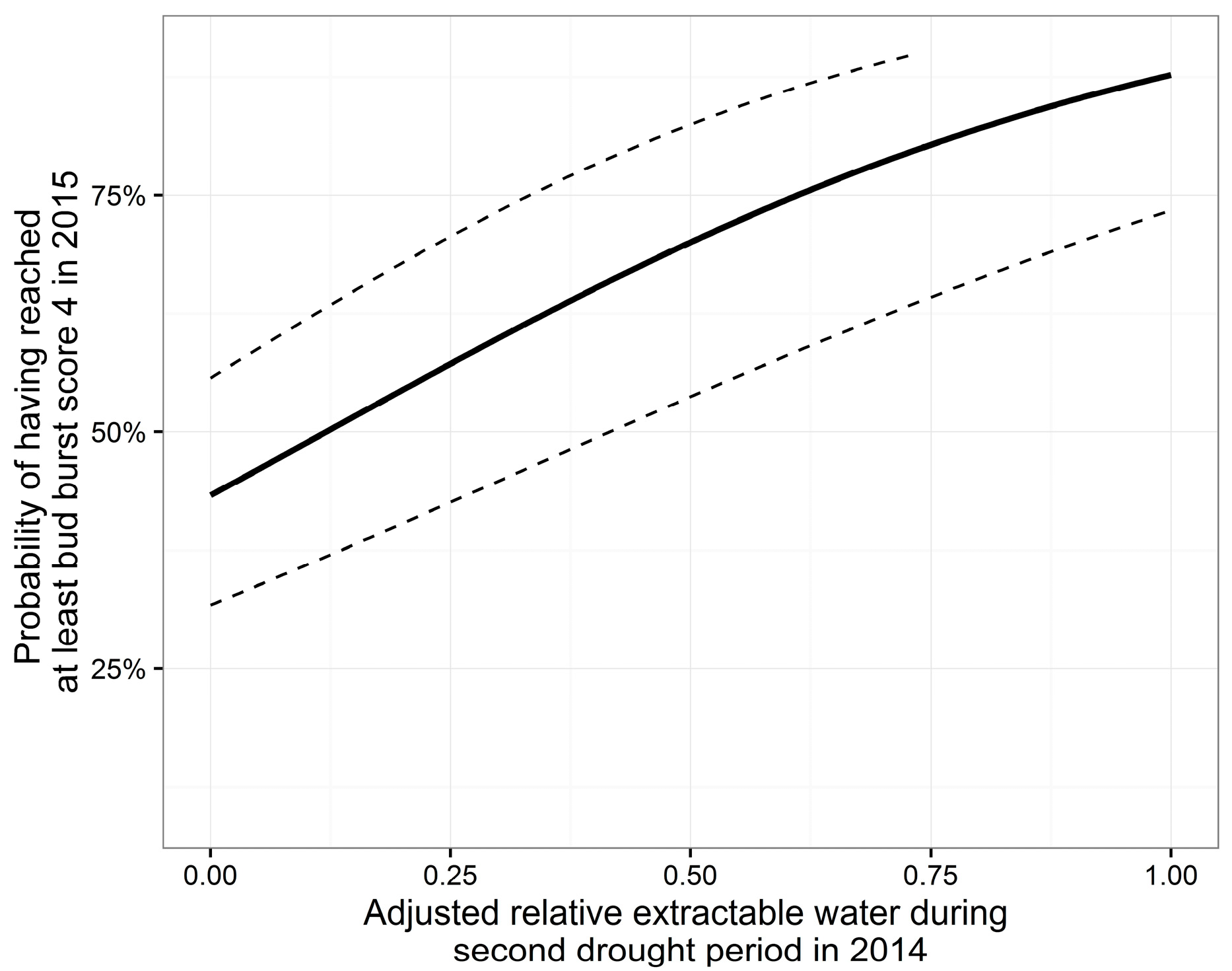
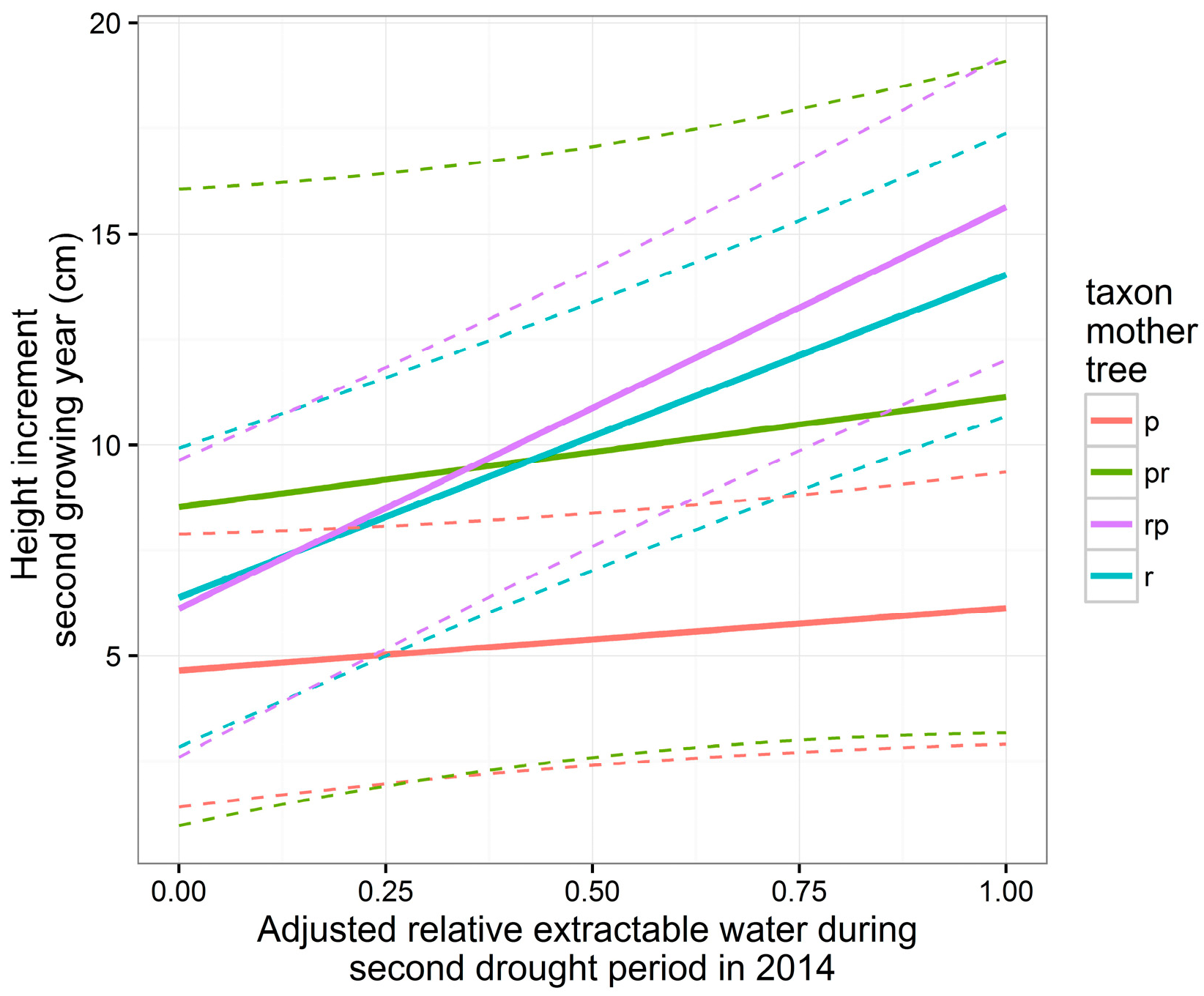

| Treatment | Taxon Mother Tree * | nt | nsu (nsu%) |
|---|---|---|---|
| control | p | 69 | 68 (99) |
| pr | 13 | 13 (100) | |
| rp | 55 | 55 (100) | |
| r | 58 | 58 (100) | |
| drought | p | 74 | 50 (68) |
| pr | 14 | 11 (79) | |
| rp | 58 | 38 (66) | |
| r | 51 | 36 (71) |
| Covariate | Estimate | St. er. | z Value | p Value |
|---|---|---|---|---|
| Intercept | 3.26 | 0.59 | 5.52 | <0.001 *** |
| H | −0.28 | 0.06 | −4.98 | <0.001 *** |
| A | −3.66 | 1.95 | −1.88 | 0.061 |
| H:A | 2.49 | 0.53 | 4.69 | <0.001 *** |
| Leaf Senescence (2014 and 2015) | Bud Burst (2015) | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Estimate | St. er. | z Value | p Value | Estimate | St. er. | z Value | p Value |
| D | −0.19 | 0.008 | −25.20 | <0.001 *** | ||||
| Y | 4.80 | 0.295 | 16.25 | <0.001 *** | ||||
| A | 1.09 | 0.252 | 4.33 | <0.001 *** | −2.24 | 0.30 | −7.40 | <0.001 *** |
| H | −0.07 | 0.013 | −5.14 | <0.001 *** | ||||
| Y:A | −1.58 | 0.331 | −4.78 | <0.001 *** | ||||
| Height Growth in 2015 | Diameter Growth in 2015 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Covariate | Estimate | St. er. | DF | t Value | p Value | Estimate | St. er. | DF | t Value | p Value |
| Intercept | 5.86 | 1.72 | 296 | 3.40 | <0.001 *** | 123.7 | 16.1 | 297 | 7.71 | <0.001 *** |
| A | 1.48 | 1.25 | 296 | 1.18 | 0.238 | −15.9 | 19.1 | 297 | −0.83 | 0.40 |
| Tpr | 3.87 | 4.19 | 14 | 0.92 | 0.371 | 26.4 | 38.4 | 14 | 0.69 | 0.50 |
| Tr | 1.73 | 2.46 | 14 | 0.70 | 0.494 | −25.2 | 24.6 | 14 | −1.03 | 0.32 |
| Trp | 1.47 | 2.44 | 14 | 0.60 | 0.557 | −9.3 | 23.7 | 14 | −0.39 | 0.70 |
| H | −0.15 | 0.08 | 296 | −1.92 | 0.056 | |||||
| A:Tpr | 1.13 | 3.10 | 296 | 0.37 | 0.716 | −1.45 | 47.5 | 297 | −0.03 | 0.98 |
| A:Tr | 6.17 | 1.84 | 296 | 3.35 | <0.001 *** | 61.1 | 27.9 | 297 | 2.19 | 0.029 * |
| A:Trp | 8.03 | 1.90 | 296 | 4.23 | <0.001 *** | 56.8 | 29.0 | 297 | 1.96 | 0.051 |
| Response Variable | Variance Component | ||
|---|---|---|---|
| Mother Taxon | Mother ID | Residual | |
| Survival 1 | <0.0001 | 0.23 | - |
| Bud burst 2 | <0.0001 | 0.52 | - |
| Senescence 2 | <0.0001 | 0.55 | - |
| Height 3 | 5.63 | 12.50 | 25.36 |
| Diameter 3 | 0.55 | 6.72 | 59.72 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vander Mijnsbrugge, K.; Turcsán, A.; Maes, J.; Duchêne, N.; Meeus, S.; Van der Aa, B.; Steppe, K.; Steenackers, M. Taxon-Independent and Taxon-Dependent Responses to Drought in Seedlings from Quercus robur L., Q. petraea (Matt.) Liebl. and Their Morphological Intermediates. Forests 2017, 8, 407. https://doi.org/10.3390/f8110407
Vander Mijnsbrugge K, Turcsán A, Maes J, Duchêne N, Meeus S, Van der Aa B, Steppe K, Steenackers M. Taxon-Independent and Taxon-Dependent Responses to Drought in Seedlings from Quercus robur L., Q. petraea (Matt.) Liebl. and Their Morphological Intermediates. Forests. 2017; 8(11):407. https://doi.org/10.3390/f8110407
Chicago/Turabian StyleVander Mijnsbrugge, Kristine, Arion Turcsán, Jorne Maes, Nils Duchêne, Steven Meeus, Beatrijs Van der Aa, Kathy Steppe, and Marijke Steenackers. 2017. "Taxon-Independent and Taxon-Dependent Responses to Drought in Seedlings from Quercus robur L., Q. petraea (Matt.) Liebl. and Their Morphological Intermediates" Forests 8, no. 11: 407. https://doi.org/10.3390/f8110407





