Post-Fire Salvage Logging Imposes a New Disturbance that Retards Succession: The Case of Bryophyte Communities in a Macaronesian Laurel Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Post-Management Habitat Characteristics
2.3. Community Composition
2.4. Data Analysis
3. Results
3.1. Post-Management Habitat Characteristics
3.2. Community Composition
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Frequency (in Percent Cover) of Bryophyte Species Recorded in the Study Site
| Species code | Species name | SL | NS | RE |
|---|---|---|---|---|
| Bryuarge | Bryum argenteum (C) | 8.9 | ||
| Camppili | Campilopus pilifer (C) | 3.8 | 16.1 | 5.0 |
| Cephdiva | Cephaloziella divaricata (L) | 2.6 | 12.6 | |
| Cerapurp | Ceratodon purpureus (C) | 25.5 | 25.6 | |
| Chilprof | Chiloscyphus profundus (L) | 27.5 | ||
| Dicrscot | Dicranum scottianum (L) | 5.0 | ||
| Didyinsu | Didymodon insulanus (C) | 15.7 | 15.0 | |
| Didyvine | Didymodon vinealis (C) | 18.5 | 26.7 | |
| Fissbryo | Fissidens bryoides (C) | 26.0 | ||
| Frulmicr | Frullania microphylla (L) | 20.0 | ||
| Frultama | Frullania tamarisci (L) | 2.1 | 47.8 | |
| Frultene | Frullania teneriffae (L) | 39.5 | ||
| Funahygr | Funaria hygrometrica (F) | 23.7 | 6.7 | |
| Gongeric | Gongylanthus ericetorum (L) | 3.0 | 12.0 | 52.5 |
| Homamand | Homalothecium mandonii (P) | 25.0 | ||
| Hypncupr | Hypnum cupressiforme (P) | 37.0 | 45.7 | |
| Hypnunci | Hypnum uncinulatum (P) | 48.3 | ||
| Isotmyos | Isothecium myosuroides (P) | 40.0 | ||
| Lejelama | Lejeunea lamacerina (S) | 50.0 | ||
| Leptlong | Leptodon longisetus (P) | 1.0 | ||
| Micrulic | Microlejeunea ulicina (S) | 16.5 | ||
| Plagrupe | Plagiochasma rupestre (S) | 5.0 | . | |
| Plagpunc | Plagiochilla punctata (P) | 20.0 | ||
| Plasmeri | Plasteurhynchium meridionale (P) | 16.7 | ||
| Polyjuni | Polytrichum juniperinum (C) | 15.0 | . | |
| Porecana | Porella canariensis (L) | 31.7 | ||
| Ptergrac | Pterigonium gracille (P) | 10.1 | ||
| Ptyccapi | Ptychostomum capillare (C) | 33.3 | 16.7 | 0.1 |
| Ptycimbr | Ptychostomum imbricatulum (C) | 23.2 | 23.8 | 2.0 |
| Radulind | Radula lindenbergiana (S) | 20.0 | ||
| Rhynconf | Rhynchostegium confertum (P) | 20.0 | ||
| Scapcomp | Scapania compacta (L) | 6.5 | ||
| Scletour | Scleropodium touretii (P) | 60.0 | ||
| Semasubs | Sematophyllum substrumulosum (P) | 9.4 | ||
| Tortniti | Tortella nitida (C) | 16.0 | 1.0 | 14.4 |
| Tricbrac | Trichostomum brachydontium (C) | 15.7 |
Appendix B. Estimated Species Number after Rarefaction

| Clench Model. Sn = a × n/(1 + b × n) | |||||
|---|---|---|---|---|---|
| Treatment | a | b | slope | %species | r2 |
| Salvage logging | 1.59 | 0.125 | 0.0002 | 94 | 0.98 |
| Non salvaged | 1.88 | 0.092 | 0.001 | 73 | 0.99 |
| Reference ecosystem | 2.20 | 0.049 | 0.001 | 64 | 0.99 |
References
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.M.J.S.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.S.; Doyle, J.C.; Pyne, S.J. Fire in the Earth system. Science 2009, 324, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Motta, R.; Berretti, R.; Lingua, E.; Piussi, P. Coarse woody debris, forest structure and regeneration in the Valbona Forest Reserve, Paneveggio, Italian Alps. For. Ecol. Manag. 2006, 235, 155–163. [Google Scholar] [CrossRef]
- Santana, V.M.; González-Pelayo, O.; Maia, P.A.; Varela, M.E.; Valdecantos, A.; Vallejo, V.R.; Kaizer, J.J. Effects of fire recurrence and different salvage logging techniques on carbón storage in Pinus pinaster forests from northern Portugal. Eur. J. For. Res. 2016, 135, 1107–1117. [Google Scholar] [CrossRef]
- White, A.M.; Manley, P.N.; Tarbill, G.L.; Richardson, T.W.; Russell, R.E.; Safford, H.D.; Dobrowski, S.Z. Avian community responses to post-fire forest structure: Implications for fire management in mixed conifer forests. Anim. Conserv. 2016, 19, 256–264. [Google Scholar] [CrossRef]
- Castro, J.; Puerta-Piñero, C.; Leverkus, A.B.; Moreno-Rueda, G.; Sánchez-Miranda, A. Post-fire salvage logging alters a key plant-animal interaction for forest regeneration. Ecosphere 2012, 3, 1–12. [Google Scholar] [CrossRef]
- Spanos, I.; Raftoyannis, Y.; Goudelis, G.; Xanthopoulou, E.; Samara, T.; Tsiontsis, A. Effects of postfire logging on soil and vegetation recovery in a Pinus halepensis Mill. forest of Greece. Plant Soil 2005, 278, 171–179. [Google Scholar] [CrossRef]
- Donato, D.C.; Fontaine, J.B.; Campbell, J.L.; Robinson, W.D.; Kauffman, J.B.; Law, B.E. Post-fire logging hinders regeneration and increases fire risk. Science 2006, 311, 352. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Allen, C.D.; Molina-Morales, M.; Marañón-Jiménez, S.; Sánchez-Miranda, A.; Zamora, R. Salvage logging versus the use of burnt wood as a nurse object to promote post-fire tree seedling establishment. Restor. Ecol. 2011, 19, 537–544. [Google Scholar] [CrossRef]
- Marañón-Jiménez, S.; Castro, J. Effect of decomposing burnt wood on soil fertility and nutrient availability in a Mediterranean ecosystem. Biogeochemistry 2013, 112, 519–535. [Google Scholar] [CrossRef]
- Serrano-Ortiz, P.; Marañón-Jiménez, S.; Reverter, B.R.; Sánchez-Cañete, E.P.; Castro, J.; Zamora, R.; Kowalski, A.S. Post-fire salvage logging reduces carbon sequestration in Mediterranean coniferous forest. For. Ecol. Manag. 2011, 262, 2287–2296. [Google Scholar] [CrossRef]
- Lindenmayer, D.; Burton, P.; Franklin, J. Salvage Logging and Its Ecological Consequences; Island Press: Washington, DC, USA, 2008. [Google Scholar]
- Thorn, S.; Bässler, C.; Brandl, R.; Burton, P.J.; Cahall, R.; Campbell, J.L.; Castro, J.; Choi, C.-Y.; Cobb, T.; Donato, D.C.; et al. Impacts of salvage logging on biodiversity—A meta-analysis. J. Appl. Ecol. 2017, in press. [Google Scholar] [CrossRef]
- García-Orenes, F.; Arcenegui, V.; Chrenkova, K.; Mataix-Solera, J.; Moltó, J.; Jara-Navarro, A.B.; Torres, M.P. Effects of salvage logging on soil properties and vegetation recovery in a fire-affected Mediterranean forest: A two year monitoring research. Sci. Total Environ. 2017, 586, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Leverkus, A.; Castro, J. An ecosystem services approach to the ecological effects of salvage logging: Valuation of seed dispersal. Ecol. Appl. 2017, 27, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.; Thorn, S.; Banks, S. Please do not disturb ecosystems further. Nat. Ecol. Evol. 2017, 1, 31. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Noss, R.F. Salvage logging, ecosystem processes, and biodiversity conservation. Conserv. Biol. 2006, 20, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Stuart, J.D.; Grifantini, M.C.; Fox, L., III. Early successional pathways following wildfire and subsequent silvicultural treatment in Douglas-Fir/Hardwood Forests, NW California. For. Sci. 1993, 39, 561–572. [Google Scholar]
- McIver, J.D.; Starr, L. (Eds.) Environmental Effects of Postfire Logging: Literature Review and Annotated Bibliography; Gen. Tech. Rep. PNW-GTR-486; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, Oregon, USA, 2000; p. 72.
- Castro, J.; Moreno-Rueda, G.; Hódar, J.A. Experimental Test of Postfire Management in Pine Forests: Impact of Salvage Logging versus Partial Cutting and Nonintervention on Bird–Species Assemblages. Conserv. Biol. 2010, 24, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Thorn, S.; Werner, S.A.B.; Wohlfahrt, J.; Bässler, C.; Seibold, S.; Quillfeldt, P.; Müller, J. Response of bird assemblages to windstorm and salvage logging—Insights from analyses of functional guild and indicator species. Ecol. Indic. 2016, 65, 142–148. [Google Scholar] [CrossRef]
- Blair, D.P.; McBurney, L.M.; Blanchard, W.; Banks, S.C.; Lindenmayer, D.B. Disturbance gradient shows logging affects plant functional groups more than fire. Ecol. Appl. 2016, 26, 2280–2301. [Google Scholar] [CrossRef] [PubMed]
- Morissette, J.L.; Cobb, T.P.; Brigham, R.M.; James, P.C. The response of boreal songbird communities to fire and postfire harvesting. Can. J. For. Res. 2002, 32, 2169–2183. [Google Scholar] [CrossRef]
- D’Amato, A.W.; Fraver, S.; Palik, B.J.; Bradford, J.B.; Patty, L. Singular and interactive effects of blowdown, salvage logging, and wildfire in sub-boreal pine systems. For. Ecol. Manag. 2011, 262, 2070–2078. [Google Scholar] [CrossRef]
- Buma, B.; Wessman, C.A. Differential species responses to compounded perturbations and implications for landscape heterogeneity and resilience. For. Ecol. Manag. 2012, 266, 25–33. [Google Scholar] [CrossRef]
- Waldron, K.; Ruel, J.C.; Gauthier, S.; De Grandpré, L.; Peterson, C.J. Effects of post-windthrow salvage logging on microsites, plant composition and regeneration. Appl. Veg. Sci. 2014, 17, 323–337. [Google Scholar] [CrossRef]
- Fontaine, J.B.; Donato, D.C.; Robinson, W.D.; Law, B.E.; Kauffman, J.B. Bird communities following high-severity fire: Response to single and repeat fires in a mixed-evergreen forest, Oregon, USA. For. Ecol. Manag. 2009, 257, 1496–1504. [Google Scholar] [CrossRef]
- Paine, R.T.; Tegner, M.J.; Johnson, T.A. Compounded perturbations yield ecological surprises. Ecosystems 1998, 1, 535–545. [Google Scholar] [CrossRef]
- Turner, M.G. Disturbance and landscape dynamics in a changing world. Ecology 2010, 9, 2833–2849. [Google Scholar] [CrossRef]
- Kishchuk, B.E.; Thiffault, E.; Lorente, M.; Quideau, S.; Keddy, T.; Sidders, D. Decadal soil and stand response to fire, harvest, and salvage-logging disturbances in the western boreal mixedwood forest of Alberta, Canada. Can. J. For. Res. 2014, 45, 141–152. [Google Scholar] [CrossRef]
- Ruiz-Jaen, M.C.; Aide, T.M. Restoration success: How is it being measured? Restor. Ecol. 2005, 13, 569–577. [Google Scholar] [CrossRef]
- Jaunatre, R.; Buisson, E.; Muller, I.; Morlon, H.; Mesléard, F.; Dutoit, T. New synthetic indicators to assess community resilience and restoration success. Ecol. Indic. 2013, 29, 468–477. [Google Scholar] [CrossRef]
- Craig, M.D.; Grigg, A.H.; Hobbs, R.J.; Hardy, G.E.S.J. Does coarse woody debris density and volume influence the terrestrial vertebrate community in restored bauxite mines? For. Ecol. Manag. 2014, 318, 142–150. [Google Scholar] [CrossRef]
- Goebel, P.C.; Wyse, T.C.; Corace III, R.G. Determining reference ecosystem conditions for disturbed landscapes within the context of contemporary resource management issues. J. For. 2005, 103, 351–356. [Google Scholar]
- Cobb, P.; Hannam, K.D.; Kishchuk, B.E.; Langor, D.W.; Quideau, S.A.; Spence, J.R. Wood-feeding beetles and soil nutrient cycling in burned forests: Implications of post-fire salvage logging. Agric. For. Entomol. 2010, 12, 9–18. [Google Scholar] [CrossRef]
- Lee, E.J.; Rhim, S.-J.; Son, S.-H.; Lee, W.-S. Differences in small-mammal and stand structures between unburned and burned pine stands subjected to two different post-fire silvicultural management practices. Ann. Zool. Fenn. 2012, 49, 129–138. [Google Scholar] [CrossRef]
- Frego, K.A. Bryophytes as potential indicators of forest integrity. For. Ecol. Manag. 2007, 242, 65–75. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Laenen, B.; Gabriel, R.; González-Mancebo, J.M.; Rumsey, F.J.; Carine, M. Dispersal, diversity and evolution of the Macaronesian cryptogamic floras. In The Biology of Island Floras; Bramwell, D., Caujapé-Castells, J., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 338–364. [Google Scholar]
- Höllermannm, P. The impact of fire in Canarian ecosystems 1983–1998. Erdkunde 2000, 54, 70–75. [Google Scholar] [CrossRef]
- Instituto Canario de Estadística (ISTAC). A Partir de datos de la Viceconsejería de Medio Ambiente. Available online: http://www.gobiernodecanarias.org/istac/jaxi-istac/menu.do?uripub=urn:uuid:1c9aed0f-ad65-4b50-b2a2-63d1559cb720 (accessed on 5 July 2017).
- Del Arco, M.J.; Wildpret, W.; Pérez-de-Paz, P.L.; Rodríguez-Delgado, O.; Acebes, J.R.; García Gallo, A.; Martín, V.E.; Reyes-Betancort, J.A.; Salas, M.; Bermejo, J.A.; et al. Mapa de Vegetación de Canarias; GRAFCAN: Santa Cruz de Tenerife, Spain, 2006. [Google Scholar]
- Del Arco-Aguilar, M.J.; Rodríguez-Delgado, O.; Acebes, J.R.; García-Gallo, A.; Pérez-de-Paz, P.L.; González-Mancebo, J.M.; González-González, R.; Garzón-Machado, V. Bioclimatology and climatophilous vegetation of Gomera (Canary Islands). Ann. Bot. Fenn. 2009, 46, 161–191. [Google Scholar] [CrossRef]
- González-Mancebo, J.M.; Losada-Lima, A.; Patiño-Llorente, J.; Leal Pérez, J. Briófitos. In Hongos, líquenes y briófitos del Parque Nacional de Garajonay (La Gomera, Islas Canarias); Beltrán Tejera, E., Ed.; O.A. Parques Nacionales, Serie Técnica; Ministerio de Medio Ambiente: España, 2008; pp. 565–678. [Google Scholar]
- During, H. Life Strategies of Bryophytes: A Preliminary Review. Lindbergia 1979, 5, 2–18. [Google Scholar]
- Beguería, S.; Pueyo, Y. A comparison of simultaneous autoregressive and generalized least squares models for dealing with spatial autocorrelation. Glob. Ecol. Biogeogr. 2009, 18, 273–279. [Google Scholar] [CrossRef]
- Pebesma, E.J.; Bivand, R.S. Classes and methods for spatial data in R. R News 2005, 5, 9–13. [Google Scholar]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 2nd ed.; Sage: Thousand Oaks CA, USA, 2011. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-131; R Core Team: Vienna, Austria, 2009; Volume 3, p. 96. [Google Scholar]
- Bivand, R.; Piras, G. Comparing Implementations of Estimation Methods for Spatial Econometrics. J. Stat. Softw. 2015, 63, 1–36. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.15.6. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 13 July 2017).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R foundation for Statistical Computing: Vienna, Austria, 2008; ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 13 July 2017).
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples, version 9; 2013. Available online: http://purl.oclc.org/estimates (accessed on 13 July 2017).
- De Caceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Henderson, P.A.; Seaby, R.M.H. Community Analysis Package Version 5; Pisces Conservation Ltd.: Lymington, UK, 2014. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 473–474. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER V. 6.1.6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Aude, E.; Poulsen, R.S. Influence of management on the species composition of epiphytic cryptogams in Danish Fagus forests. Appl. Veg. Sci. 2000, 3, 81–88. [Google Scholar] [CrossRef]
- McAlister, S. Cryptogam communities of fallen logs in the Duke Forest, North Carolina. J. Veg. Sci. 1997, 8, 115–124. [Google Scholar] [CrossRef]
- Oksanen, J. Impact of habitat, substrate, and microsite classes on the epiphytic vegetation: Interpretation using exploratory and canonical correspondence analysis. Ann. Bot. Fenn. 1998, 25, 59–71. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination, version 4.5; Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- González-Mancebo, J.M.; Romaguera, F.; Losada-Lima, A.; Suárez, A. Epiphytic bryophytes growing in Laurus azorica (Seub.) Franco in three laurel forest areas in Tenerife (Canary Islands). Acta Oecol. 2004, 25, 159–167. [Google Scholar] [CrossRef]
- Bradbury, S.M. Response of the post-fire bryophyte community to salvage logging in boreal mixedwood forests of northeastern Alberta, Canada. For. Ecol. Manag. 2006, 234, 313–322. [Google Scholar] [CrossRef]
- Foster, D.R. Vegetation development following fire in Picea mariana (black spruce)-Pleurozium forest of South-Eastern Labrador, Canada. J. Ecol. 1985, 73, 517–534. [Google Scholar] [CrossRef]
- Hylander, K.; Dynesius, M.; Jonsson, B.G.; Nilsson, C. Substrate form determines the fate of bryophytes in riparian buffer strips. Ecol. Appl. 2005, 15, 674–688. [Google Scholar] [CrossRef]
- Jonsson, B.G. The bryophyte diaspore bank and its role after small-scale disturbance in a boreal forest. J. Veg. Sci. 1993, 4, 819–826. [Google Scholar] [CrossRef]
- Cornelissen, J.; ter Steege, H. Distribution and ecology of epiphytic bryophytes and lichens in dry evergreen forest of Guyana. J. Trop. Ecol. 1989, 5, 131–150. [Google Scholar] [CrossRef]
- Wolf, J.H. Diversity patterns and biomass of epiphytic bryophytes and lichens along an altitudinal gradient in the northern Andes. Ann. Mo. Bot. Garden 1993, 80, 928–960. [Google Scholar] [CrossRef]
- Acebey, A.; Gradstein, S.R.; Krömer, T. Species richness and habitat diversification of bryophytes in submontane rain forest and fallows of Bolivia. J. Trop. Ecol. 2003, 19, 9–18. [Google Scholar] [CrossRef]
- Niemelä, J. Invertebrates and Boreal Forest Management. Conserv. Biol. 1997, 11, 601–610. [Google Scholar] [CrossRef]
- Leverkus, A.B.; Castro, J.; Puerta-Piñero, C.; Rey Benayas, J.M. Suitability of the management of habitat complexity, acorn burial depth, and a chemical repellent for post-fire reforestation of oaks. Ecol. Eng. 2013, 53, 15–22. [Google Scholar] [CrossRef]
- Hutto, R.L.; Gallo, S.M. The effects of postfire salvage logging on cavity-nesting birds. Condor 2006, 108, 817–831. [Google Scholar] [CrossRef]
- Choi, C.-Y.; Lee, E.-J.; Nam, H.-Y.; Lee, W.-S.; Lim, J.-H. Temporal changes in the breeding bird community caused by post-fire treatments after the Samcheok forest fire in Korea. Landsc. Ecol. Eng. 2014, 10, 203–214. [Google Scholar] [CrossRef]
- Lee, E.-J.; Lee, W.-S.; Rhim, S.-J. Characteristics of small rodent populations in post-fire silvicultural management stands within pine forest. For. Ecol. Manag. 2008, 255, 1418–1422. [Google Scholar] [CrossRef]
- Boucher, D.; Gauthier, S.; Noël, J.; Greene, D.F.; Bergeron, Y. Salvage logging affects early post-fire tree composition in Canadian boreal forest. For. Ecol. Manag. 2014, 325, 118–127. [Google Scholar] [CrossRef]
- Leverkus, A.B.; Lorite, J.; Navarro, F.B.; Sánchez-Cañete, E.P.; Castro, J. Post-fire salvage logging alters species composition and reduces cover, richness, and diversity in Mediterranean plant communities. J. Environ. Manag. 2014, 133, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Bros, V.; Moreno-Rueda, G.; Santos, X. Does post-fire management affect the recovery of Mediterranean communities? The case study of terrestrial gastropods. For. Ecol. Manag. 2011, 261, 611–619. [Google Scholar] [CrossRef]
- Koivula, M.; Spence, J.R. Effects of post-fire salvage logging on boreal mixed-wood ground beetle assemblages (Coleoptera, Carabidae). For. Ecol. Manag. 2006, 236, 102–112. [Google Scholar] [CrossRef]
- Bottero, A.; Garbarino, M.; Long, J.N.; Motta, R. The interacting ecological effects of large disturbances and salvage logging on montane spruce forest regeneration in the western European Alps. For. Ecol. Manag. 2013, 292, 19–28. [Google Scholar] [CrossRef]
- Marzano, R.; Garbarino, M.; Marcolin, E.; Pividori, M.; Lingua, E. Deadwood anisotropic facilitation on seedling establishment after a stand-replacing wildfire in Aosta Valley (NW Italy). Ecol. Eng. 2013, 51, 117–122. [Google Scholar] [CrossRef]
- Thorn, S.; Bässler, C.; Bernhardt-Römermann, M.; Cadotte, M.; Heibl, C.; Schäfer, H.; Seibold, S.; Müller, J. Changes in the dominant assembly mechanism drive species loss caused by declining resources. Ecol. Lett. 2016, 19, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Jonášová, M.; Prach, K. The influence of bark beetles outbreak vs. salvage logging on ground layer vegetation in Central European mountain spruce forests. Biol. Conserv. 2008, 141, 1525–1535. [Google Scholar] [CrossRef]
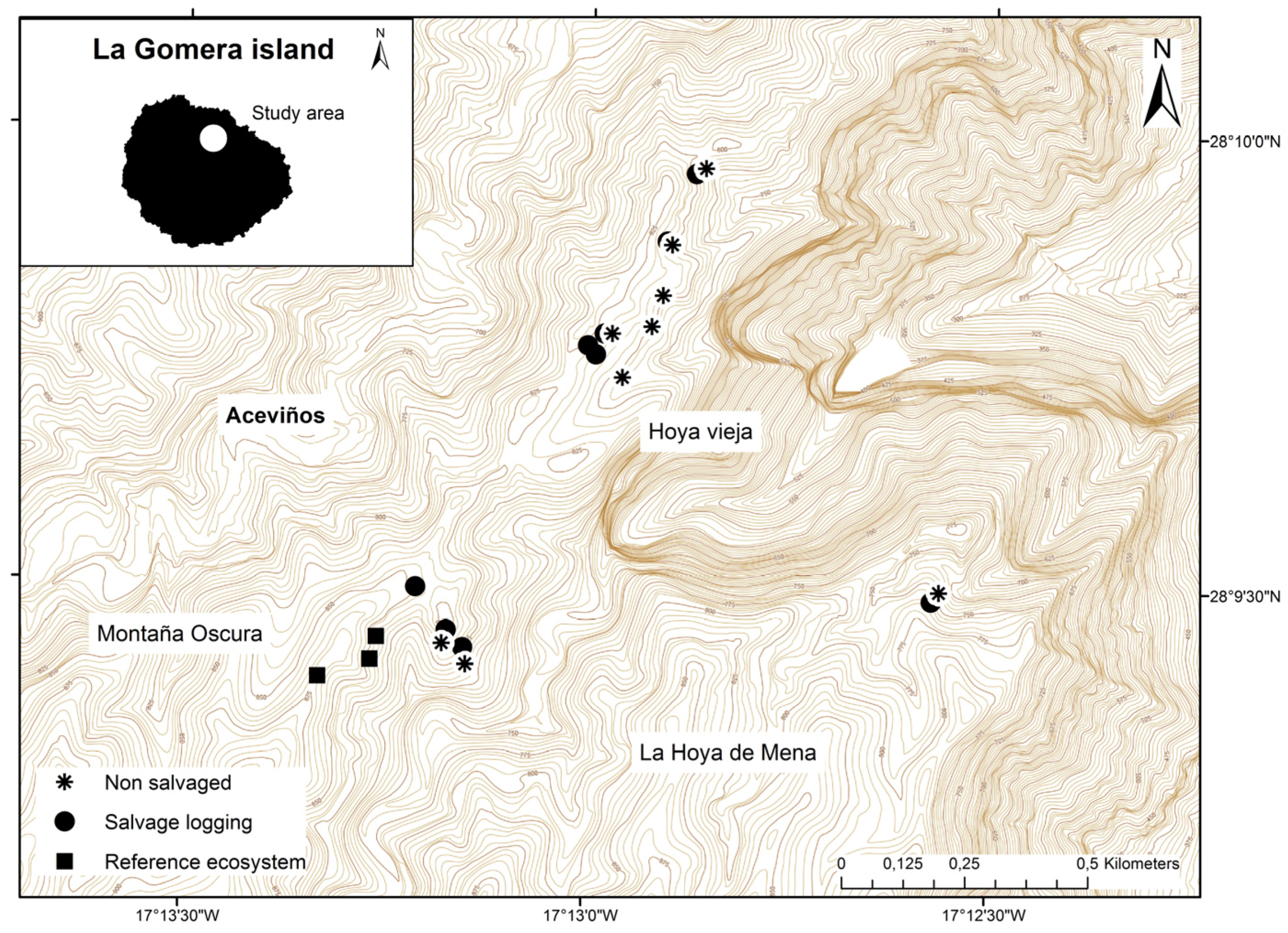
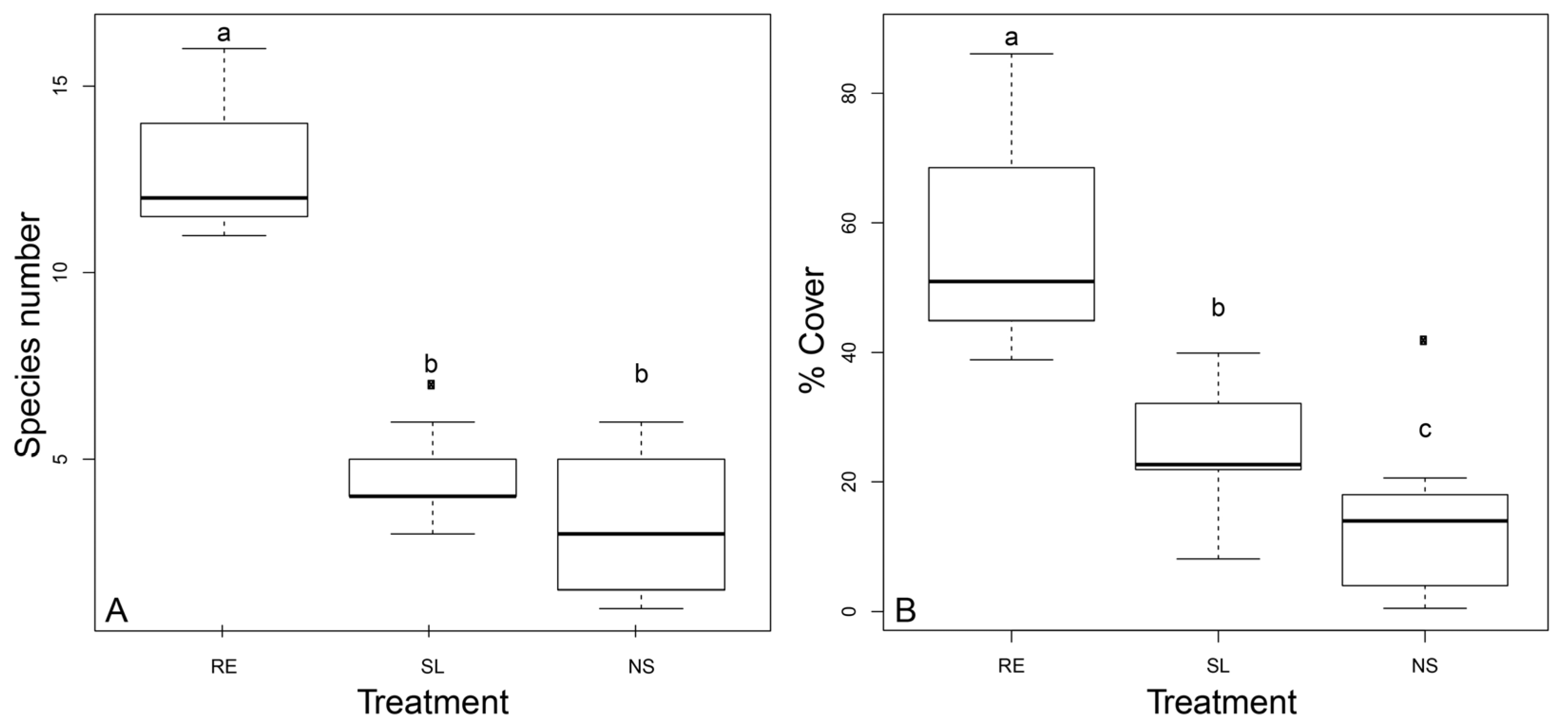
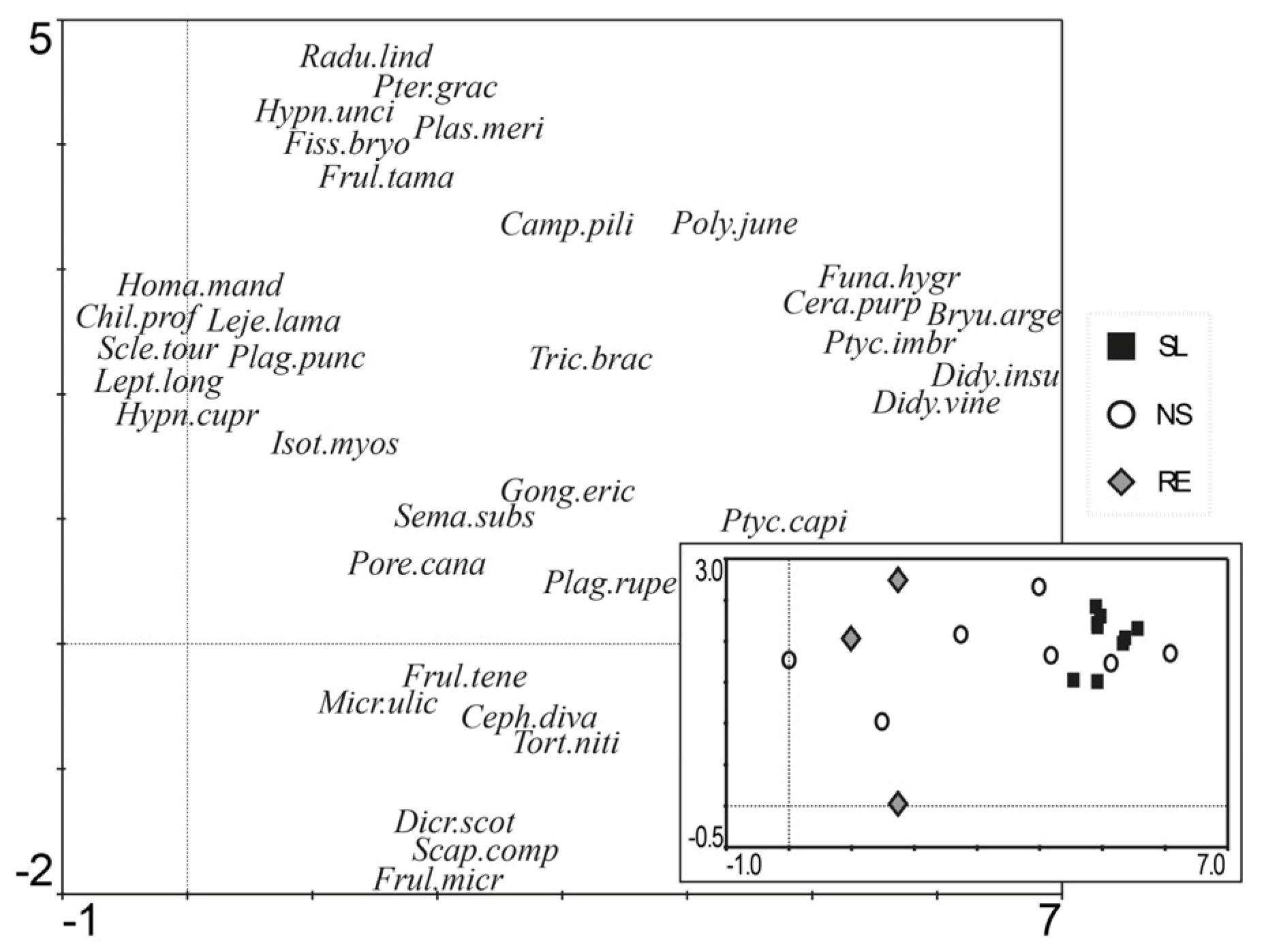


| Variable | Salvage Logged | Non-Salvaged | Reference Ecosystem | p-Value | |
|---|---|---|---|---|---|
| (SL) | (NS) | (RE) | |||
| Environmental features | |||||
| Elevation (m a.s.l.) * | 811.9 ± 7.7 | 799.9 ± 3.4 | 846.66 ± 21.07 | 0.013 | |
| Slope (°) * | 23.77 ± 3.78 | 28.88 ± 3.61 | 29.0 ± 7.9 | 0.586 | |
| Number of individuals ** | 118.66 ± 24.21 | 102.44 ± 9.45 | 117.67 ± 27.88 | 0.803 | |
| Aspect (degrees) * | 120.44 ± 53.8 | 117.88 ± 52.3 | 104.33 ± 18.92 | 0.890 | |
| Post-management stand features | |||||
| Canopy cover | 58.33 ± 7.81 | 84.77 ± 4.99 | 96.33 ± 1.33 | 0.006 | |
| Litter layer (cover %) | 27.88 ± 8.97 | 53.88 ± 11.63 | 80.00 ± 5.77 | 0.037 | |
| Depth of the litter layer (cm) | 1.79 ± 0.38 | 2.64 ± 0.62 | 3.3 ± 0.52 | 0.278 | |
| Height of the resprouted vegetation (m) *** | 3.08 ± 0.13 | 3.98 ± 0.20 | † | 0.016 | |
| Bare Soil (%) | 21.11 ± 4.98 | 9.44 ± 3.64 | 11.67 ± 6.01 | 0.169 | |
| Functional Group | Categories |
|---|---|
| Taxonomic | Liverworts |
| Mosses | |
| Canopy preference | Open canopy |
| Canopy generalists | |
| Closed Canopy | |
| Life strategy | Fugitives |
| Colonists | |
| Short-lived shuttles | |
| Long-lived shuttles | |
| Perennials | |
| Substrate preference | Epiphytes |
| Substrate generalists | |
| Saxicolous | |
| Terricolous |
| Species | % Contribution | Cumulative % | |
|---|---|---|---|
| RE | Frultene | 22.24 | 22.24 |
| Isotmyos | 20.70 | 42.94 | |
| Frulltama | 17.19 | 60.03 | |
| Porecana | 9.84 | 69.87 | |
| Hypnunci | 7.59 | 77.47 | |
| Fissbryo | 5.70 | 83.16 | |
| Semasubs | 5.23 | 88.40 | |
| Plastmeri | 3.80 | 92.19 | |
| NS | Cerapurp | 22.54 | 22.54 |
| Camppili | 19.40 | 42.00 | |
| Hypncupr | 13.16 | 55.15 | |
| Ptyccapi | 12.70 | 67.85 | |
| Ptycimbr | 9.10 | 76.95 | |
| Didyvine | 9.03 | 85.98 | |
| Didyinsu | 6.84 | 92.82 | |
| SL | Ptycimbr | 60.72 | 60.72 |
| Didyvine | 16.14 | 76.86 | |
| Cerapurp | 10.91 | 87.77 | |
| Funahygr | 5.51 | 93.28 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Hernández, R.; Castro, J.; Del Arco-Aguilar, M.; Fernández-López, Á.; González-Mancebo, J.M. Post-Fire Salvage Logging Imposes a New Disturbance that Retards Succession: The Case of Bryophyte Communities in a Macaronesian Laurel Forest. Forests 2017, 8, 252. https://doi.org/10.3390/f8070252
Hernández-Hernández R, Castro J, Del Arco-Aguilar M, Fernández-López Á, González-Mancebo JM. Post-Fire Salvage Logging Imposes a New Disturbance that Retards Succession: The Case of Bryophyte Communities in a Macaronesian Laurel Forest. Forests. 2017; 8(7):252. https://doi.org/10.3390/f8070252
Chicago/Turabian StyleHernández-Hernández, Raquel, Jorge Castro, Marcelino Del Arco-Aguilar, Ángel Fernández-López, and Juana María González-Mancebo. 2017. "Post-Fire Salvage Logging Imposes a New Disturbance that Retards Succession: The Case of Bryophyte Communities in a Macaronesian Laurel Forest" Forests 8, no. 7: 252. https://doi.org/10.3390/f8070252





