Influence of Scarification on the Germination Capacity of Acorns Harvested from Uneven-Aged Stands of Pedunculate Oak (Quercus robur L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
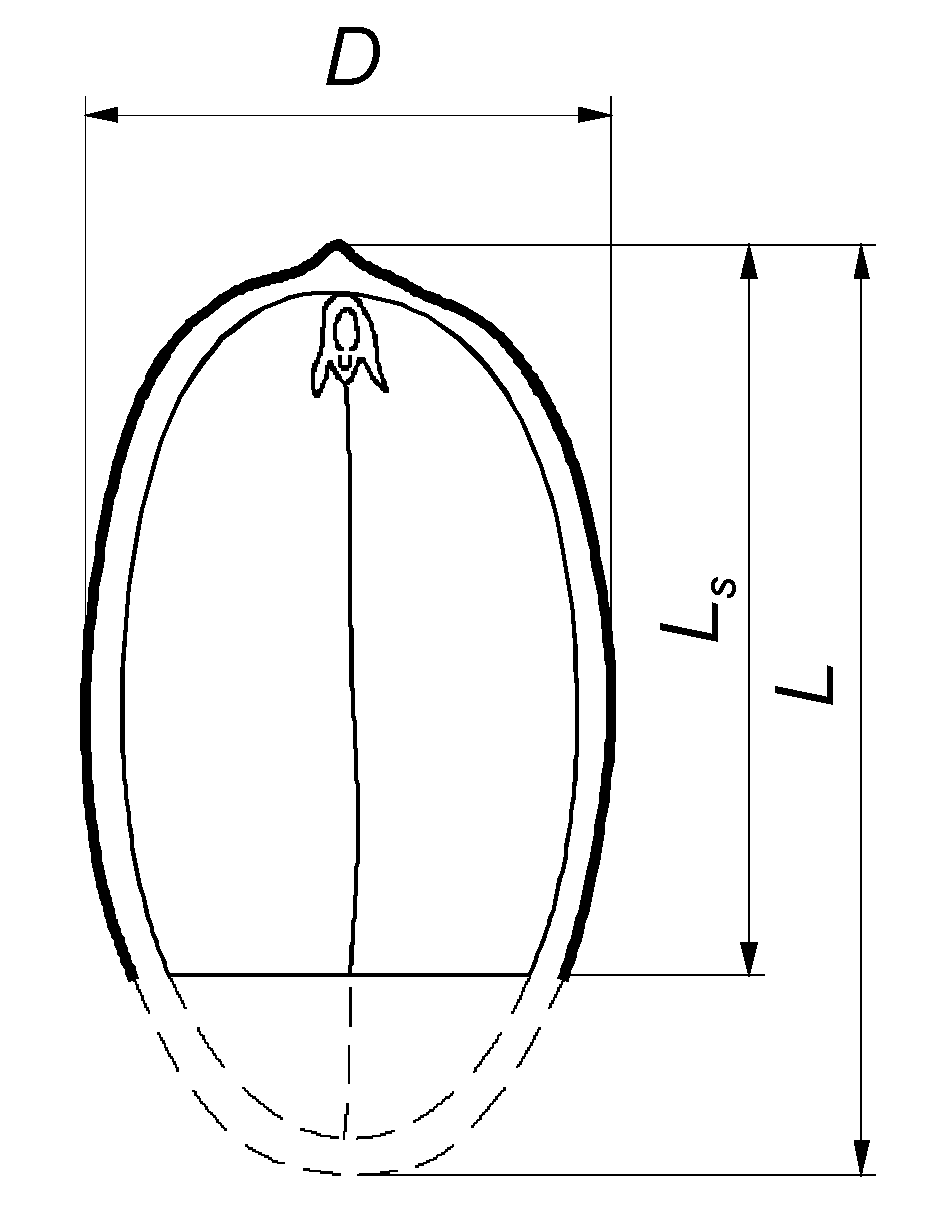
2.2. Determination of Physical Properties
- specific mass mD [27]:
- and in scarified acorns—the dimensional scarification index SL and the mass scarification index Sm:
2.3. Comparative Germination of Complete and Scarified Acorns
2.4. Statistical Analysis
3. Results
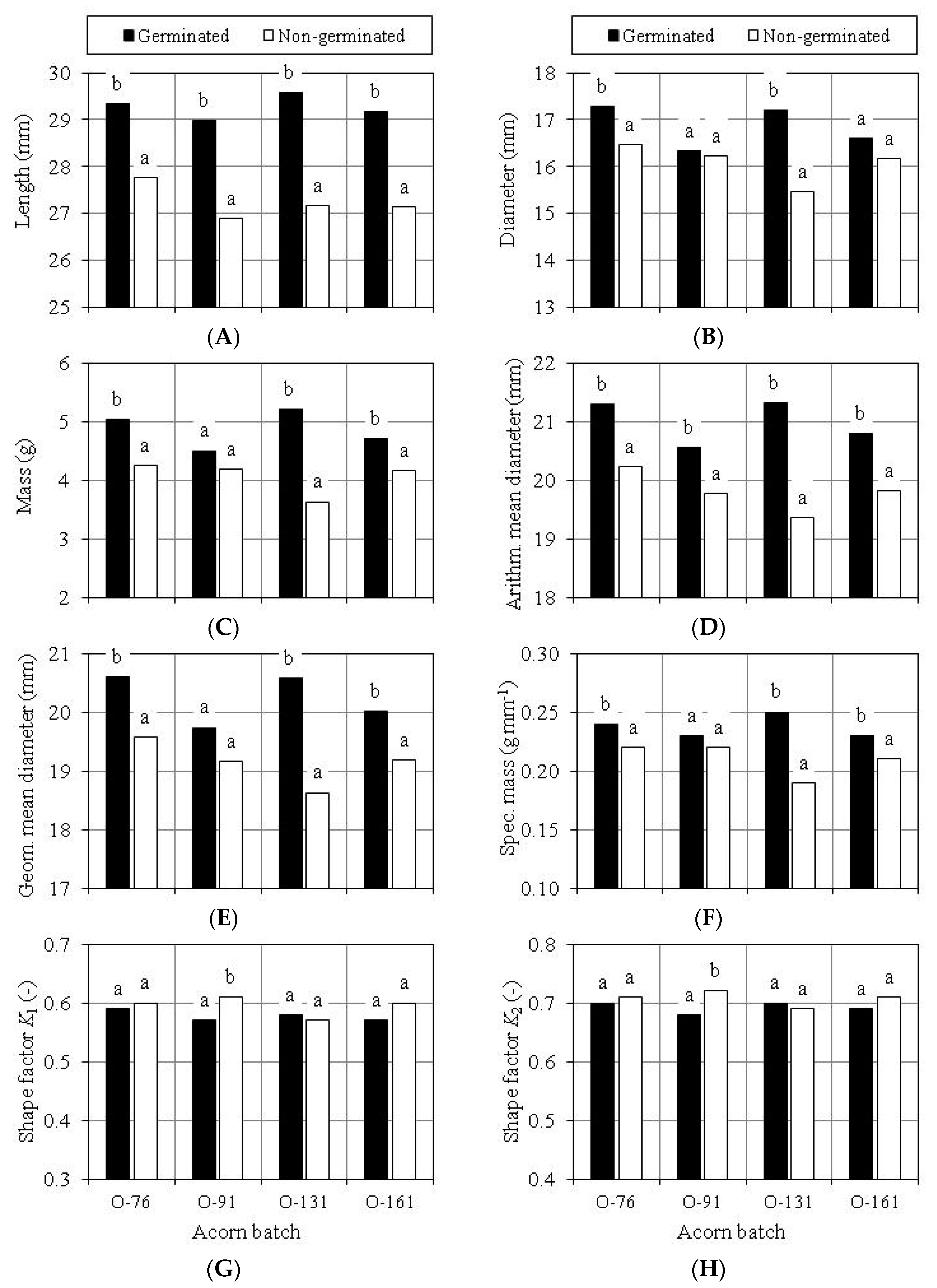
3.1. Experimental Material
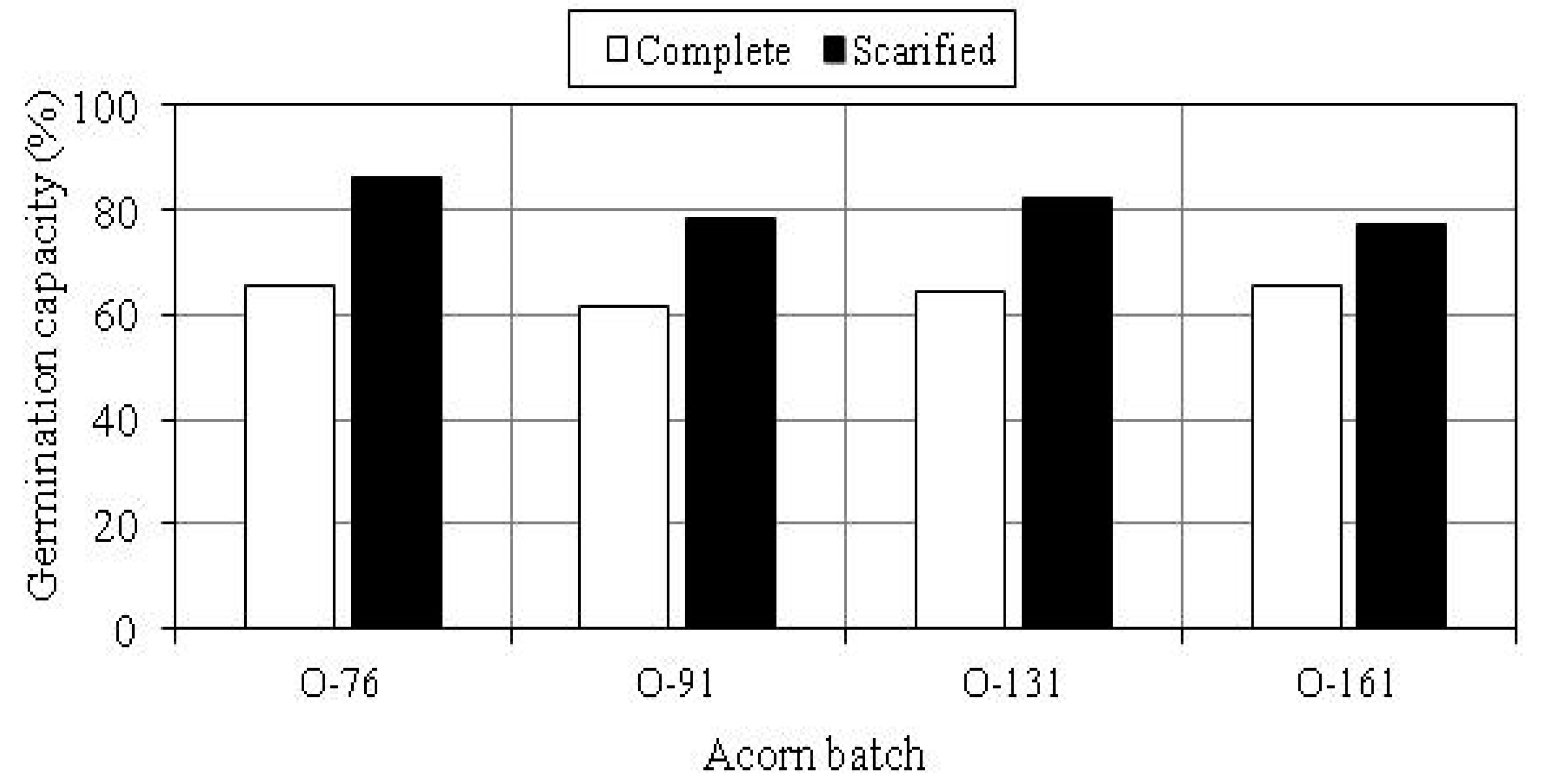
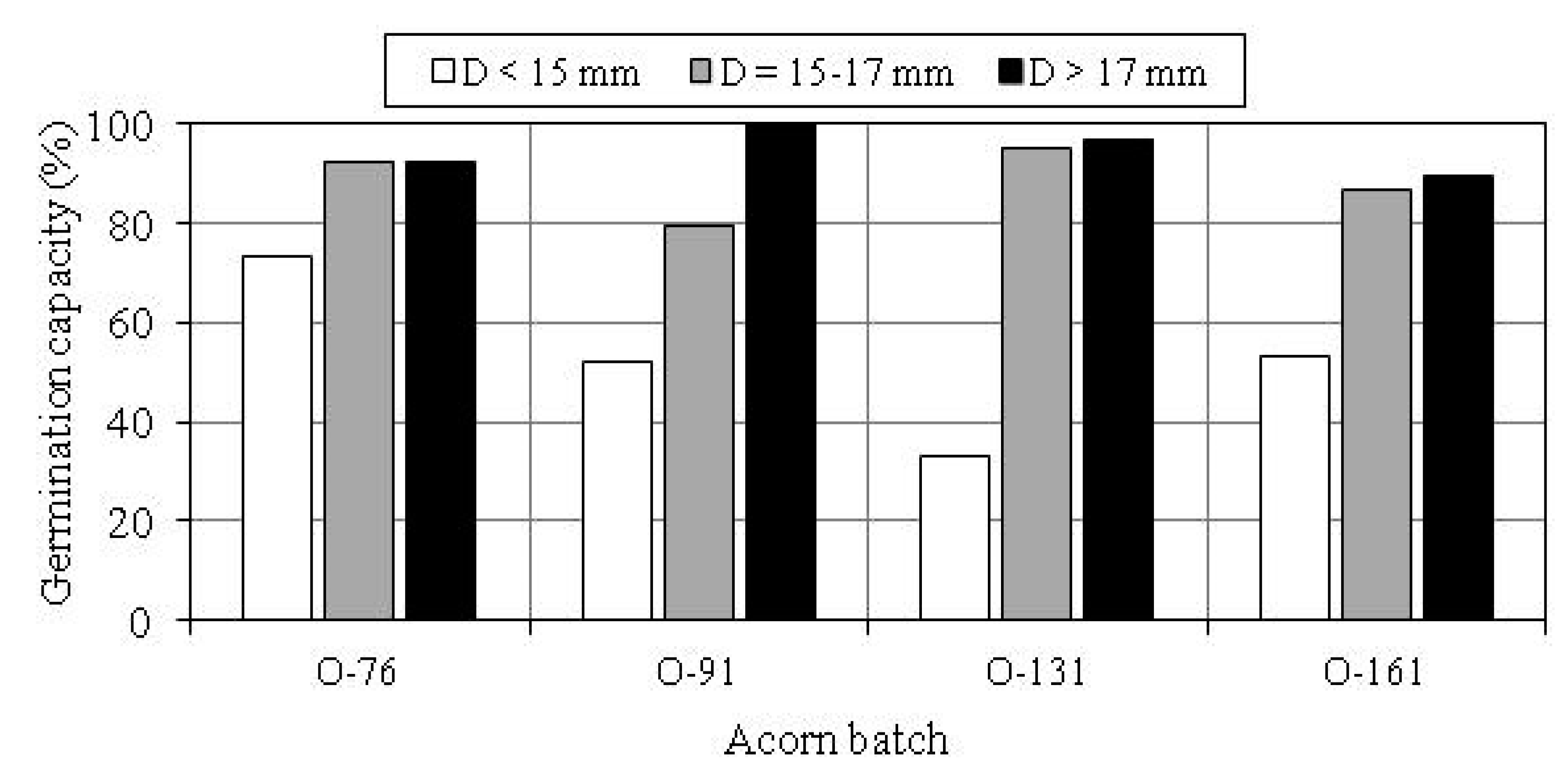
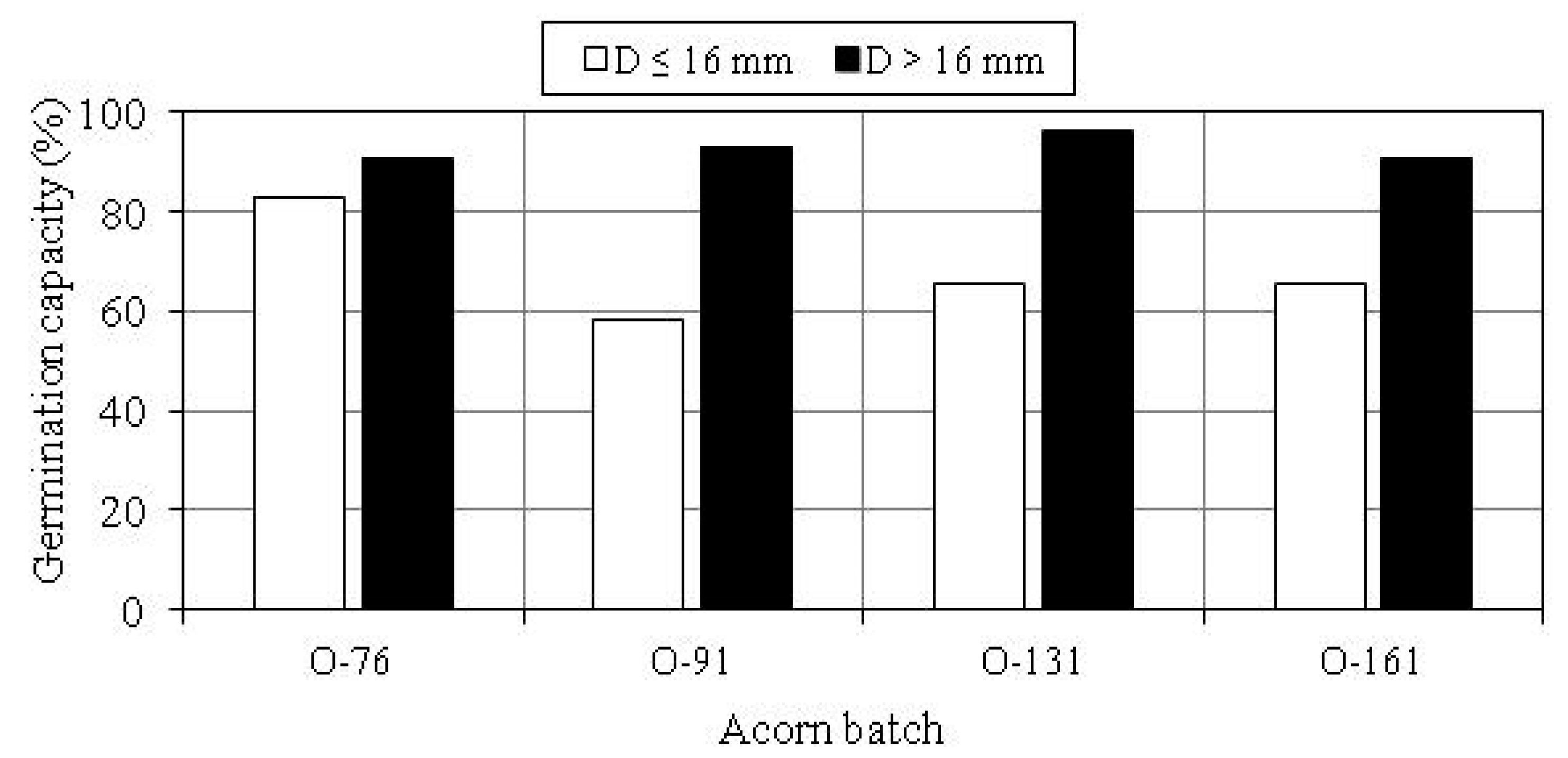
3.2. Germination Capacity of Acorns
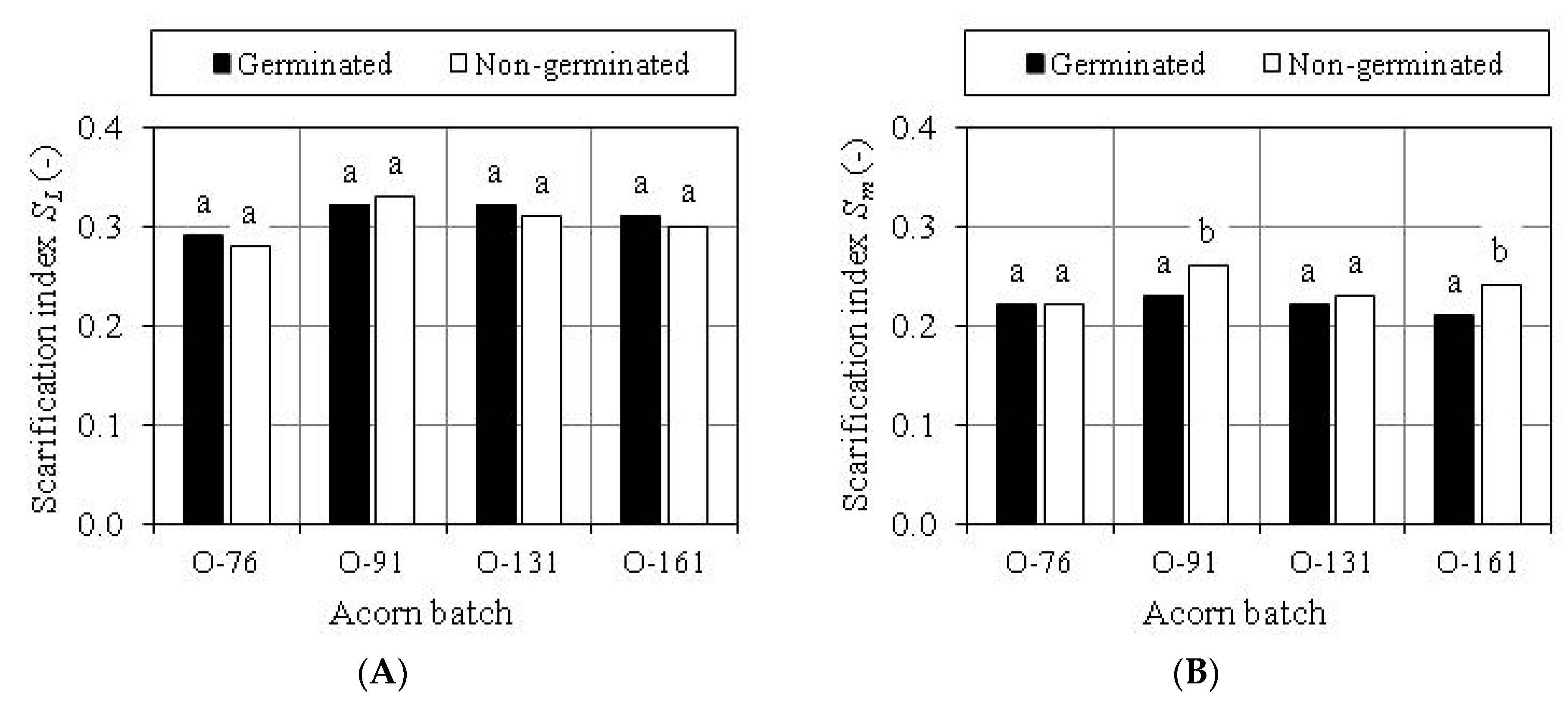
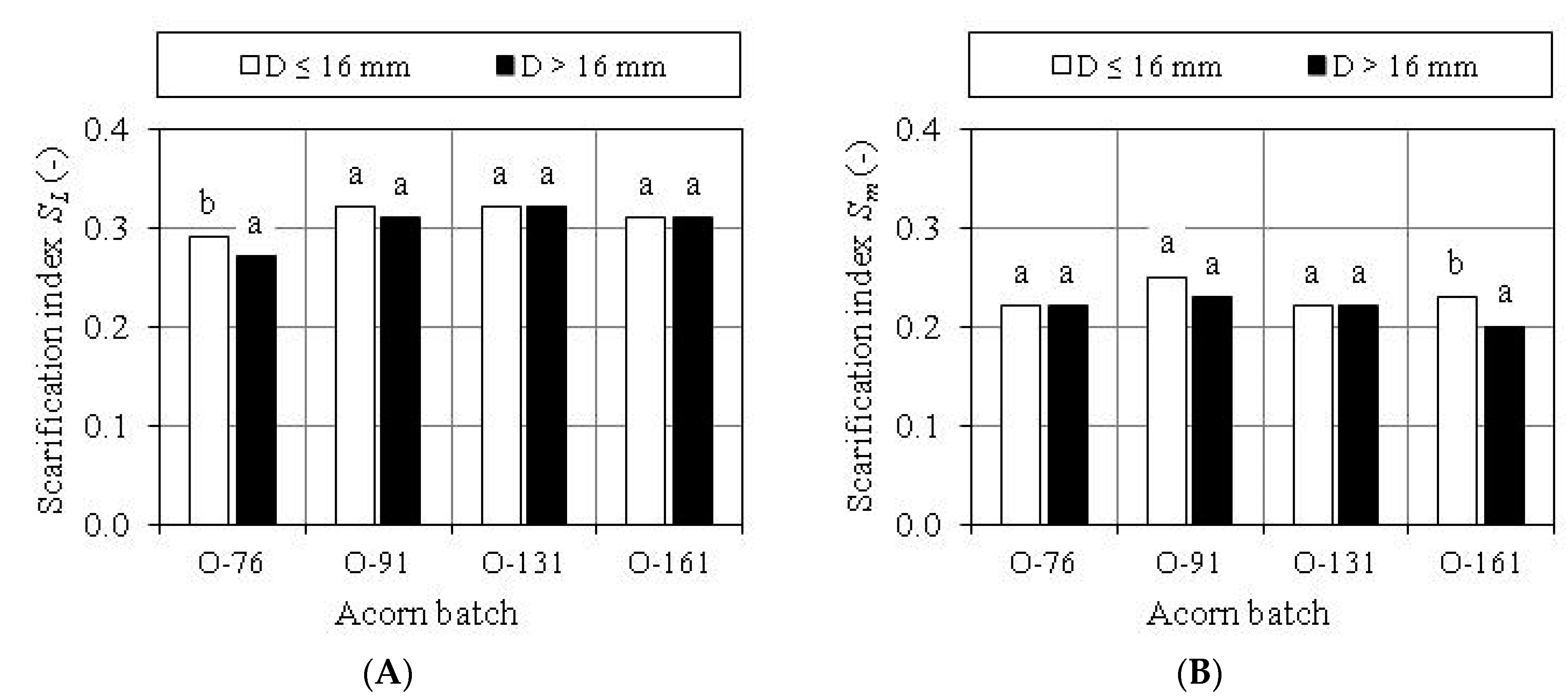
3.3. Evaluation of Scarification Treatment
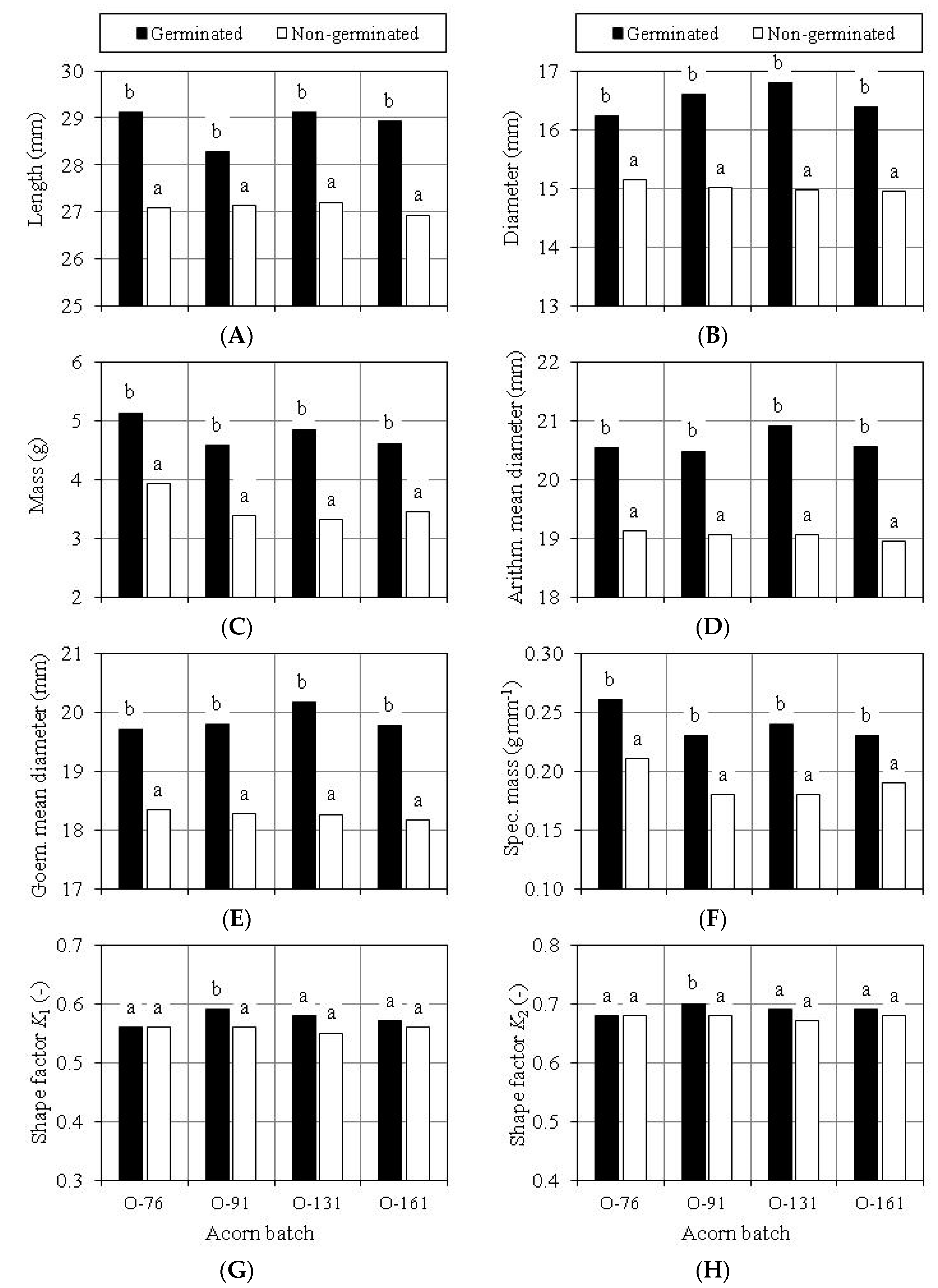
3.4. Germination Capacity of Scarified Acorns
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jaworski, A. Hodowla lasu. Tom III. Charakterystyka hodowlana drzew i krzewów leśnych (Silviculture. Volume 3. Breeding Characteristics of Forest Trees and Shrubs); PWRiL: Warszawa, Poland, 2011; pp. 242–266. ISBN 9788309010760. (In Polish) [Google Scholar]
- Suszka, B.; Muller, C.; Bonnet-Masimber, M. Nasiona leśnych drzew liściastych od zbioru do siewu (Seeds of Deciduous Forest Trees—From Harvest to Sowing); Wydawnictwo Naukowe PWN: Warszawa-Poznań, Poland, 2000; pp. 1–274. ISBN 8301133430. (In Polish) [Google Scholar]
- Rodriguez-Campos, A.; Diaz-Maroto, I.J.; Barcala-Perez, E.; Vila-Lameiro, P. Comparison of the autoecology of Quercus robur L. and Q. petraea (Mattuschka) Liebl. Stands in the Northwest of the Iberian Peninsula. Ann. For. Res. 2010, 53, 7–25. [Google Scholar]
- Brus, D.J.; Hengeveld, G.M.; Walvoort, D.J.J.; Goedhart, P.W.; Heidema, A.H.; Nabuurs, G.J.; Gunia, K. Statistical mapping of tree species over Europe. Eur. J. For. Res. 2012, 131, 145–157. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sisó, S.; Sancho-Knapik, D.; Díaz-Espejo, A.; Flexas, J.; Galmés, J.; Gil-Pelegrín, E. Leaf morphological and physiological adaptations of a deciduous oak (Quercus faginea Lam.) to the Mediterranean climate: A comparison with a closely related temperate species (Quercus robur L.). Tree Phys. 2016, 36, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, I.M.B.; Thomsen, K.A.; Jensen, B.; Poulsen, K.M. Effect of hot water treatment, biocontrol agents, disinfectants and a fungicide on storability of English oak acorns and control of the pathogen, Ciboria batschiana. For. Pathol. 2004, 1, 47–64. [Google Scholar] [CrossRef]
- Schröder, T.; Kehr, R.; Procházková, Z.; Sutherland, J.R. Practical methods for estimating the infection rate of Quercus robur acorn seedlots by Ciboria batschiana. For. Pathol. 2004, 34, 187–196. [Google Scholar] [CrossRef]
- Tadeusiewicz, R.; Tylek, P.; Adamczyk, F.; Kiełbasa, P.; Jabłoński, M.; Pawlik, P.; Piłat, A.; Walczyk, J.; Szczepaniak, J.; Juliszewski, T.; et al. Automation of the acorn scarification process as a contribution to sustainable forest management. Case study: Common oak. Sustainability 2017, 9, 2276. [Google Scholar] [CrossRef]
- Chebouti-Meziou, N.; Merabet, A.; Chebouti, Y.; Bissaad, F.Z.; Behidj-Benyounes, N.; Doumandji, S. Effect of cold and scarification on seeds germination of Pistacia atlantica L. for rapid multiplication. Pak. J. Bot. 2014, 46, 441–446. [Google Scholar]
- Asl, M.B.; Sharivivash, R.; Rahbari, A. Effect of different treatments on seed germination of honey locust (Gleditschia triacanthos). Mod. Appl. Sci. 2011, 5, 200–204. [Google Scholar]
- Ciccarelli, D.; Andreucci, A.C.; Pagni, A.M.; Garbari, F. The role of the elaiosome in the germination of seeds of Myrtus communis L. (Myrtaceae). Atti Soc. Tosc. Sci. Nat. 2004, 111, 143–146. [Google Scholar]
- Nwaoguala, C.N.C.; Osaigbovo, A.U. Enhancing seedling production of black velvet tamarind (Dialium guineense Willd). J. Appl. Nat. Sci. 2009, 1, 36–40. [Google Scholar]
- Tylkowski, T.; Grupa, R. Effectiveness of pre-sowing scarification methods of black locust seeds. Sylwan 2010, 154, 33–40. [Google Scholar]
- Missanjo, E.; Maya, C.; Kapira, D.; Banda, H.; Kamanga-Thole, G. Effect of seed size and pretreatment methods on germination of Albizia lebbeck. ISRN Bot. 2013. [Google Scholar] [CrossRef]
- Aliero, B.L. Effects of sulphuric acid, mechanical scarification and wet heat treatments on germination of seeds of African locust bean tree, Parkia biglobosa. Afric. J. Biotechnol. 2004, 3, 179–181. [Google Scholar] [CrossRef]
- Pipinis, E.; Milios, E.; Smiris, P.; Gioumousidis, C. Effect of acid scarification and cold moist stratification on the germination of Cercis siliquastrum L. seeds. Turk. J. Agric. For. 2011, 35, 259–264. [Google Scholar] [CrossRef]
- Nelson, S. Noni seed handling and seedling production. Fruit. Nut. 2005, 10, 1–4. [Google Scholar]
- Botsheleng, B.; Mathowa, T.; Mojeremane, W. Effects of pre-treatments methods on the germination of pod mahogany (Afzelia quanzensis) and mukusi (Baikiaea plurijuga) seeds. Int. J. Innov. Res. Sci. 2014, 3, 8108–8113. [Google Scholar]
- Giertych, M.J.; Suszka, J. Consequences of cutting off distal ends of cotyledons of Quercus robur acorns before sowing. Ann. For. Sci. 2011, 68, 433–442. [Google Scholar] [CrossRef]
- Jabłoński, M.; Tylek, P.; Walczyk, J.; Tadeusiewicz, R.; Piłat, A. Colour-based binary discrimination of scarified Quercus robur acorn under varying illumination. Sensors 2016, 16, 1319. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Chrząstowska, J.; Kwiecień, J.; Drożdż, M.; Bubliński, Z.; Tadeusiewicz, R.; Szczepaniak, J.; Walczyk, J.; Tylek, P. Comparison of selected classification methods in automated oak seed sorting. J. Res. Appl. Agric. Eng. 2017, 62, 31–33. [Google Scholar]
- Pawlik, P.; Jabłoński, M.; Bubliński, Z.; Tadeusiewicz, R.; Walczyk, J.; Tylek, P.; Juliszewski, T.; Adamczyk, F. Use of Harris detector for determination of orientation of acorns in the process of automated scarification. J. Res. Appl. Agric. Eng. 2017, 62, 163–165. [Google Scholar]
- Przybyło, J.; Jabłoński, M.; Pociecha, D.; Tadeusiewicz, R.; Piłat, A.; Walczyk, J.; Kiełbasa, P.; Szczepaniak, J.; Adamczyk, F. Application of model-based design in algorithms’ prototyping for experimental acorn scarification rig. J. Res. Appl. Agric. Eng. 2017, 62, 166–170. [Google Scholar]
- Tadeusiewicz, R.; Tylek, P.; Adamczyk, F.; Kiełbasa, P.; Jabłoński, M.; Bubliński, Z.; Grabska-Chrząstowska, J.; Kaliniewicz, Z.; Walczyk, J.; Szczepaniak, J.; et al. Assessment of selected parameters of the automatic scarification device as an example of a device for sustainable forest management. Sustainability 2017, 9, 2370. [Google Scholar] [CrossRef]
- Greń, J. Statystyka matematyczna. Modele i zadania (Mathematical Statistics. Models and Tasks); PWN: Warszawa, Poland, 1984; pp. 237–280. ISBN 8301036990. (In Polish) [Google Scholar]
- Mohsenin, N.N. Physical Properties of Plant and Animal Materials; Gordon and Breach Science Public: New York, NY, USA, 1986; pp. 1–891. ISBN 9780677213705. [Google Scholar]
- Kaliniewicz, Z.; Tylek, P.; Markowski, P.; Anders, A.; Rawa, T.; Jóźwiak, K.; Fura, S. Correlations between the germination capacity and selected physical properties of Scots pine (Pinus sylvestris L.) seeds. Balt. For. 2013, 19, 201–211. [Google Scholar]
- Grochowicz, J. Maszyny do czyszczenia i sortowania nasion (Seed Cleaning and Sorting Machines); Akademia Rolnicza: Lublin, Poland, 1994; pp. 25–28. ISBN 839016129X. (In Polish) [Google Scholar]
- Tylkowski, T.; Bujarska-Borkowska, B. Effect of acorn size and sowing depth on Quercus robur and Q. petraea seedling emergence and height. Sylwan 2011, 155, 159–170. (In Polish) [Google Scholar]
- Rabiej, M. Statystyka z programem Statistica (Statisctics in Statistica Software); Helion: Gliwice, Poland, 2012; pp. 1–344. ISBN 9788324641109. (In Polish) [Google Scholar]
- García-Mozo, H.; Gómez-Casero, M.T.; Domínguez, E.; Galán, C. Influence of pollen emission and weather-related factors on variations in holm-oak (Quercus ilex subsp. ballota) acorn production. Environ. Exp. Bot. 2007, 61, 35–40. [Google Scholar] [CrossRef]
- Koenig, W.D.; Knops, J.M.H.; Carmen, W.J.; Sage, D. No trade-off between seed size and number in the valley oak Quercus lobata. Am. Nat. 2009, 173, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Alejano, R.; Vázquez-Piqué, J.; Carevic, F.; Fernández, M. Do ecological and silvicultural factors influence acorn mass in Holm Oak (southwestern Spain)? Agroforest Syst. 2011, 83, 25–39. [Google Scholar] [CrossRef]
- Kaliniewicz, Z.; Rawa, T.; Tylek, P.; Markowski, P.; Anders, A.; Fura, S. The effect of the age of Scots pine (Pinus sylvestris L.) stands on the physical properties of seeds and the operating parameters of cleaning machines. Tech. Sci. 2013, 16, 63–72. [Google Scholar]
- Kaliniewicz, Z.; Markowski, P.; Anders, A.; Tylek, P.; Krzysiak, Z.; Fura, S. Influence of the age of parent stand on selected physical properties of Norway spruce seeds. Sylwan 2017, 161, 548–557. (In Polish) [Google Scholar]
- Nikolić, N.P.; Orlović, S. Genotypic variability of morphological characteristics of English oak (Quercus robur L.) acorn. Zb. Matice Srp. Prir. Nauk. 2002, 102, 53–58. [Google Scholar] [CrossRef]
- Tylek, P. Size and shape as separation properties of pedunculate oak seeds (Quercus robur L.). Acta Agrophys. 2012, 19, 673–687. (In Polish) [Google Scholar]
- Rakić, S.; Povrenović, D.; Tešević, V.; Simić, M.; Maletić, R. Oak acorn, polyphenols and antioxidant activity in functional food. J. Food Eng. 2006, 74, 416–423. [Google Scholar] [CrossRef]
- Aizen, M.A.; Patterson, W.A. Acorn size and geographical range in the North American oaks (Quercus L.). J. Biogeogr. 1990, 17, 327–332. [Google Scholar] [CrossRef]
- Oleksyn, J.; Reich, P.B.; Tjoelker, M.G.; Chalupka, W. Biogeographic differences in shoot elongation pattern among European Scots pine populations. For. Ecol. Manag. 2001, 148, 207–220. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Latitude, seed predation and seed mass. J. Biogeogr. 2003, 30, 105–128. [Google Scholar] [CrossRef]
- Tylek, P.; Kaliniewicz, Z.; Kiełbasa, P.; Zagrobelny, T. Mass and density as separation criteria of pedunculate oak (Quercus robur L.) seeds. Electron. J. Pol. Agric. Univ. Ser. For. 2015, 18. Available online: http://www.ejpau.media.pl/volume18/issue4/art-05.html (accessed on 5 February 2018).
- Kaliniewicz, Z.; Tylek, P.; Anders, A.; Markowski, P.; Rawa, T.; Ołdakowski, M.; Wąsowski, L. An analysis of the physical properties of seeds of selected deciduous tree species. Balt. For. 2016, 22, 169–174. [Google Scholar]
- Kleinschmit, J. Intraspecific variation of growth and adaptive traits in European oak species. Ann. For. Sci. 1993, 50, 166–185. [Google Scholar] [CrossRef]
- Kormanik, P.P.; Sung, S.S.; Kormanik, T.L.; Schlarbaum, S.E.; Zarnoch, S.J. Effect of acorn size on development of northern red oak 1-0 seedlings. Can. J. For. Res. 1998, 28, 1805–1813. [Google Scholar] [CrossRef]
- Quero, J.L.; Villar, R.; Marañón, T.; Zamora, R.; Poorter, L. Seed-mass effects in four Mediterranean Quercus species (Fagaceae) growing in contrasting light environments. Am. J. Bot. 2007, 94, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Tilki, F. Influence of acorn size and storage duration on moisture content, germination and survival of Quercus petraea (Mattuschka). J. Environ. Biol. 2010, 31, 325–328. [Google Scholar] [PubMed]
- Yi, X.; Zhang, J.; Wang, Z. Large and small acorns contribute equally to early-stage oak seedlings: A multiple species study. Eur. J. For. Res. 2015, 134, 1019–1026. [Google Scholar] [CrossRef]
- Llanderal-Mendoza, J.; Gugger, P.F.; Oyama, K.; Uribe-Salas, D.; González-Rodríguez, A. Climatic determinants of acorn size and germination percentage of Quercus rucosa (Fagaceae) along a latitudinal gradient in Mexico. Bot. Sci. 2017, 95, 37–45. [Google Scholar] [CrossRef]
- García-Cebrián, F.; Esteso-Martínez, J.; Gil-Pelegrín, E. Influence of cotyledon removal on early seedling growth in Quercus robur L. Ann. For. Sci. 2003, 60, 69–73. [Google Scholar] [CrossRef]
- Shi, W.; Bloomberg, M.; Li, G.; Su, S.; Jia, L. Combined effects of cotyledon excision and nursery fertilization on root growth, nutrient status and outplanting performance of Quercus variabilis container seedlings. PLoS ONE 2017, 12, e0177002. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yi, X.; Yang, Y.; Liu, W. Acorn germination and seedling survival of Q. variabilis: Effects of cotyledon excision. Ann. For. Sci. 2010, 67. [Google Scholar] [CrossRef]
| Property/Indicator | Acorn Batch | |||
|---|---|---|---|---|
| O-76 | O-91 | O-131 | O-161 | |
| Length (mm) | 28.82 ± 2.20 b | 28.10 ± 2.36 a | 28.74 ± 2.41 ab | 28.49 ± 2.88 ab |
| Diameter (mm) | 16.54 ± 1.62 a | 16.27 ± 1.52 a | 16.53 ± 1.53 a | 16.25 ± 1.56 a |
| Mass (g) | 4.87 ± 1.16 b | 4.35 ± 0.98 a | 4.61 ± 1.22 ab | 4.43 ± 1.12 a |
| Arithm. mean diameter (mm) | 20.63 ± 1.50 a | 20.21 ± 1.39 a | 20.60 ± 1.53 a | 20.33 ± 1.57 a |
| Geom. mean diameter (mm) | 19.88 ± 1.55 a | 19.49 ± 1.41 a | 19.86 ± 1.53 a | 19.56 ± 1.55 a |
| Specific mass (g mm−1) | 0.24 ± 0.04 a | 0.22 ± 0.04 a | 0.23 ± 0.04 a | 0.22 ± 0.04 a |
| Shape factor K1 (-) | 0.58 ± 0.06 a | 0.58 ± 0.07 a | 0.58 ± 0.06 a | 0.58 ± 0.07 a |
| Shape factor K2 (-) | 0.69 ± 0.05 a | 0.70 ± 0.05 a | 0.69 ± 0.05 a | 0.69 ± 0.06 a |
| Scarification Index | Acorn Batch | Value of Trait | Standard Deviation of Trait | Coefficient of Trait Variability (%) | ||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Average | ||||
| Dimensional SL | O-76 | 0.15 | 0.39 | 0.28 a | 0.046 | 16.18 |
| O-91 | 0.19 | 0.40 | 0.32 b | 0.041 | 12.69 | |
| O-131 | 0.24 | 0.42 | 0.32 b | 0.031 | 9.77 | |
| O-161 | 0.22 | 0.38 | 0.31 b | 0.030 | 9.54 | |
| Mass Sm | O-76 | 0.12 | 0.35 | 0.22 a | 0.043 | 19.47 |
| O-91 | 0.13 | 0.33 | 0.24 b | 0.043 | 18.16 | |
| O-131 | 0.13 | 0.33 | 0.22 a | 0.034 | 15.64 | |
| O-161 | 0.14 | 0.33 | 0.22 a | 0.042 | 19.23 | |
| Acorn Batch | Size Group | Germination Capacity of Acorns with Dimensional Scarification Index SL: | |||
|---|---|---|---|---|---|
| ≤0.25 | 0.26–0.30 | 0.31–0.35 | >0.35 | ||
| O-76 | D ≤ 16 mm | 60.0 | 83.3 | 92.7 | 75.0 |
| D > 16 mm | 100.0 | 84.2 | 92.2 | - | |
| Total | 88.2 | 83.6 | 92.3 | 75.0 | |
| O-91 | D ≤ 16 mm | 0 | 63.6 | 57.9 | 60.0 |
| D > 16 mm | 100.0 | 88.9 | 95.8 | 88.9 | |
| Total | 80.0 | 79.3 | 79.1 | 73.7 | |
| O-131 | D ≤ 16 mm | - | 50.0 | 70.4 | 100.0 |
| D > 16 mm | 100.0 | 94.4 | 96.3 | 100.0 | |
| Total | 100.0 | 76.7 | 81.8 | 100.0 | |
| O-161 | D ≤ 16 mm | 33.3 | 64.7 | 64.0 | 100.0 |
| D > 16 mm | - | 81.0 | 100.0 | 100.0 | |
| Total | 33.3 | 73.2 | 78.6 | 100.0 | |
| Acorn Batch | Size Group | Germination Capacity of Acorns with Mass Scarification Index Sm: | ||||
|---|---|---|---|---|---|---|
| ≤0.15 | 0.16–0.20 | 0.21–0.25 | 0.26–0.30 | >0.30 | ||
| O-76 | D ≤ 16 mm | 66.6 | 82.3 | 82.6 | 88.9 | 100.0 |
| D > 16 mm | 100.0 | 100.0 | 81.0 | 100.0 | 100.0 | |
| Total | 83.3 | 90.3 | 81.8 | 91.7 | 100.0 | |
| O-91 | D ≤ 16 mm | 100.0 | 66.7 | 66.7 | 42.9 | 50.0 |
| D > 16 mm | 100.0 | 92.3 | 96.4 | 90.0 | 66.7 | |
| Total | 100.0 | 87.5 | 84.8 | 62.5 | 57.1 | |
| O-131 | D ≤ 16 mm | 0 | 93.3 | 40.0 | 63.6 | - |
| D > 16 mm | 100.0 | 100.0 | 93.1 | 100.0 | 100.0 | |
| Total | 50.0 | 97.1 | 75.0 | 73.3 | 100.0 | |
| O-161 | D ≤ 16 mm | 100.0 | 50.0 | 79.2 | 57.1 | 0 |
| D > 16 mm | 100.0 | 100.0 | 81.3 | 75.0 | - | |
| Total | 100.0 | 82.8 | 80.0 | 61.1 | 0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaliniewicz, Z.; Tylek, P. Influence of Scarification on the Germination Capacity of Acorns Harvested from Uneven-Aged Stands of Pedunculate Oak (Quercus robur L.). Forests 2018, 9, 100. https://doi.org/10.3390/f9030100
Kaliniewicz Z, Tylek P. Influence of Scarification on the Germination Capacity of Acorns Harvested from Uneven-Aged Stands of Pedunculate Oak (Quercus robur L.). Forests. 2018; 9(3):100. https://doi.org/10.3390/f9030100
Chicago/Turabian StyleKaliniewicz, Zdzisław, and Paweł Tylek. 2018. "Influence of Scarification on the Germination Capacity of Acorns Harvested from Uneven-Aged Stands of Pedunculate Oak (Quercus robur L.)" Forests 9, no. 3: 100. https://doi.org/10.3390/f9030100
APA StyleKaliniewicz, Z., & Tylek, P. (2018). Influence of Scarification on the Germination Capacity of Acorns Harvested from Uneven-Aged Stands of Pedunculate Oak (Quercus robur L.). Forests, 9(3), 100. https://doi.org/10.3390/f9030100






