The Relationship between Sap Flow Density and Environmental Factors in the Yangtze River Delta Region of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sap Flow Measurements
2.3. Meteorological and Soil Water Measurements
2.4. Statistical Analyses
3. Results
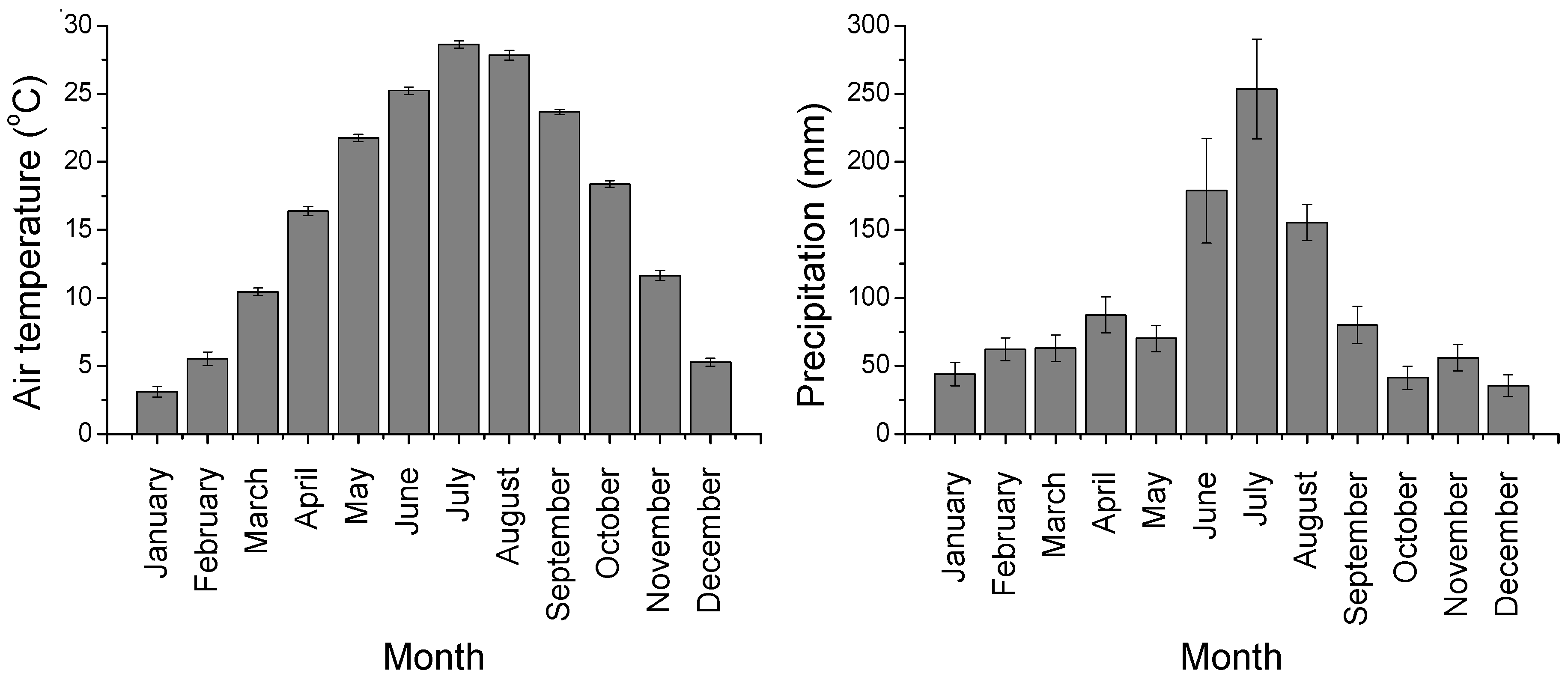
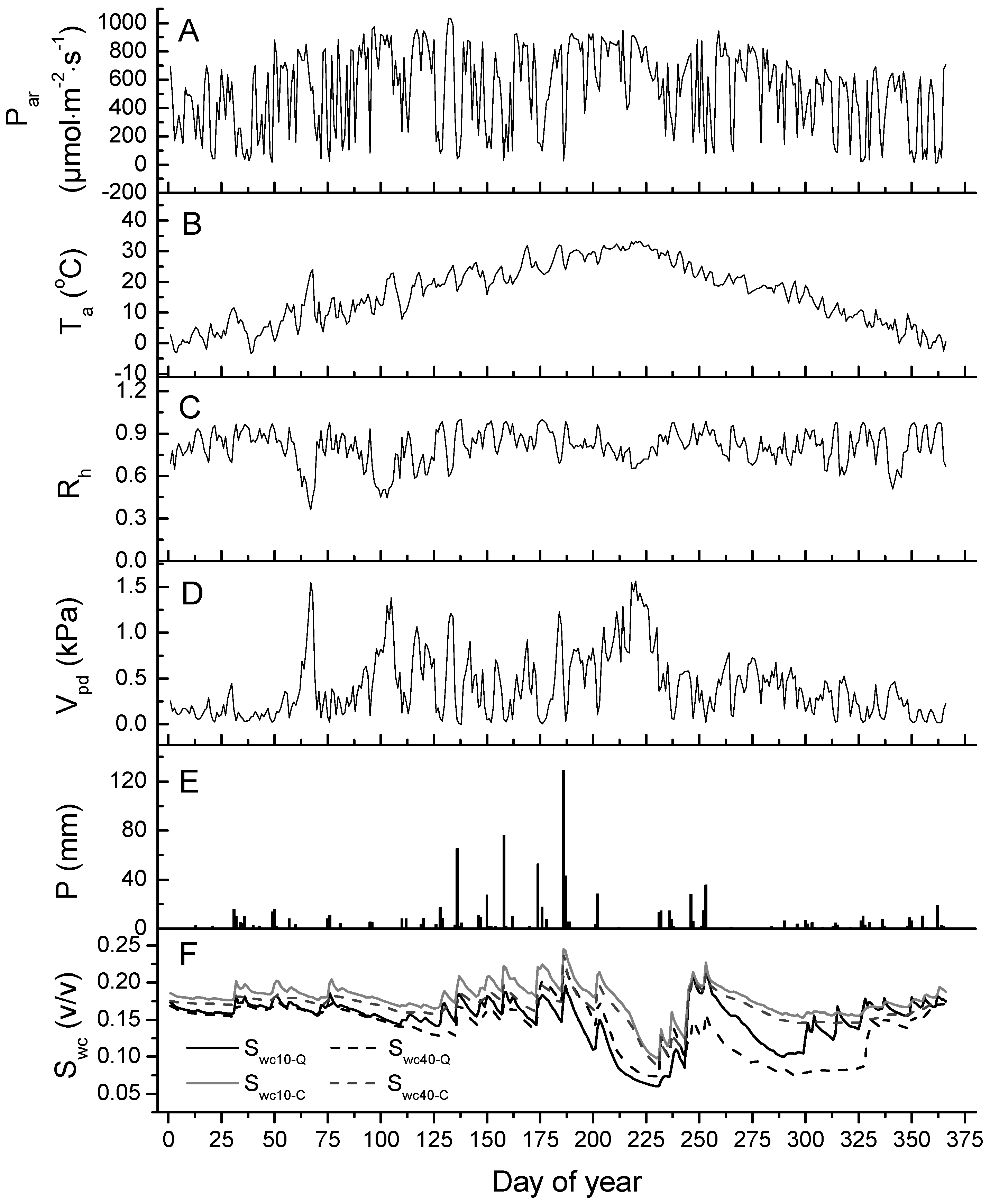
3.1. Environmental Variables
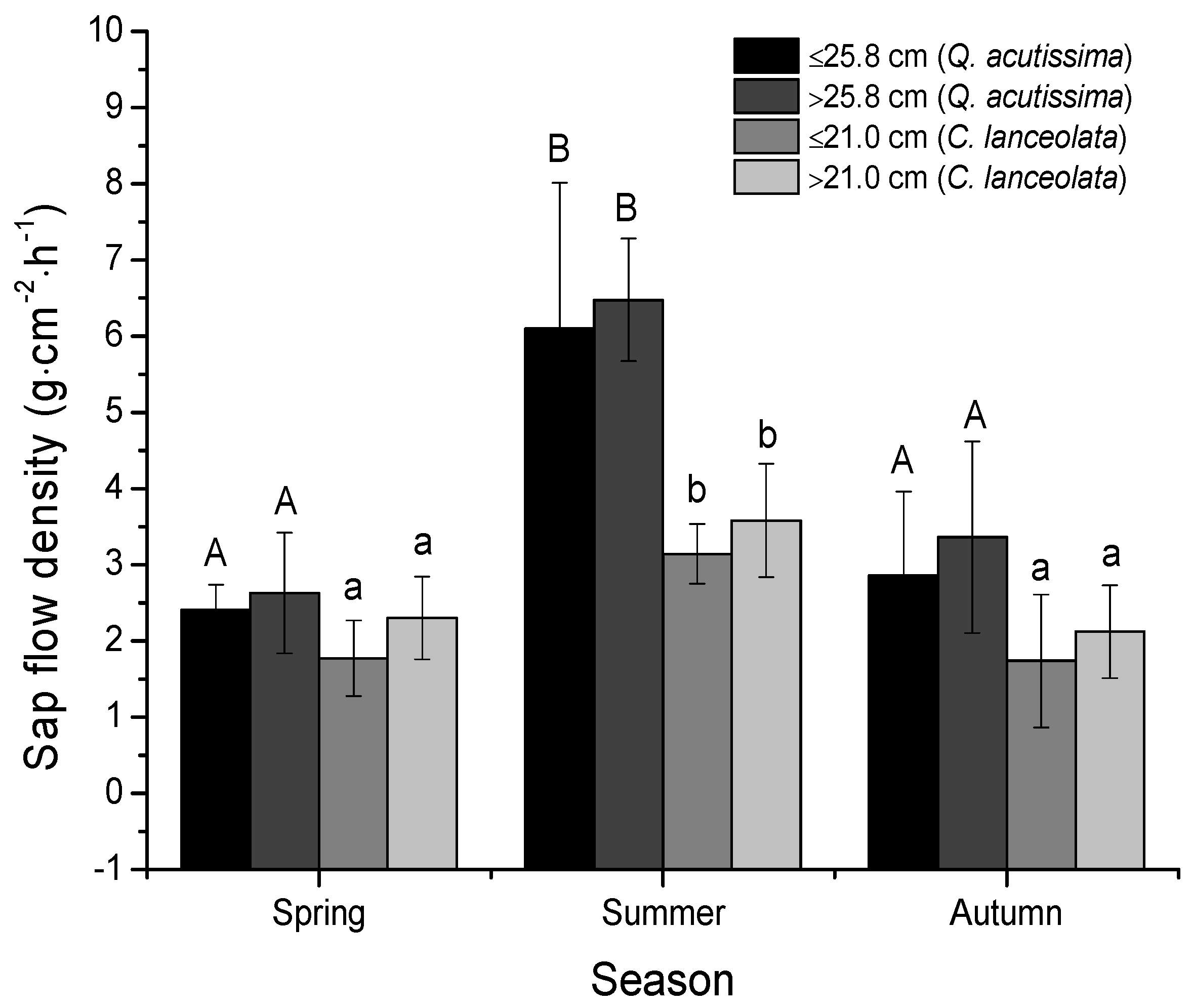
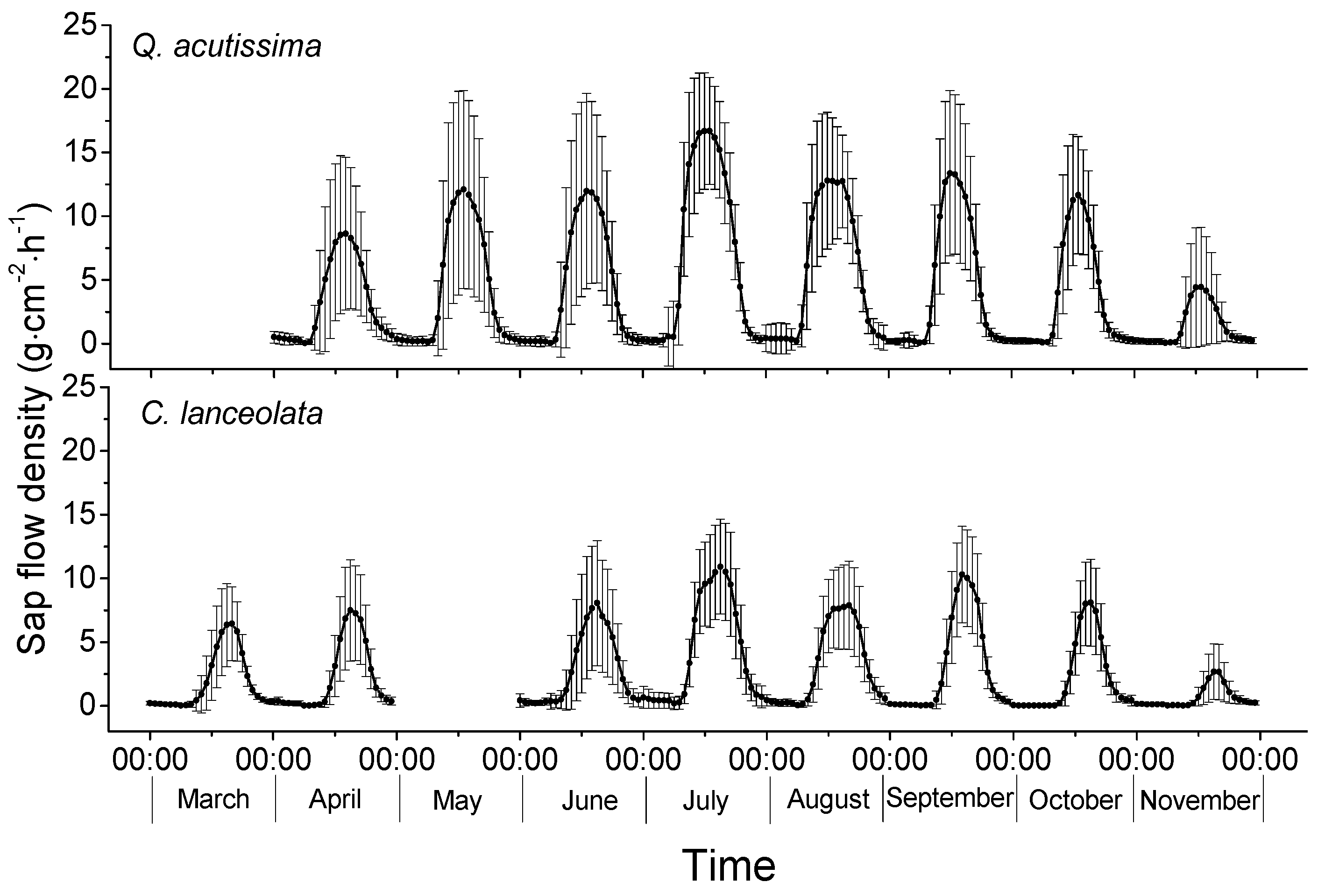
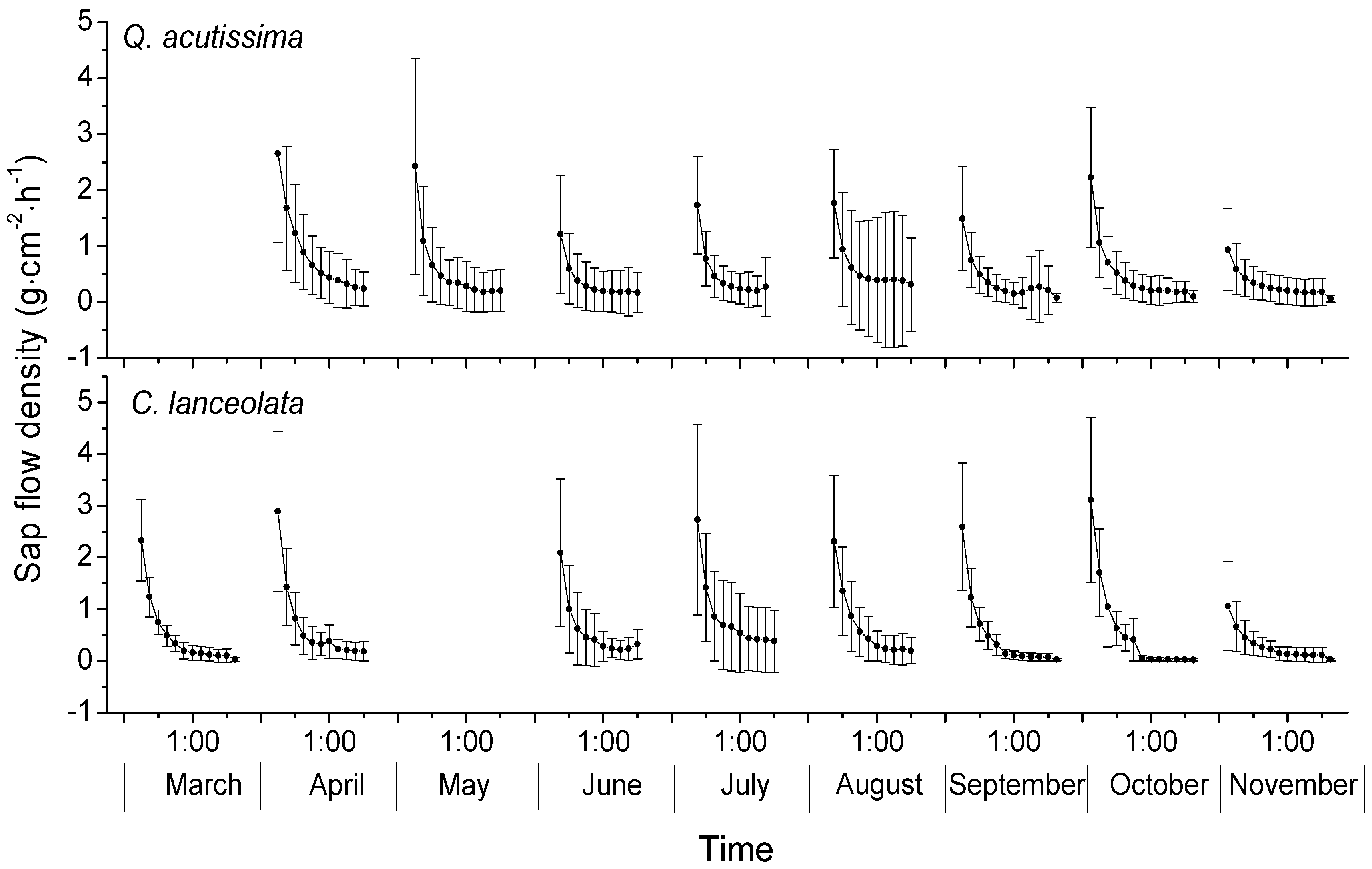
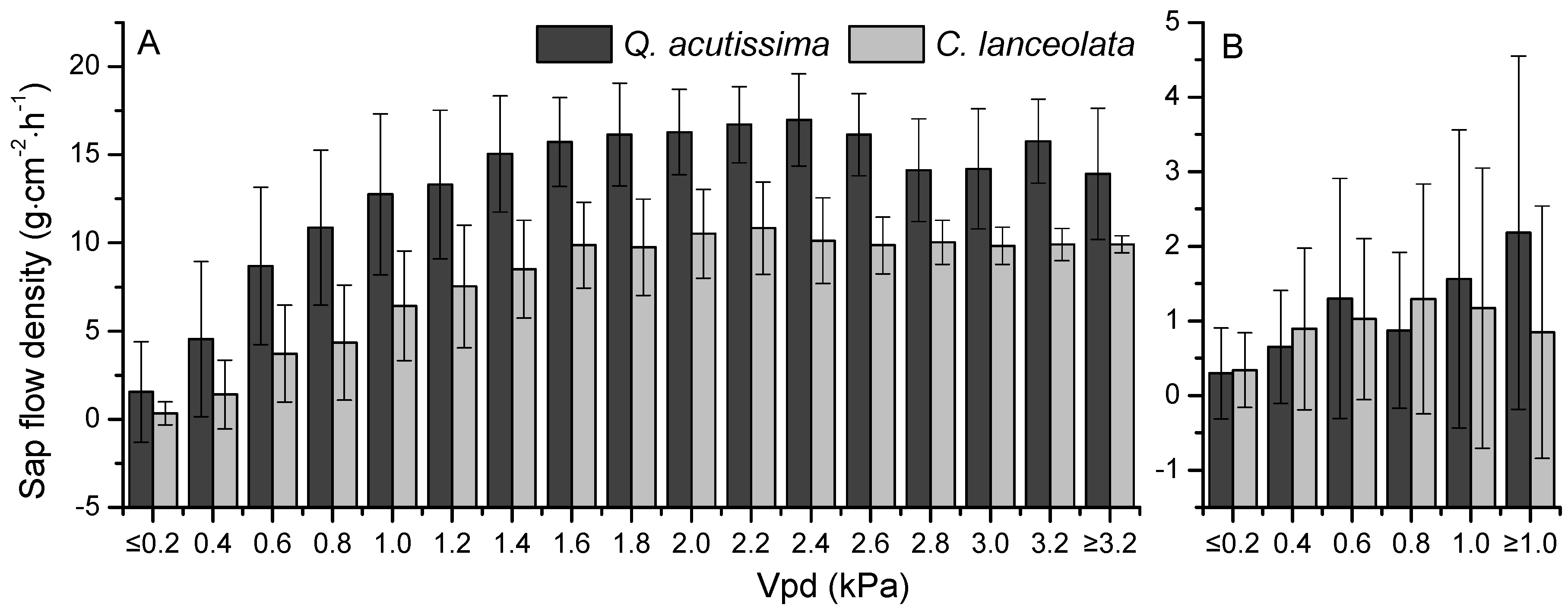
3.2. Sap Flow Densities
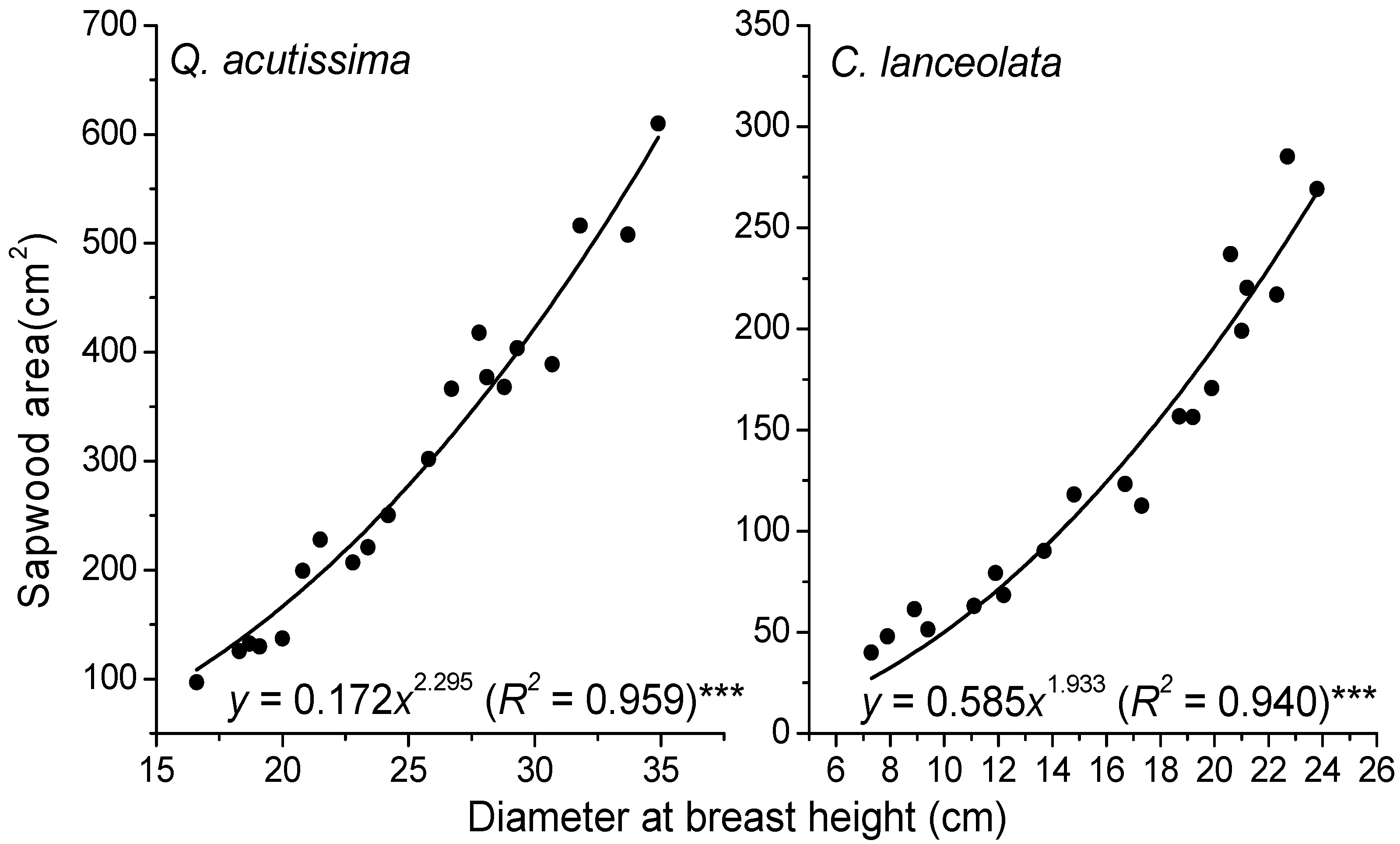
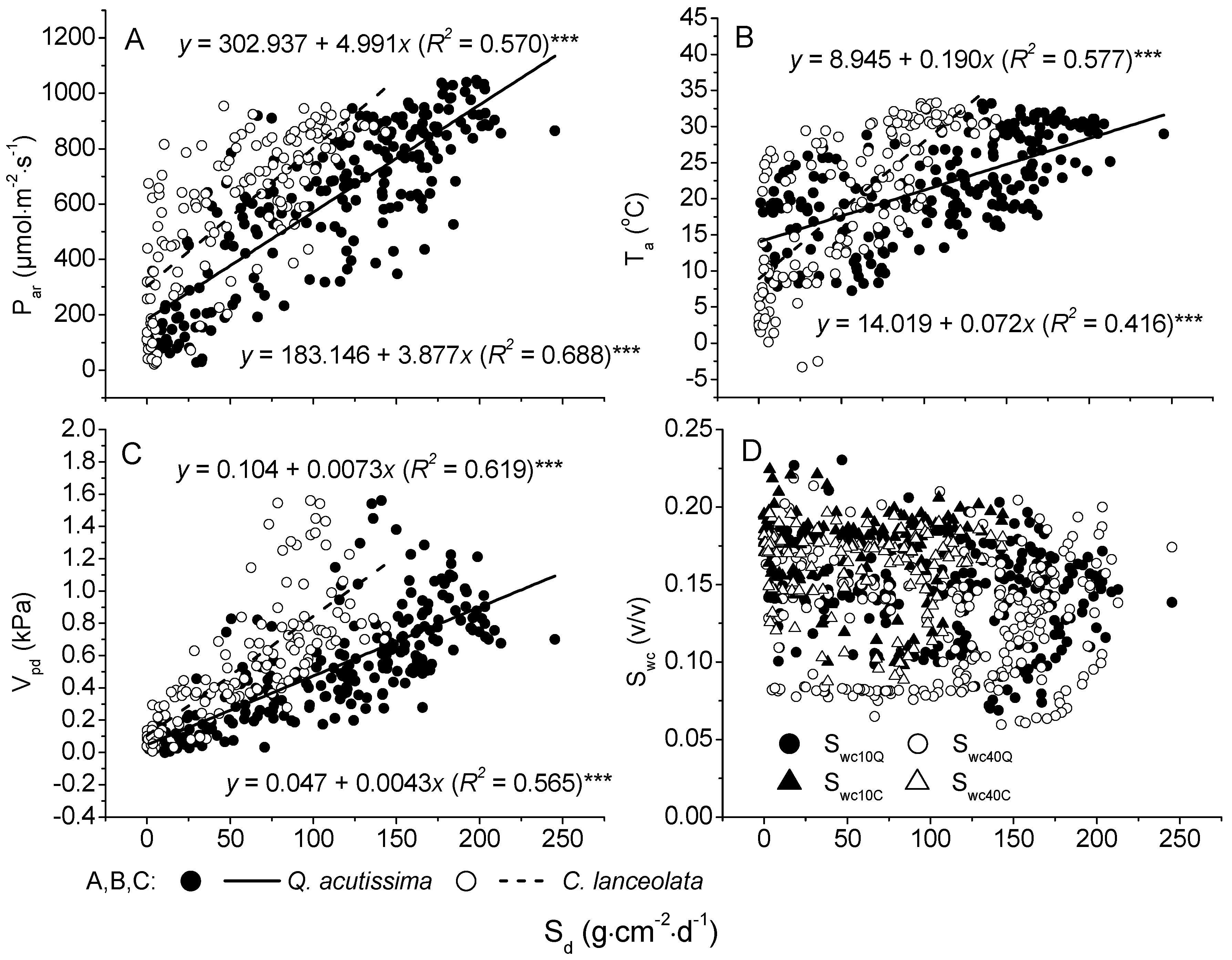
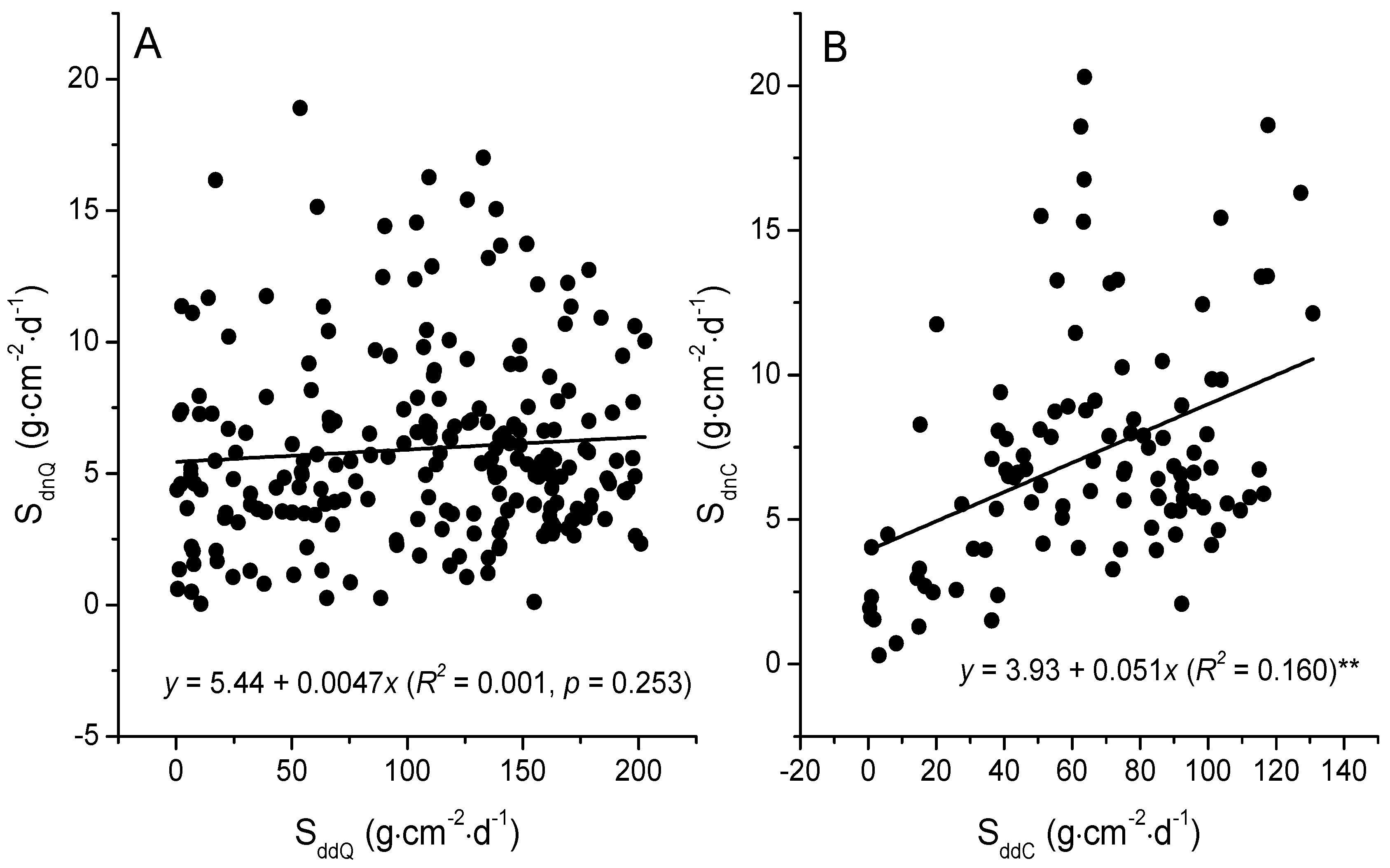
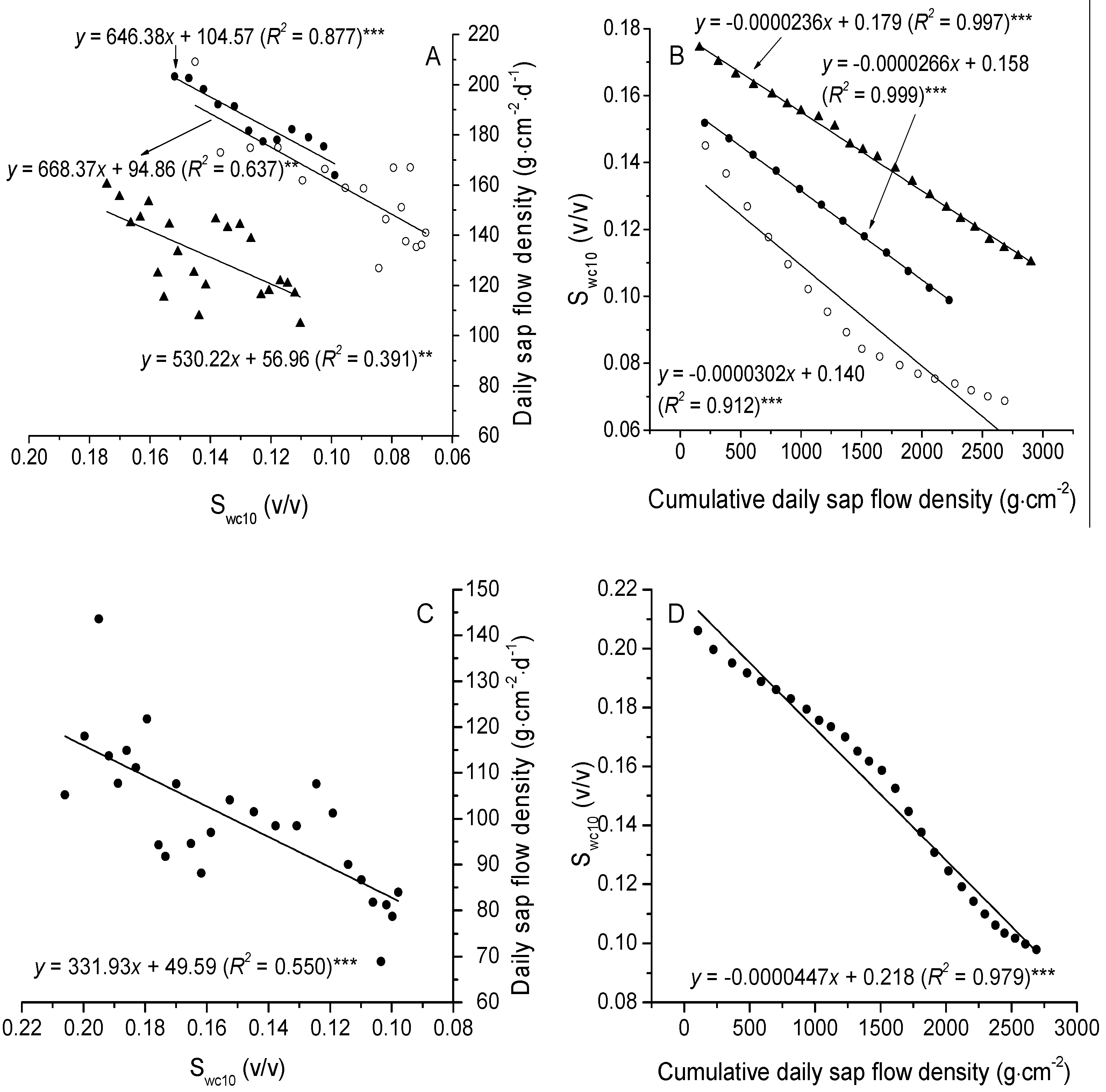
3.3. The Relationships between Sap Flow Densities and Environmental Factors
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baumgarten, M.; Weis, W.; Kühn, A.; May, K.; Matyssek, R. Forest transpiration-targeted through xylem sap flux assessment versus hydrological modeling. Eur. J. For. Res. 2014, 133, 677–690. [Google Scholar] [CrossRef]
- Ehrler, W.L.; Nakayama, F.S.; Bavel, C.V. Cyclic changes in water balance and transpiration of cotton leaves in a steady environment. Physiol. Plant 1965, 18, 766–775. [Google Scholar] [CrossRef]
- Belmans, C.; Wesseling, J.G.; Feddes, R.A. Simulation model of the water balance of a cropped soil: SWATRE. J. Hydrol. 1983, 63, 271–286. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Jasechko, S. Transpiration in the global water cycle. Agric. For. Meteorol. 2014, 189, 115–117. [Google Scholar] [CrossRef]
- Manoli, G.; Domec, J.C.; Novick, K.; Oishi, A.C.; Noormets, A.; Marani, M.; Katul, G. Soil-plant-atmosphere conditions regulating convective cloud formation above southeastern US pine plantations. Glob. Change Biol. 2016, 22, 2238–2254. [Google Scholar] [CrossRef] [PubMed]
- Tor-ngern, P.; Oren, R.; Oishi, A.C.; Uebelherr, J.M.; Palmroth, S.; Tarvainen, L.; Näsholm, T. Ecophysiological variation of transpiration of pine forests: Synthesis of new and published results. Ecol. Appl. 2017, 27, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.L.; Waring, R.H. Coupling of environment to plant response: A simulation model of transpiration. Ecology 1974, 55, 62–72. [Google Scholar] [CrossRef]
- Brito, P.; Lorenzo, J.R.; González-Rodríguez, Á.M.; Morales, D.; Wieser, G.; Jiménez, M.S. Canopy transpiration of a semi arid Pinus canariensis forest at a treeline ecotone in two hydrologically contrasting years. Agric. For. Meteorol. 2015, 201, 120–127. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Zha, T.; Mo, K.; Zhang, Y.; Fang, X. Soil water affects transpiration response to rainfall and vapor pressure deficit in poplar plantation. New For. 2014, 45, 235–250. [Google Scholar] [CrossRef]
- Chang, X.; Zhao, W.; He, Z. Radial pattern of sap flow and response to microclimate and soil moisture in Qinghai spruce (Picea crassifolia) in the upper Heihe River Basin of arid northwestern China. Agric. For. Meteorol. 2014, 187, 14–21. [Google Scholar] [CrossRef]
- Haijun, L.; Cohen, S.; Lemcoff, J.H.; Israeli, Y.; Tanny, J. Sap flow, canopy conductance and microclimate in a banana screenhouse. Agric. For. Meteorol. 2015, 201, 165–175. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Liu, S.; Wei, X.; Wang, X. Response of relative sap flow to meteorological factors under different soil moisture conditions in rainfed jujube (Ziziphus jujuba Mill.) plantations in semiarid Northwest China. Agric. Water Manag. 2014, 136, 23–33. [Google Scholar] [CrossRef]
- Monteith, J.L. Evaporation and environment. Symp. Soc. Experimental Biol. 1964, 19, 205–234. [Google Scholar]
- Ritchie, J.T. Atmospheric and soil water influences on the plant water balance. Agric. Meteorol. 1974, 14, 183–198. [Google Scholar] [CrossRef]
- Caird, M.A.; Richards, J.H.; Donovan, L.A. Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol. 2007, 143, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Forster, M.A. How significant is nocturnal sap flow? Tree Physiol. 2014, 34, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E.; Burgess, S.S.; Tu, K.P.; Oliveira, R.S.; Santiago, L.S.; Fisher, J.B.; Ambrose, A.R. Nighttime transpiration in woody plants from contrasting ecosystems. Tree Physiol. 2007, 27, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Ballestrini, C.; Tezara, W. Nocturnal sap flow in the C3-CAM species, Clusia minor. Trees 2008, 22, 491–497. [Google Scholar] [CrossRef]
- Si, J.; Feng, Q.; Yu, T.; Zhao, C. Nighttime sap flow and its driving forces for Populus euphratica in a desert riparian forest, Northwest China. J. Arid Land 2015, 7, 665–674. [Google Scholar] [CrossRef]
- Daley, M.J.; Phillips, N.G. Interspecific variation in nighttime transpiration and stomatal conductance in a mixed New England deciduous forest. Tree Physiol. 2006, 26, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Caird, M.A.; Richards, J.H.; Hsiao, T.C. Significant transpirational water loss occurs throughout the night in field-grown tomato. Funct. Plant Biol. 2007, 34, 172–177. [Google Scholar] [CrossRef]
- Tian, D.; Fang, X.; Xiang, W. Carbon density of the Chinese fir plantation ecosystem at Huitong, Hu'nan Province. Acta Ecol. Sin. 2004, 24, 2382–2386. (In Chinese) [Google Scholar]
- Shi, Z.; Li, Y.; Wang, S.; Wang, G.; Ruan, H.; He, R.; Zhang, Z. Accelerated soil CO2 efflux after conversion from secondary oak forest to pine plantation in southeastern China. Ecol.Res. 2009, 24, 1257–1265. [Google Scholar] [CrossRef]
- Chen, H. Phosphatase activity and P fractions in soils of an 18-year-old Chinese fir (Cunninghamia lanceolata) plantation. For. Ecol. Manag. 2003, 178, 301–310. [Google Scholar] [CrossRef]
- Yang, Y.S.; Guo, J.; Chen, G.; Xie, J.; Gao, R.; Li, Z.; Jin, Z. Carbon and nitrogen pools in Chinese fir and evergreen broadleaved forests and changes associated with felling and burning in mid-subtropical China. For. Ecol. Manag. 2005, 216, 216–226. [Google Scholar] [CrossRef]
- Kim, Y.W.; Youn, Y.; Noh, E.R.; Kim, J.C. Somatic embryogenesis and plant regeneration from immature embryos of five families of Quercus acutissima. Plant Cell Rep. 1997, 16, 869–873. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Fang, Y.M.; Mukui, K.Y.; Li, X.X.; Xia, T. Molecular characterization and genetic structure of Quercus acutissima germplasm in China using microsatellites. Mol. Biol. Rep. 2013, 40, 4083–4090. [Google Scholar] [CrossRef] [PubMed]
- Dye, P.J. Climate, forest and stream flow relationships in South African afforested catchments. Commonw. For. Rev. 1996, 75, 31–38. [Google Scholar]
- Feng, R.F.; Yang, W.Q.; Zhang, J. Artificial forest management for global change mitigation. Acta Ecol. Sin. 2006, 26, 3870–3877. (In Chinese) [Google Scholar]
- Benyon, R.G.; Marcar, N.E.; Crawford, D.F.; Nicholson, A.T. Growth and water use of Eucalyptus camaldulensis and E.occidentalis on a saline discharge site near Wellington, NSW, Australia. Agric. Water Manag. 1999, 39, 229–244. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Kang, W.X.; Tian, D.L.; Xiang, W.H.; Yan, W.D. Sap flow rate and its relationship with environmental factors of Chinese fir plantation in Huitong, Hunan Province. Sci. Silvae Sin. 2009, 45, 127–132. (In Chinese) [Google Scholar]
- Wilson, K.B.; Hanson, P.J.; Mulholland, P.J.; Baldocchi, D.D.; Wullschleger, S.D. A comparison of methods for determining forest evapotranspiration and its components: Sap-flow, soil water budget, eddy covariance and catchment water balance. Agric. For. Meteorol. 2001, 106, 153–168. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Zhang, W.H. Sap flow of Abies georgei var. smithii and its relationship with the environment factors in the Tibetan subalpine region, China. J. Mt. Sci. 2015, 12, 1373–1382. [Google Scholar] [CrossRef]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Zeppel, M.J.; Tissue, D.; Taylor, D.T.; Macinnis-Ng, C.M.; Eamus, D. Rates of nocturnal transpiration in two evergreen temperate woodland species with differing water-use strategies. Tree Physiol. 2010, 30, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Fan, J.; Wang, Q.; Warrington, D. Discrepancy of sap flow in Salix matsudana grown under different soil textures in the water-wind erosion crisscross region on the Loess Plateau. Plant Soil 2015, 390, 383–399. [Google Scholar] [CrossRef]
- Oishi, A.C.; Oren, R.; Stoy, P.C. Estimating components of forest evapotranspiration: A footprint approach for scaling sap flux measurements. Agric. For. Meteorol. 2008, 148, 1719–1732. [Google Scholar] [CrossRef]
- Laiju, N.; Otieno, D.; Jung, E.Y.; Lee, B.; Tenhunen, J.; Lim, J.H.; Kang, S. Environmental controls on growing-season sap flow density of Quercus serrata Thunb in a temperate deciduous forest of Korea. J. Ecol. Environ. 2012, 35, 213–225. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, Y.; Yin, L.; Wenninger, J.; Zhang, J.; Hou, G.; Uhlenbrook, S. Climatic controls on sap flow dynamics and used water sources of Salix psammophila in a semi-arid environment in northwest China. Environ. Earth Sci. 2015, 73, 289–301. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Zeppel, M.; Liu, C.; Guo, J.; Zhu, J.; Zha, T. Response of transpiration to rain pulses for two tree species in a semiarid plantation. Int. J. Biometeorol. 2014, 58, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Guan, J.H.; Shi, W.Y.; Yamanaka, N.; Du, S. Interannual variation in stand transpiration estimated by sap flow measurement in a semi-arid black locust plantation, Loess Plateau, China. Ecohydrology 2015, 8, 137–147. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Q. Seasonal and annual variation in transpiration of a dominant desert species, Haloxylon ammodendron, in Central Asia up-scaled from sap flow measurement. Ecohydrology 2015, 8, 948–960. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, J.; Savenije, H.H. A simple approach to assess water scarcity integrating water quantity and quality. Ecol. Indic. 2013, 34, 441–449. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, W.; Blanckaert, K.; Ma, J.; Huang, G. Optimization of water quality monitoring network in a large river by combining measurements, a numerical model and matter-element analyses. J. Environ. Manag. 2012, 110, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ding, G.; Gao, G.; Wu, B.; Zhang, Y.; Qin, S.; Fan, W. The Sap flow dynamics and response of Hedysarum scoparium to environmental factors in semiarid northwestern China. PLoS ONE 2015, 10, e0131683. [Google Scholar] [CrossRef] [PubMed]
- Barbeta, A.; Ogaya, R.; Peñuelas, J. Comparative study of diurnal and nocturnal sap flow of Quercus ilex and Phillyrea latifolia in a Mediterranean holm oak forest in Prades (Catalonia, NE Spain). Trees 2012, 26, 1651–1659. [Google Scholar] [CrossRef]
- Doronila, A.I.; Forster, M.A. Performance measurement via sap flow monitoring of three Eucalyptus species for mine site and dryland salinity phytoremediation. Int. J. Phytoremediation. 2015, 17, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Wang, Y.; Kume, T.; Zhang, J.; Otsuki, K.; Yamanaka, N.; Liu, G. Sap flow characteristics and climatic responses in three forest species in the semiarid Loess Plateau region of China. Agric. For. Meteorol. 2011, 151, 1–10. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Lewis, J.D.; Phillips, N.G.; Tissue, D.T. Consequences of nocturnal water loss: A synthesis of regulating factors and implications for capacitance, embolism and use in models. Tree Physiol. 2014, 34, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Dragoni, D.; Lakso, A.N.; Piccioni, R.M. Transpiration of apple trees in a humid climate using heat pulse sap flow gauges calibrated with whole-canopy gas exchange chambers. Agric. For. Meteorol. 2005, 130, 85–94. [Google Scholar] [CrossRef]
- Liu, C.; Du, T.; Li, F.; Kang, S.; Li, S.; Tong, L. Trunk sap flow characteristics during two growth stages of apple tree and its relationships with affecting factors in an arid region of northwest China. Agric. Water Manag. 2012, 104, 193–202. [Google Scholar] [CrossRef]
- Rosado, B.H.; Oliveira, R.S.; Joly, C.A.; Aidar, M.P.; Burgess, S.S. Diversity in nighttime transpiration behavior of woody species of the Atlantic Rain Forest, Brazil. Agric. For. Meteorol. 2012, 158, 13–20. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, J.; Sun, Z.; Niu, J.; Zhou, C.; Gu, D.; Zhao, P. Suppression of nighttime sap flux with lower stem photosynthesis in Eucalyptus trees. Int. J. Biometeorol. 2016, 60, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Mahadevan, M.; Bonada, M.; Skewes, M.A.; Cox, J.W. Night-time sap flow is parabolically linked to midday water potential for field-grown almond trees. Irrig. Sci. 2013, 31, 1265–1276. [Google Scholar] [CrossRef]
- Nakano, S.; Tacarindua, C.R.; Nakashima, K.; Homma, K.; Shiraiwa, T. Evaluation of the effects of increasing temperature on the transpiration rate and canopy conductance of soybean by using the sap flow method. J. Agric. Meteorol. 2015, 71, 98–105. [Google Scholar] [CrossRef]

| No. | DBH (mm) | Depth (mm) and Aspects | SA (cm2) | DBH (mm) | Depth (mm) and Aspects | SA (cm2) |
|---|---|---|---|---|---|---|
| Q. acutissima | C. lanceolata | |||||
| 1 | 187 | 0–25 at N | 142.69 | 122 | 0–20 at N | 73.64 |
| 2 | 200 | 0–25 at N | 166.49 | 137 | 0–20 at N | 92.14 |
| 3 | 242 | 0–25 at N | 257.86 | 188 | 0–20 at N | 169.86 |
| 4 | 252 | 0–25 at N | 282.97 | 196 | 0–20 at N | 184.11 |
| 5 | 268 | 0–25 at N | 325.91 | 204 | 0–20 at N | 198.92 |
| 6 | 278 | 0–25 at N | 354.50 | 208 | 0–20 at N | 206.52 |
| 7 | 308 | 0–25 at N | 448.49 | 215 | 0–20 at N | 220.17 |
| 8 | 318 | 0–25 at N | 482.61 | 218 | 0–20 at N | 226.15 |
| 9 | - | - | - | 223 | 0–20 at N | 236.28 |
| 10 | - | - | - | 227 | 0–20 at N | 244.54 |
| 11 | - | - | - | 249 | 0–20 at N | 292.42 |
| The Percentage of Amounts | The Percentage of Events | |
|---|---|---|
| <10 mm | 26.86% | 77.39% |
| 10–25 mm | 23.28% | 14.78% |
| 25–50 mm | 16.67% | 4.35% |
| 50–100 mm | 19.93% | 2.61% |
| >100 mm | 13.26% | 0.87% |
| Month | Q. acutissima | R2 | C. lanceolata | R2 |
|---|---|---|---|---|
| March | / | / | y = 3.438 n–1.57 | 0.927 |
| April | y = 3.281 n–1.04 | 0.978 | y = 2.889 n–1.18 | 0.979 |
| May | y = 2.274 n–1.08 | 0.986 | / | / |
| June | y = 2.037 n–1.11 | 0.929 | y = 1.878 n–0.96 | 0.932 |
| July | y = 1.468 n–0.94 | 0.937 | y = 2.547 n–-0.87 | 0.986 |
| August | y = 1.497 n–0.69 | 0.937 | y = 2.671 n–1.16 | 0.979 |
| September | y = 1.351 n–0.93 | 0.829 | y = 3.861 n–1.76 | 0.951 |
| October | y = 2.268 n–1.09 | 0.975 | y = 7.988 n–2.31 | 0.883 |
| November | y = 1.041 n–0.81 | 0.882 | y = 1.428 n–1.14 | 0.862 |
| Month | Q. acutissima | C. lanceolata |
|---|---|---|
| March | / | 25.25 |
| April | 28.18 | 27.46 |
| May | 41.16 | 43.34 |
| June | 40.23 | 35.47 |
| July | 62.72 | 56.52 |
| August | 58.10 | 39.71 |
| September | 40.57 | 38.56 |
| October | 33.52 | 28.87 |
| November | 11.49 | 8.38 |
| Total | 315.99 | 303.55 |
| Factors | SddQ | SdnQ | SddC | SdnC | ||||
|---|---|---|---|---|---|---|---|---|
| PC | VE (%) | PC | VE (%) | PC | VE (%) | PC | VE (%) | |
| Par | 0.51 | 25.91 | –0.07 | 0.49 | ||||
| Ta | 0.46 | 20.88 | 0.01 | 0.00 | 0.25 | 6.45 | 0.09 | 0.76 |
| Vpd | 0.26 | 6.71 | 0.44 | 19.45 | 0.59 | 35.28 | 0.20 | 3.88 |
| Swc10 | 0.08 | 0.67 | 0.26 | 6.92 | 0.06 | 0.35 | 0.00 | 0.00 |
| Swc40 | –0.05 | 0.23 | –0.14 | 1.93 | 0.03 | 0.08 | –0.01 | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, B.; Zhuang, J.; Han, C.; Zhai, L.; Zhao, W.; Zhang, J. The Relationship between Sap Flow Density and Environmental Factors in the Yangtze River Delta Region of China. Forests 2017, 8, 74. https://doi.org/10.3390/f8030074
Liu X, Zhang B, Zhuang J, Han C, Zhai L, Zhao W, Zhang J. The Relationship between Sap Flow Density and Environmental Factors in the Yangtze River Delta Region of China. Forests. 2017; 8(3):74. https://doi.org/10.3390/f8030074
Chicago/Turabian StyleLiu, Xin, Bo Zhang, Jia‐Yao Zhuang, Cheng Han, Lu Zhai, Wen‐Rui Zhao, and Jin‐Chi Zhang. 2017. "The Relationship between Sap Flow Density and Environmental Factors in the Yangtze River Delta Region of China" Forests 8, no. 3: 74. https://doi.org/10.3390/f8030074





