Biomass Losses Caused by Teratosphaeria Leaf Disease in Eucalyptus globulus Short Rotation Forestry
Abstract
:1. Introduction
2. Materials and Methods
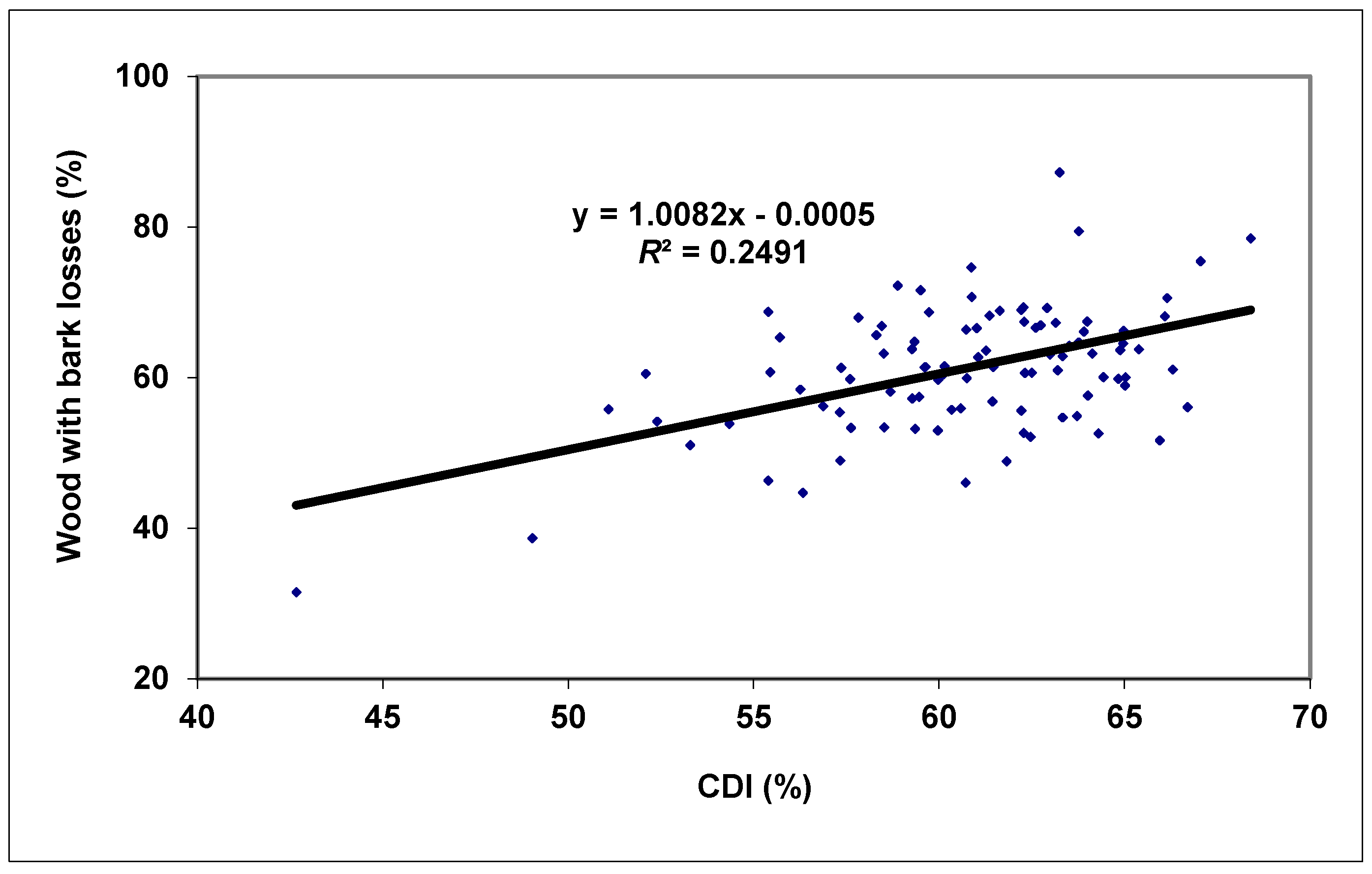
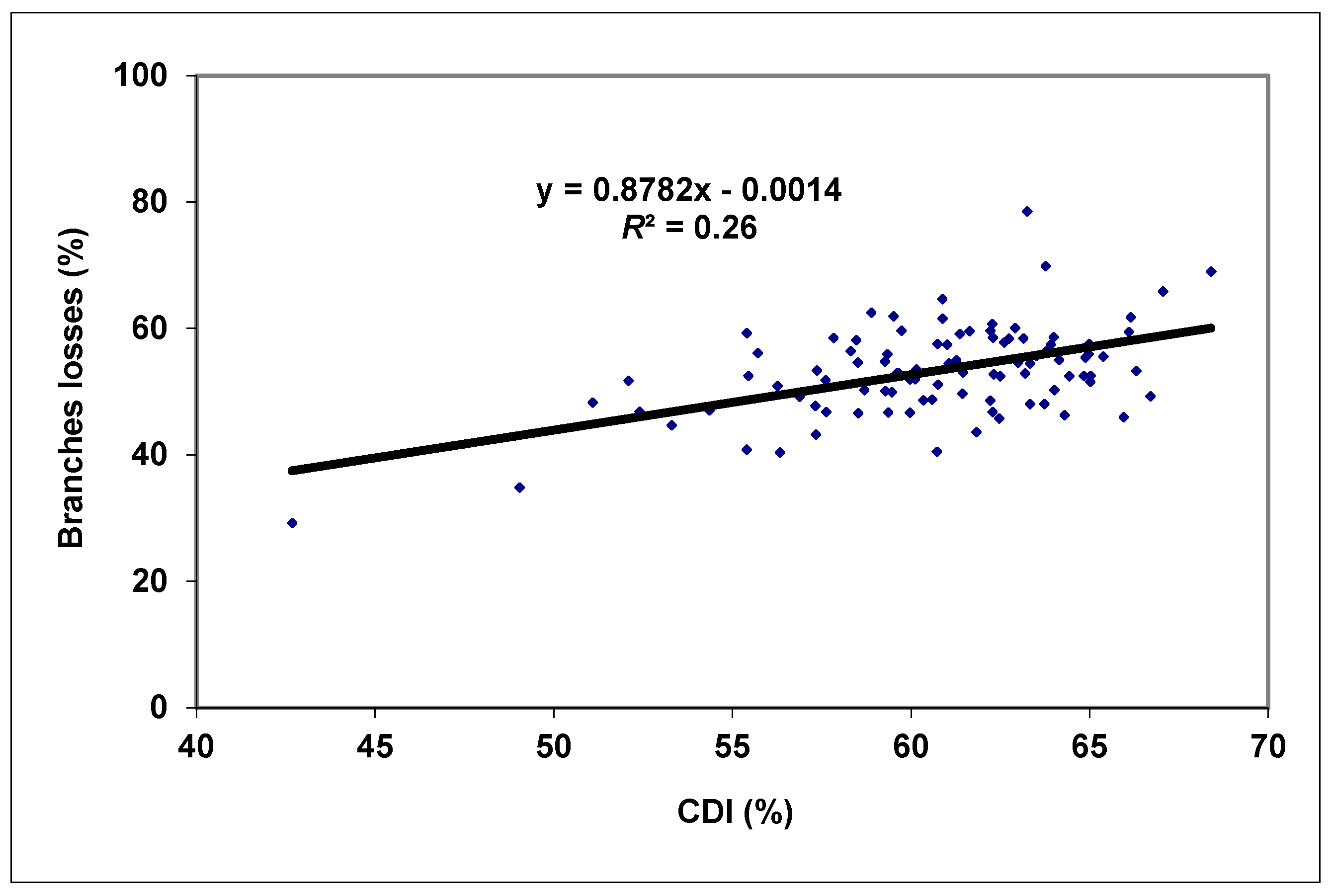
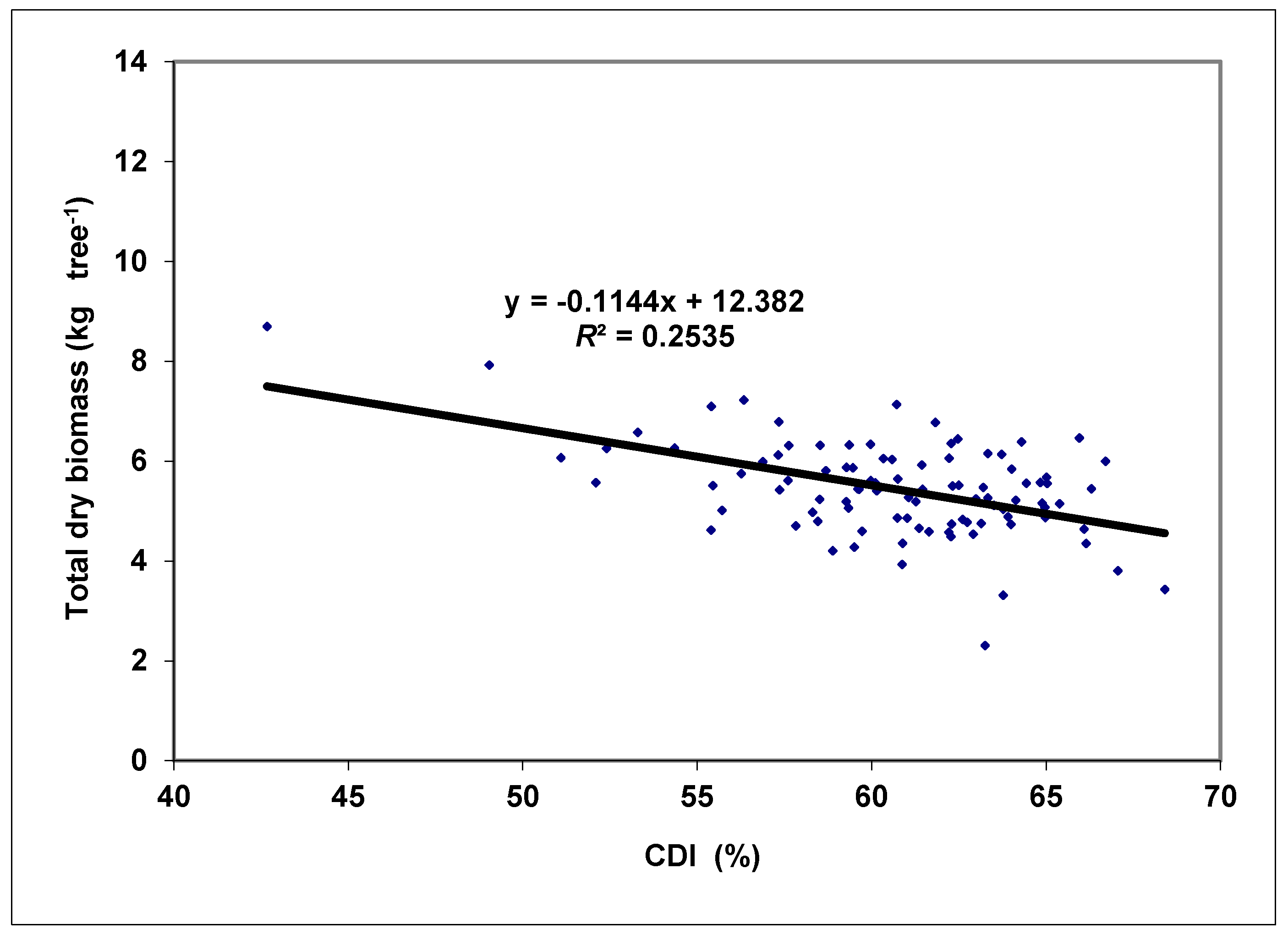
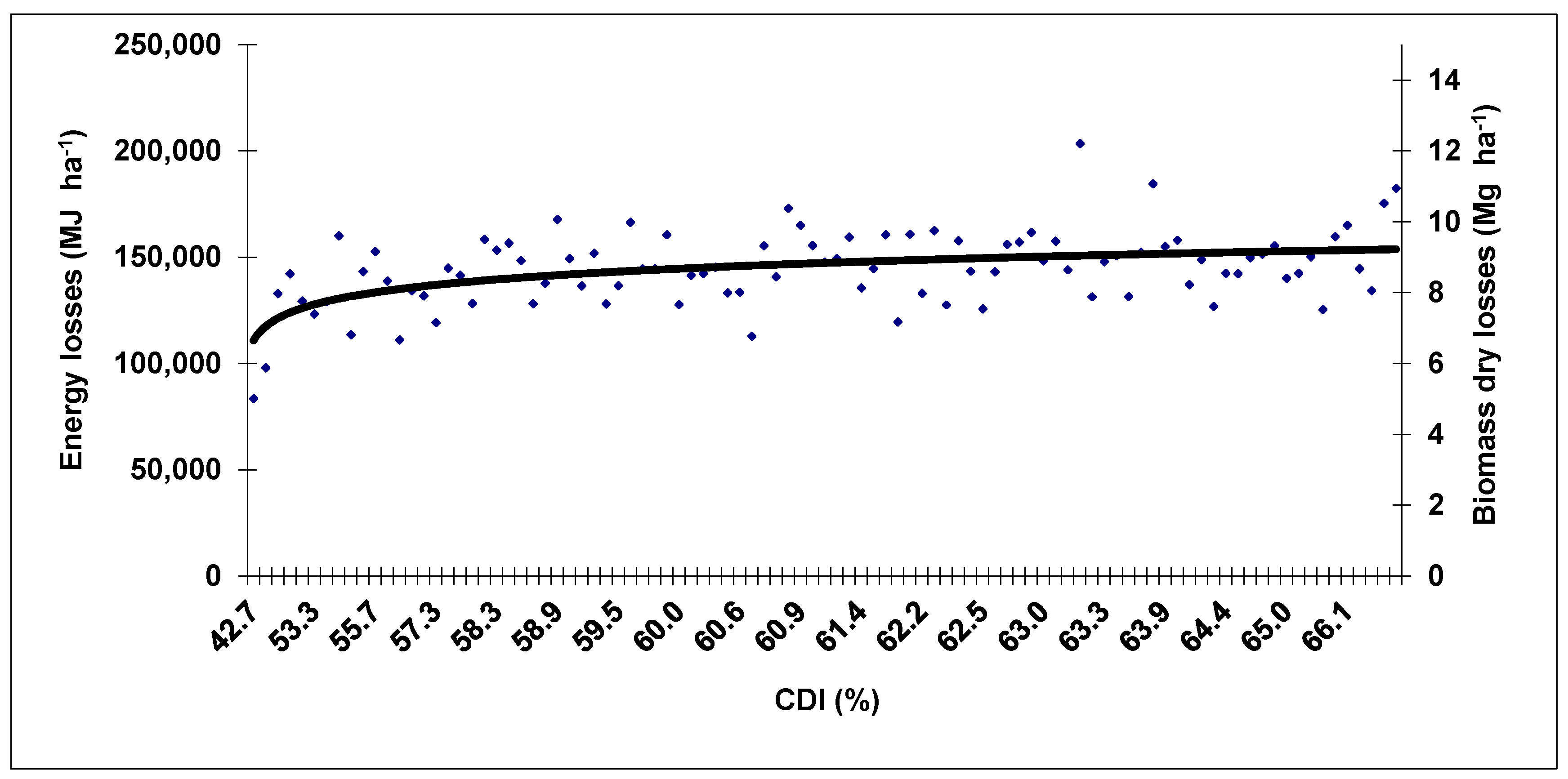
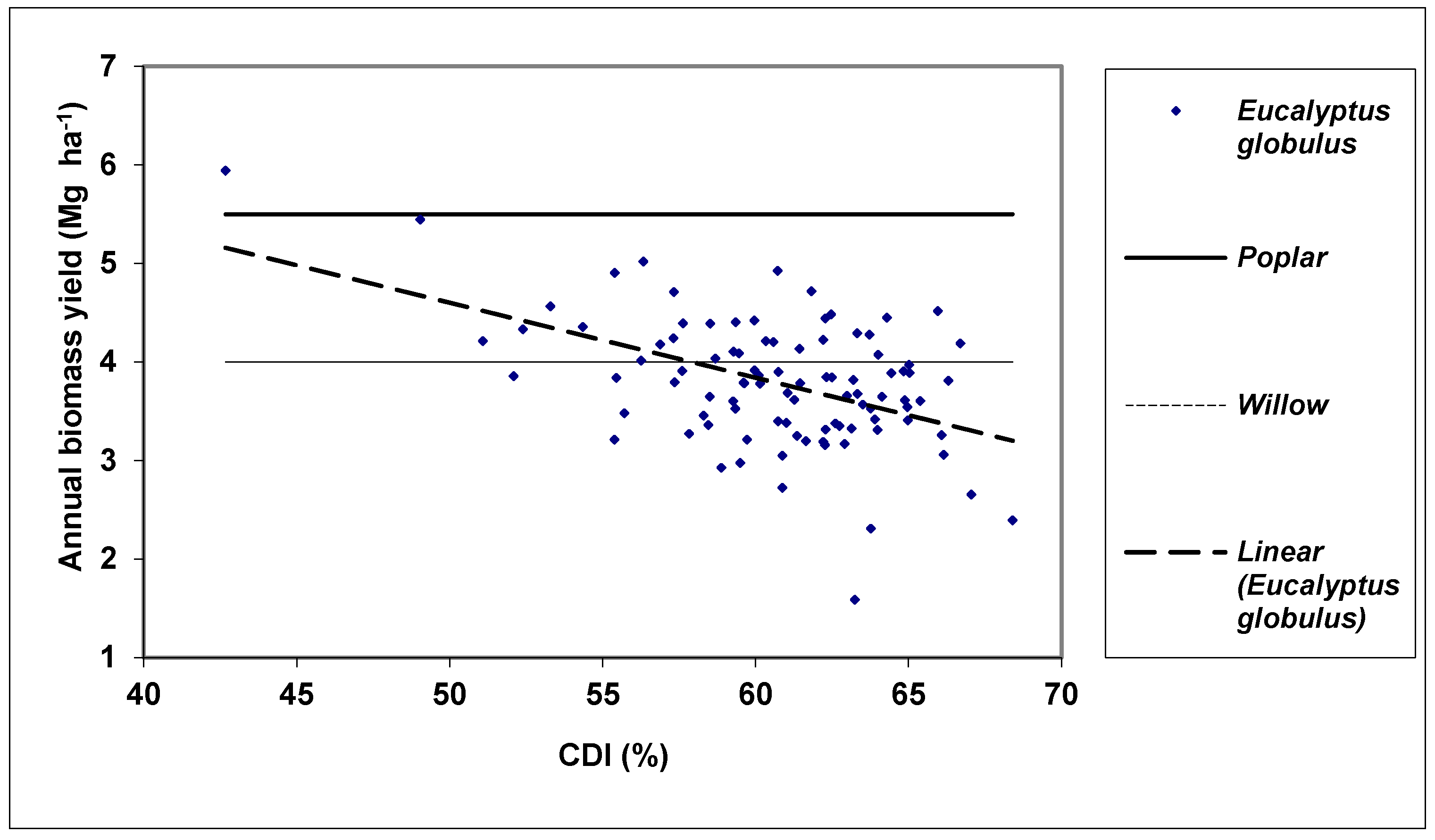
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pacala, S.; Socolow, R. Stabilization wedges: Solving the climate problem for the next 50 years with current technologies. Science 2004, 305, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Werther, J.; Saenger, M.; Harthge, U.; Ogada, T.; Siagi, Z. Combustion of agricultural residues. Prog. Energy Combust. Sci. 2000, 26, 1–27. [Google Scholar] [CrossRef]
- Strelher, A. Technologies of wood combustion. Ecol. Eng. 2000, 16, 25–40. [Google Scholar] [CrossRef]
- Walle, I.V.; Camp, N.V.; Van de Casteele, L.; Verheyen, K.; Lemeur, R. Short-rotation forestry of birch, maple, poplar and willow in Flanders (Belgium) II. Energy production and CO2 emission reduction potential. Biomass Bioenergy 2007, 31, 276–283. [Google Scholar] [CrossRef]
- Sims, R.E.; Senelwa, K.; Maiava, T.; Bullock, B.T. Eucalyptus species for biomass energy in New Zealand—Part II: Coppice performance. Biomass Bioenergy 1999, 17, 333–343. [Google Scholar] [CrossRef]
- Zewdie, M.; Olsson, M.; Verwijst, T. Above-ground biomass production and allometric relations of Eucalyptus globulus Labill coppice plantations along a chronosequence in the central highlands of Ethiopia. Biomass Bioenergy 2009, 33, 421–428. [Google Scholar] [CrossRef]
- Sixto, H.; Hernández, M.; Barrio, M.; Carrasco, J.; Cañellas, I. Plantaciones del género Populus para producción de biomasa con fines energéticos: Revisión. Investig. Agrar. Sist. Recur. For. 2007, 16, 277–294. [Google Scholar] [CrossRef]
- Hofmann-Schielle, C.; Jug, A.; Makeschin, F.; Rehfuess, K. Short-rotation plantations of balsam poplars, aspen and willows on former arable land in the Federal Republic of Germany. I. Site-growth relationships. For. Ecol. Manag. 1999, 121, 67–83. [Google Scholar] [CrossRef]
- Misra, R.K.; Turnbull, C.R.A.; Cromer, R.N.; Gibbons, A.K.; LaSala, A.V. Bellow-and above ground growth of Eucalyptus nitens in a young plantation I. Biomass. For. Ecol. Manag. 1998, 106, 283–293. [Google Scholar] [CrossRef]
- Van den Broek, R.; Vleeshouwers, L.; Hoogwijk, M. The energy crop growth model SILVA: Description and application to Eucalyptus plantations in Nicaragua. Biomass Bioenergy 2001, 21, 335–349. [Google Scholar] [CrossRef]
- Sochacki, S.J.; Harper, R.J.; Smettem, K.R.J. Estimation of woody biomass production from a short-rotation bio-energy system in semi-arid Australia. Biomass Bioenergy 2007, 31, 608–616. [Google Scholar] [CrossRef]
- Rockwood, D.L.; Rudie, A.W.; Ralph, S.A. Energy product options for Eucalyptus species grown as short rotation woody crops. Int. J. Mol. Sci. 2008, 9, 1361–1378. [Google Scholar] [CrossRef] [PubMed]
- Sims, R.E.H.; Maiava, T.G.; Bullock, B.T. Short rotation coppice tree species selection for woody biomass production in New Zealand. Biomass Bioenergy 2001, 20, 329–335. [Google Scholar] [CrossRef]
- Gasol, C.M.; Gabarrilla, X.; Antón, A.; Rigolad, M.; Carrascoe, J.; Ciriae, P.; Rieradevalla, J. LCA of poplar bioenergy system compared with Brassica carinata energy crop and natural gas in regional scenario. Biomass Bioenergy 2009, 33, 119–129. [Google Scholar] [CrossRef]
- Zalesny, R.S.; Wiese, A.H.; Bauer, E.O.; Riemenschneider, D.E. Ex situ growthand biomass of Populus bioenergy crops irrigatedand fertilized with landfill leachate. Biomass Bioenergy 2009, 33, 62–69. [Google Scholar] [CrossRef]
- Coylea, D.R.; Colemana, M.D.; Durante, J.A.; Newman, L.A. Survival and growth of 31 Populus clones in South Carolina. Biomass Bioenergy 2006, 30, 750–758. [Google Scholar] [CrossRef]
- Ceulemans, R.; Mcdonaldt, A.J.S.; Pereira, J.S.A. A comparison among eucalypt, poplar and willow characteristics with particular reference to a coppice, growth-modelling approach. Biomass Bioenergy 1996, 11, 215–231. [Google Scholar] [CrossRef]
- Arevalo, C.B.M.; Volk, T.A.; Bevilacqua, E.; Abrahamson, L. Development and validation of aboveground biomass estimations for four Salix clones in central New York. Biomass Bioenergy 2007, 31, 1–12. [Google Scholar] [CrossRef]
- Aravanopoulos, F.A.; Kimb, K.H.; Zsuffa, L. Genetic diversity of superior Salix clones selected for intensive forestry plantations. Biomass Bioenergy 1999, 16, 249–255. [Google Scholar] [CrossRef]
- Kidanu, S.; Mamo, T.; Stroosnijder, L. Biomass production of Eucalyptus boundary plantations and their effect on crop productivity on Ethiopian highland vertisols. Agrofor. Syst. 2005, 63, 281–290. [Google Scholar] [CrossRef]
- Tejedor, C. Basic density selection for Eucalyptus globulus in Northern Spain. Within-tree and between-tree variation. In Proceedings of the IUFRO Conference Eucalyptus in a Changing World, Aveiro, Portugal, 11–15 October 2004. [Google Scholar]
- Faúndez, P. Potential costs of four short-rotation silvicultural regimes used for the production of energy. Biomass Bioenergy 2003, 24, 373–380. [Google Scholar] [CrossRef]
- Pérez, S.; Renedo, C.J.; Ortiz, A.; Mañana, M.; Silió, D. Energy evaluation of the Eucalyptus globulus and the Eucalyptus nitens in the North of Spain (Cantabria). Thermochim. Acta 2006, 451, 57–64. [Google Scholar] [CrossRef]
- Hunter, G.C.; Crous, P.W.; Carnegie, A.J.; Burgess, T.I.; Wingfield, M.J. Mycosphaerella and Teratosphaeria diseases of Eucalyptus: Easily confused and with serious consequences. Fungal Divers. 2011, 50, 145–166. [Google Scholar] [CrossRef]
- Park, R.F.; Keane, P.J.; Wingfield, M.J.; Crous, P.W. Fungal disease of eucalypt foliage. In Diseases and Pathogens of Eucalypts; Keane, P.J., Kile, G.A., Podger, F.D., Brown, B.N., Eds.; CSIRO Publishing: Collingwood, Australia, 2000; pp. 153–239. [Google Scholar]
- Crous, P.W.; Hong, L.; Wingfield, B.D.; Wingfield, M.J. ITS rDNA phylogeny of selected Mycosphaerella species and their anamorphs ocurring on Myrtaceae. Mycol. Res. 2001, 105, 425–431. [Google Scholar] [CrossRef]
- Milgate, A.W.; Potts, B.M.; Joyce, K.; Mohammed, C.; Vaillancourt, R.E. Genetic variation in Eucalyptus globulus for susceptibility to Mycosphaerella nubilosa and its association with tree growth. Australas. Plant Pathol. 2005, 34, 11–18. [Google Scholar] [CrossRef]
- Park, R.F.; Keane, P.J. Three Mycosphaerella species from leaf diseases of Eucalyptus. Trans. Br. Mycol. Soc. 1982, 79, 95–100. [Google Scholar] [CrossRef]
- Royle, D.J.; Hubbes, M. Diseases and pests in energy crop plantations. Biomass Bioenergy 1992, 2, 45–54. [Google Scholar] [CrossRef]
- Royle, D.J.; Ostry, M.E. Disease and pest control in the bioenergy crops Poplar and Willow. Biomass Bioenergy 1995, 9, 69–79. [Google Scholar] [CrossRef]
- Sage, R.B. Short rotation coppice for energy: Towards guidelines. Biomass Bioenergy 1998, 15, 39–47. [Google Scholar] [CrossRef]
- Pinkard, E.A.; Mohammed, C.L. Photosynthesis of Eucalyptus globulus with Mycosphaerella leaf disease. New Phytol. 2006, 170, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Eyles, A.; Barry, K.; Quentin, A.; Pinkard, E. Impact of defoliation in temperate Eucalypt plantations: Physiological perspectives and management. For. Ecol. Manag. 2013, 304, 49–64. [Google Scholar] [CrossRef]
- Tuskan, G.A. Short-Rotation woody crop supply systems in the United States: What do we know and what do we need to know? Biomass Bioenergy 1998, 14, 307–315. [Google Scholar] [CrossRef]
- Dickmann, D.I. Silviculture and biology of short-rotation woody crops in temperate regions: Then and now. Biomass Bioenergy 2006, 30, 696–705. [Google Scholar] [CrossRef]
- Park, R.F. Epidemiology of Mycosphaerella nubilosa and M. cryptica on Eucalyptus spp. in South-Eastern Australia. Trans. Br. Mycol. Soc. 1988, 91, 261–266. [Google Scholar] [CrossRef]
- Carnegie, A.J.; Addes, P.K. The proportion of leaf spots caused by Mycosphaerella cryptica and M. nubilosa on Eucalyptus globulus, E. nitens and their F1 hybrids in a family trial in Tasmania, Australia. Australas. Mycol. 2002, 21, 53–63. [Google Scholar]
- Maxwell, A. The Taxonomy Philogeny and Impact of Mycosphaerella Species on Eucalypts in South-Western Australia. Ph.D. Thesis, School of Biothecnology and Biological Science, Murdoch University, Murdoch, Australia, 2004. [Google Scholar]
- Carnegie, A.J.; Keane, P.J.; Ades, P.K.; Smith, I.W. Variation in susceptibility of Eucalyptus globulus provenances to Mycosphaerella leaf disease. Can. J. For. Res. 1994, 24, 1751–1757. [Google Scholar] [CrossRef]
- Lundquist, J.E.; Purnell, R.C. Effects of Mycosphaerella Leaf Spot on growth of Eucalyptus nitens. Plant Dis. 1987, 71, 1025–1029. [Google Scholar] [CrossRef]
- Stone, C.; Matsuki, M.; Carnegie, A.J. Pest and Disease Assessment in Young Eucalypt Plantations: Field Manual for Using the Crown Damage Index; Parson, M., Ed.; National Forest Inventory, Bureau of Rural Sciences: Canberra, Australia, 2003. [Google Scholar]
- Pita, P.A. Tablas de CubicacióN Por DiáMetros Normales y Alturas Totales; Instituto Forestal de Investigaciones y Experiencias (I.F.I.E. Ministerio de Agricultura): Madrid, Spain, 1967. [Google Scholar]
- Reed, D.; Tomé, M. Total aboveground biomass and net dry matter accumulation by plant component in young Eucalyptus globulus in response to irrigation. For. Ecol. Manag. 1998, 103, 21–32. [Google Scholar] [CrossRef]
- Hubbard, W.N.; Scott, D.W.; Waddinton, G. Experimental Thermochemistry; Rossini, F.D., Ed.; Interscience: New York, NY, USA, 1956. [Google Scholar]
- Brañas, J.; González-Río, E.; Merino, A. Content and distribution of nutrients in Eucalyptus globulus plantations in Northwestern Spain. Investig. Agrar. 2000, 2, 317–355. [Google Scholar]
- Singh, R.P.; Sharma, V.K. Biomass estimation in five different aged plantations of Eucalyptus tereticornis Smith in Western Uttar Pradesh. In Oslo Biomass Studies; College of Life Sciences and Agriculture, University of Maine: Orono, ME, USA, 1976; pp. 143–161. [Google Scholar]
- Soria, S. Especies de Mycosphaerella, y Teratosphaeria en Plantaciones Jóvenes de Eucalyptus globulus: Evaluación de Daño y Control. Ph.D. Thesis, Facultad de Ciencias, Universidad de Uruguay, Montevideo, Uruguay, 2016. [Google Scholar]
- Dungey, H.S.; Potts, B.M.; Carnegie, A.J.; Ades, P.K. Mycosphaerella leaf disease: Genetic variation in damage to Eucalyptus nitens, Eucalyptus globulus, and their F1 hybrid. Can. J. For. Res. 1997, 27, 750–759. [Google Scholar] [CrossRef]
- Balmelli, G.; Altier, N.; Marroni, V. Daños provocados por enfermedades foliares y por heladas en E. globulus I. Efecto fenotípico sobre el comportamiento productivo posterior. Bol. CIDEU 2007, 3, 67–75. [Google Scholar]
- Vande Walle, I.; Van Camp, N.; Van de Casteele, L.; Verheyen, K.; Lemeur, R. Short-rotation forestry of birch, maple, poplar and willow in Flanders (Belgium) I—Biomass production after 4 years of tree growth. Biomass Bioenergy 2007, 31, 267–275. [Google Scholar] [CrossRef]
- Pérez, S.; Renedo, C.J.; Ortiz, A.; Ortiz, F.; Tejedor, C. Strategies to combat Mycosphaerella leaf disease in Eucalyptus globulus plantations in northern Spain. Forests 2016, 7, 190. [Google Scholar] [CrossRef]

| Fraction | α | β | γ |
|---|---|---|---|
| Total biomass | −2.8982 | 0.1984 | 1.7425 |
| Leaves | 0.7897 | 0.2921 | 0.8769 |
| Wood + bark | −6.8579 | 0.2474 | 2.2294 |
| Rest | −2.5669 | 0.3346 | 1.3349 |
| Code | H (m) | Std Dev | Std Err Mean | D × 102 (m) | Std Dev | Std Err Mean | V × 103 (m3) | Std Dev | Std Err Mean | CDI (%) | Std Dev | Std Err Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 32 | 4.22 | 1.06 | 0.249 | 3.34 | 1.07 | 0.252 | 6.32 | 2.69 | 0.635 | 67.05 | 7.42 | 1.749 |
| 65 | 5.19 | 1.14 | 0.238 | 4.33 | 1.31 | 0.272 | 9.65 | 4.44 | 0.926 | 56.26 | 15.46 | 3.225 |
| 68 | 4.93 | 1.31 | 0.272 | 3.97 | 1.36 | 0.284 | 8.60 | 3.96 | 0.826 | 64.88 | 8.66 | 1.806 |
| 86 | 4.64 | 1.34 | 0.292 | 3.76 | 1.48 | 0.323 | 7.97 | 4.50 | 0.981 | 59.72 | 14.30 | 3.121 |
| 89 | 4.61 | 1.04 | 0.216 | 3.74 | 1.06 | 0.220 | 7.47 | 2.92 | 0.609 | 62.91 | 6.65 | 1.387 |
| 90 | 5.07 | 1.25 | 0.272 | 4.10 | 1.26 | 0.274 | 8.94 | 3.81 | 0.831 | 66.30 | 8.18 | 1.785 |
| 92 | 4.88 | 1.08 | 0.221 | 3.88 | 1.02 | 0.207 | 8.03 | 2.95 | 0.602 | 63.77 | 8.30 | 1.694 |
| 96 | 4.72 | 0.95 | 0.203 | 3.82 | 0.96 | 0.205 | 7.68 | 2.99 | 0.637 | 62.29 | 8.64 | 1.842 |
| 101 | 4.80 | 0.92 | 0.196 | 3.87 | 1.15 | 0.246 | 8.00 | 3.52 | 0.751 | 63.90 | 5.90 | 1.257 |
| 102 | 5.03 | 1.58 | 0.372 | 4.65 | 1.64 | 0.397 | 10.82 | 5.93 | 1.439 | 52.09 | 19.65 | 4.632 |
| 104 | 5.61 | 0.97 | 0.206 | 4.89 | 1.18 | 0.251 | 11.44 | 4.02 | 0.856 | 57.33 | 12.87 | 2.744 |
| 105 | 6.00 | 1.27 | 0.259 | 5.65 | 1.80 | 0.367 | 14.99 | 7.68 | 1.568 | 49.03 | 17.98 | 3.670 |
| 152 | 5.05 | 1.96 | 0.408 | 4.76 | 1.63 | 0.355 | 11.47 | 5.71 | 1.245 | 60.75 | 9.97 | 2.125 |
| 213 | 4.02 | 1.24 | 0.358 | 3.04 | 1.06 | 0.305 | 5.70 | 2.83 | 0.816 | 68.40 | 8.28 | 2.391 |
| 216 | 4.76 | 0.99 | 0.197 | 3.89 | 0.93 | 0.187 | 7.84 | 2.81 | 0.561 | 62.60 | 7.31 | 1.462 |
| 223 | 5.38 | 1.32 | 0.269 | 4.44 | 1.47 | 0.301 | 10.40 | 5.73 | 1.170 | 63.33 | 5.42 | 1.107 |
| 225 | 5.65 | 0.89 | 0.182 | 4.68 | 0.93 | 0.189 | 10.78 | 3.45 | 0.704 | 61.83 | 8.71 | 1.779 |
| 232 | 4.89 | 1.19 | 0.239 | 4.06 | 1.16 | 0.232 | 8.55 | 3.69 | 0.738 | 63.50 | 7.49 | 1.497 |
| 235 | 5.23 | 0.87 | 0.186 | 4.36 | 0.86 | 0.183 | 9.39 | 3.12 | 0.665 | 64.01 | 7.00 | 1.493 |
| 238 | 5.31 | 1.36 | 0.278 | 4.49 | 1.30 | 0.265 | 10.24 | 4.73 | 0.965 | 60.58 | 10.91 | 2.227 |
| 239 | 5.71 | 1.39 | 0.279 | 5.25 | 1.32 | 0.269 | 13.13 | 6.13 | 1.251 | 60.72 | 8.54 | 1.744 |
| 241 | 5.40 | 1.15 | 0.235 | 4.63 | 1.13 | 0.231 | 10.48 | 3.93 | 0.803 | 54.35 | 13.29 | 2.712 |
| 246 | 5.32 | 1.00 | 0.199 | 4.49 | 1.05 | 0.210 | 9.97 | 3.94 | 0.788 | 62.22 | 6.81 | 1.362 |
| 248 | 4.76 | 1.27 | 0.253 | 3.79 | 1.23 | 0.246 | 7.92 | 3.55 | 0.710 | 58.46 | 12.70 | 2.540 |
| 255 | 5.29 | 1.30 | 0.265 | 4.68 | 1.60 | 0.326 | 10.96 | 6.28 | 1.281 | 51.09 | 13.83 | 2.823 |
| 256 | 4.73 | 0.95 | 0.198 | 3.82 | 0.95 | 0.197 | 7.66 | 2.58 | 0.537 | 63.14 | 7.15 | 1.491 |
| 257 | 4.76 | 1.00 | 0.204 | 3.72 | 1.08 | 0.220 | 7.65 | 3.45 | 0.705 | 62.74 | 6.96 | 1.420 |
| 259 | 4.91 | 1.19 | 0.254 | 4.15 | 1.16 | 0.247 | 8.77 | 4.00 | 0.852 | 61.26 | 8.78 | 1.873 |
| 261 | 5.43 | 0.89 | 0.186 | 4.60 | 1.00 | 0.209 | 10.31 | 3.72 | 0.775 | 57.62 | 11.73 | 2.447 |
| 265 | 5.48 | 1.01 | 0.207 | 4.68 | 1.25 | 0.255 | 10.76 | 3.93 | 0.802 | 62.47 | 8.70 | 1.775 |
| 267 | 5.82 | 1.18 | 0.245 | 4.89 | 1.23 | 0.257 | 11.91 | 4.99 | 1.041 | 56.33 | 9.26 | 1.932 |
| 270 | 5.30 | 1.06 | 0.226 | 4.40 | 0.98 | 0.209 | 9.69 | 3.86 | 0.822 | 56.88 | 18.17 | 3.874 |
| 271 | 4.98 | 1.18 | 0.240 | 4.10 | 1.17 | 0.245 | 8.93 | 4.16 | 0.866 | 61.05 | 11.97 | 2.444 |
| 275 | 5.02 | 0.97 | 0.199 | 4.35 | 1.12 | 0.228 | 9.16 | 3.24 | 0.661 | 59.63 | 8.16 | 1.665 |
| 279 | 5.15 | 0.93 | 0.198 | 4.05 | 0.84 | 0.179 | 8.60 | 2.82 | 0.600 | 64.83 | 6.15 | 1.311 |
| 282 | 4.96 | 1.28 | 0.280 | 4.14 | 1.28 | 0.280 | 8.91 | 3.88 | 0.847 | 63.33 | 9.76 | 2.130 |
| 283 | 6.24 | 1.19 | 0.248 | 6.18 | 1.63 | 0.340 | 17.13 | 8.22 | 1.714 | 42.67 | 16.59 | 3.460 |
| 286 | 5.06 | 1.24 | 0.259 | 4.33 | 1.26 | 0.262 | 9.46 | 4.61 | 0.962 | 55.45 | 12.91 | 2.691 |
| 287 | 4.93 | 0.95 | 0.202 | 4.20 | 1.20 | 0.256 | 8.86 | 3.86 | 0.822 | 58.50 | 9.80 | 2.090 |
| 338 | 5.11 | 1.01 | 0.212 | 4.36 | 1.26 | 0.263 | 9.55 | 4.88 | 1.017 | 57.60 | 10.10 | 2.107 |
| 339 | 5.26 | 1.12 | 0.228 | 4.30 | 1.24 | 0.253 | 9.62 | 4.30 | 0.878 | 59.28 | 14.57 | 2.973 |
| 340 | 4.87 | 0.70 | 0.146 | 4.02 | 0.77 | 0.160 | 8.10 | 2.42 | 0.505 | 64.96 | 8.38 | 1.747 |
| 341 | 5.36 | 1.16 | 0.237 | 4.82 | 1.31 | 0.267 | 11.04 | 4.46 | 0.911 | 52.40 | 10.80 | 2.204 |
| 342 | 4.44 | 1.62 | 0.324 | 3.76 | 1.79 | 0.358 | 8.19 | 6.24 | 1.247 | 59.50 | 14.21 | 2.843 |
| 343 | 4.41 | 1.32 | 0.270 | 3.71 | 1.12 | 0.233 | 7.44 | 3.38 | 0.706 | 58.88 | 12.36 | 2.577 |
| 345 | 4.93 | 1.29 | 0.268 | 3.93 | 1.42 | 0.297 | 8.64 | 4.83 | 1.006 | 65.38 | 7.97 | 1.662 |
| 346 | 4.78 | 1.12 | 0.233 | 4.20 | 1.30 | 0.270 | 8.78 | 4.32 | 0.902 | 58.30 | 10.57 | 2.204 |
| 347 | 4.64 | 1.58 | 0.322 | 3.48 | 1.57 | 0.321 | 7.66 | 4.97 | 1.014 | 62.27 | 8.35 | 1.704 |
| 348 | 5.32 | 1.25 | 0.272 | 4.31 | 1.19 | 0.259 | 9.71 | 4.28 | 0.933 | 66.70 | 5.90 | 1.287 |
| 349 | 4.88 | 1.17 | 0.245 | 4.34 | 1.15 | 0.239 | 9.09 | 3.85 | 0.803 | 59.27 | 10.44 | 2.226 |
| 350 | 5.13 | 0.97 | 0.199 | 4.09 | 0.94 | 0.191 | 8.74 | 3.18 | 0.650 | 65.03 | 6.02 | 1.228 |
| 351 | 4.62 | 1.00 | 0.224 | 3.96 | 1.29 | 0.288 | 8.03 | 3.56 | 0.797 | 55.39 | 13.16 | 2.944 |
| 352 | 5.41 | 1.59 | 0.331 | 4.71 | 1.78 | 0.372 | 11.42 | 5.56 | 1.159 | 58.52 | 11.18 | 2.331 |
| 353 | 5.46 | 1.46 | 0.291 | 4.53 | 1.26 | 0.253 | 10.56 | 5.60 | 1.119 | 59.96 | 9.56 | 1.912 |
| 354 | 4.77 | 1.09 | 0.227 | 3.91 | 1.00 | 0.209 | 7.95a | 3.08 | 0.643 | 60.73 | 9.30 | 1.939 |
| 355 | 5.13 | 1.03 | 0.215 | 4.25 | 1.11 | 0.232 | 9.20 | 3.79 | 0.790 | 59.97 | 10.46 | 2.182 |
| 356 | 5.06 | 1.13 | 0.230 | 4.34 | 1.32 | 0.270 | 9.47 | 4.09 | 0.834 | 62.50 | 6.26 | 1.277 |
| 357 | 5.23 | 1.17 | 0.239 | 4.45 | 1.28 | 0.262 | 10.03 | 5.40 | 1.103 | 59.46 | 10.21 | 2.084 |
| 358 | 5.02 | 1.67 | 0.340 | 4.32 | 1.42 | 0.296 | 9.87 | 5.65 | 1.178 | 61.46 | 9.22 | 1.883 |
| 359 | 4.79 | 1.05 | 0.215 | 3.85 | 1.09 | 0.222 | 7.92 | 3.23 | 0.659 | 64.98 | 7.18 | 1.466 |
| 360 | 5.02 | 0.77 | 0.157 | 4.35 | 0.92 | 0.188 | 9.07 | 2.90 | 0.593 | 59.60 | 7.56 | 1.544 |
| 361 | 5.18 | 1.45 | 0.295 | 4.20 | 1.50 | 0.307 | 9.67 | 5.85 | 1.194 | 65.01 | 7.51 | 1.534 |
| 362 | 4.72 | 0.96 | 0.192 | 3.79 | 1.02 | 0.205 | 7.66 | 3.00 | 0.600 | 63.99 | 10.66 | 2.131 |
| 363 | 4.61 | 1.42 | 0.295 | 3.87 | 1.45 | 0.302 | 8.10 | 4.24 | 0.883 | 62.21 | 7.37 | 1.538 |
| 364 | 5.71 | 0.77 | 0.157 | 5.17 | 1.19 | 0.244 | 12.31 | 4.63 | 0.945 | 55.40 | 14.07 | 2.872 |
| 365 | 4.56 | 1.11 | 0.226 | 3.43 | 1.03 | 0.210 | 6.86a | 2.67 | 0.545 | 66.15 | 10.11 | 2.063 |
| 366 | 4.65 | 1.47 | 0.300 | 4.06 | 1.48 | 0.303 | 8.60 | 4.66 | 0.951 | 57.82 | 8.73 | 1.782 |
| 367 | 4.85 | 1.23 | 0.251 | 4.12 | 1.34 | 0.274 | 8.77 | 4.30 | 0.877 | 59.33 | 11.42 | 2.382 |
| 368 | 5.54 | 0.87 | 0.177 | 4.45 | 0.82 | 0.167 | 9.98 | 3.13 | 0.638 | 65.95 | 5.10 | 1.040 |
| 370 | 4.74 | 1.13 | 0.303 | 4.04 | 1.17 | 0.311 | 8.30 | 3.66 | 0.978 | 61.01 | 4.22 | 1.127 |
| 372 | 4.80 | 0.85 | 0.185 | 4.24 | 1.31 | 0.287 | 8.80 | 4.00 | 0.872 | 55.71 | 11.00 | 2.401 |
| 377 | 4.53 | 0.94 | 0.200 | 3.57 | 0.97 | 0.206 | 7.03 | 3.00 | 0.639 | 60.88 | 7.29 | 1.555 |
| 379 | 5.28 | 0.97 | 0.194 | 4.36 | 1.05 | 0.210 | 9.60 | 3.95 | 0.790 | 61.44 | 8.64 | 1.728 |
| 380 | 5.36 | 1.48 | 0.309 | 4.50 | 1.04 | 0.221 | 10.37 | 3.78 | 0.806 | 63.72 | 9.33 | 1.946 |
| 381 | 5.30 | 0.89 | 0.186 | 4.81 | 1.07 | 0.223 | 10.64 | 3.64 | 0.759 | 57.32 | 7.76 | 1.619 |
| 384 | 5.06 | 1.22 | 0.244 | 4.48 | 1.52 | 0.305 | 9.97 | 4.92 | 0.984 | 60.10 | 11.18 | 2.237 |
| 388 | 5.13 | 1.35 | 0.276 | 4.13 | 1.31 | 0.267 | 9.13 | 3.69 | 0.753 | 64.43 | 9.02 | 1.881 |
| 389 | 5.50 | 1.11 | 0.222 | 4.38 | 1.10 | 0.220 | 10.05 | 4.58 | 0.916 | 62.28 | 7.69 | 1.538 |
| 390 | 4.94 | 1.15 | 0.239 | 4.16 | 1.01 | 0.210 | 8.70 | 3.39 | 0.707 | 62.99 | 7.89 | 1.645 |
| 391 | 5.47 | 0.99 | 0.203 | 4.59 | 1.10 | 0.225 | 10.46 | 4.13 | 0.842 | 64.30 | 4.30 | 0.878 |
| 393 | 4.24 | 1.45 | 0.303 | 3.64 | 1.54 | 0.327 | 7.46 | 4.34 | 0.925 | 60.87 | 10.10 | 2.106 |
| 395 | 5.31 | 0.89 | 0.239 | 4.51 | 0.94 | 0.252 | 9.89 | 3.79 | 1.014 | 60.34 | 8.06 | 2.153 |
| 402 | 4.70 | 1.15 | 0.235 | 3.63 | 1.11 | 0.227 | 7.44 | 3.27 | 0.667 | 66.09 | 7.17 | 1.464 |
| 403 | 5.09 | 1.13 | 0.247 | 4.19 | 1.28 | 0.279 | 9.14 | 3.98 | 0.868 | 62.31 | 12.54 | 2.736 |
| 404 | 4.67 | 1.11 | 0.232 | 3.82 | 1.21 | 0.253 | 7.84 | 3.64 | 0.759 | 61.36 | 9.14 | 1.906 |
| 405 | 5.05 | 1.16 | 0.231 | 4.14 | 1.13 | 0.225 | 8.91 | 3.90 | 0.779 | 57.36 | 14.28 | 2.856 |
| 406 | 4.62 | 1.36 | 0.284 | 3.86 | 1.49 | 0.312 | 8.18 | 5.12 | 1.067 | 61.64 | 8.99 | 1.875 |
| 407 | 5.04 | 1.47 | 0.307 | 4.12 | 1.25 | 0.261 | 9.03 | 4.50 | 0.938 | 60.14 | 12.51 | 2.609 |
| 408 | 5.06 | 1.09 | 0.227 | 4.24 | 1.34 | 0.280 | 9.22 | 3.93 | 0.819 | 63.20 | 7.07 | 1.474 |
| 410 | 4.96 | 1.25 | 0.267 | 4.00 | 1.36 | 0.291 | 8.75 | 5.00 | 1.065 | 64.13 | 11.95 | 2.547 |
| 411 | 5.52 | 1.35 | 0.270 | 4.83 | 1.62 | 0.325 | 11.80 | 6.83 | 1.366 | 53.29 | 16.84 | 3.368 |
| 412 | 5.44 | 1.09 | 0.233 | 4.58 | 1.35 | 0.289 | 10.68 | 5.78 | 1.232 | 59.35 | 11.57 | 2.467 |
| 423 | 5.18 | 1.35 | 0.296 | 4.54 | 1.50 | 0.327 | 10.35 | 5.22 | 1.139 | 58.69 | 11.58 | 2.526 |
| 424 | 3.94 | 1.46 | 0.344 | 3.02 | 1.27 | 0.298 | 5.81 | 3.70 | 0.872 | 63.77 | 13.40 | 3.159 |
| 425 | 3.28 | 0.44 | 0.139 | 2.28 | 0.43 | 0.137 | 3.74 | 0.74 | 0.235 | 63.25 | 7.46 | 2.359 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, S.; Renedo, C.J.; Ortiz, A.; Ortiz, F.; Santisteban, A. Biomass Losses Caused by Teratosphaeria Leaf Disease in Eucalyptus globulus Short Rotation Forestry. Forests 2017, 8, 447. https://doi.org/10.3390/f8110447
Pérez S, Renedo CJ, Ortiz A, Ortiz F, Santisteban A. Biomass Losses Caused by Teratosphaeria Leaf Disease in Eucalyptus globulus Short Rotation Forestry. Forests. 2017; 8(11):447. https://doi.org/10.3390/f8110447
Chicago/Turabian StylePérez, Severiano, Carlos J. Renedo, Alfredo Ortiz, Félix Ortiz, and Agustín Santisteban. 2017. "Biomass Losses Caused by Teratosphaeria Leaf Disease in Eucalyptus globulus Short Rotation Forestry" Forests 8, no. 11: 447. https://doi.org/10.3390/f8110447
APA StylePérez, S., Renedo, C. J., Ortiz, A., Ortiz, F., & Santisteban, A. (2017). Biomass Losses Caused by Teratosphaeria Leaf Disease in Eucalyptus globulus Short Rotation Forestry. Forests, 8(11), 447. https://doi.org/10.3390/f8110447






