Evaluation of the Susceptibility of Several Czech Conifer Provenances to Fusarium circinatum
Abstract
:1. Introduction
2. Material and Methods
2.1. Fungal Isolates and Plant Material
2.2. Pathogenicity Tests
2.3. Statistical Analyses
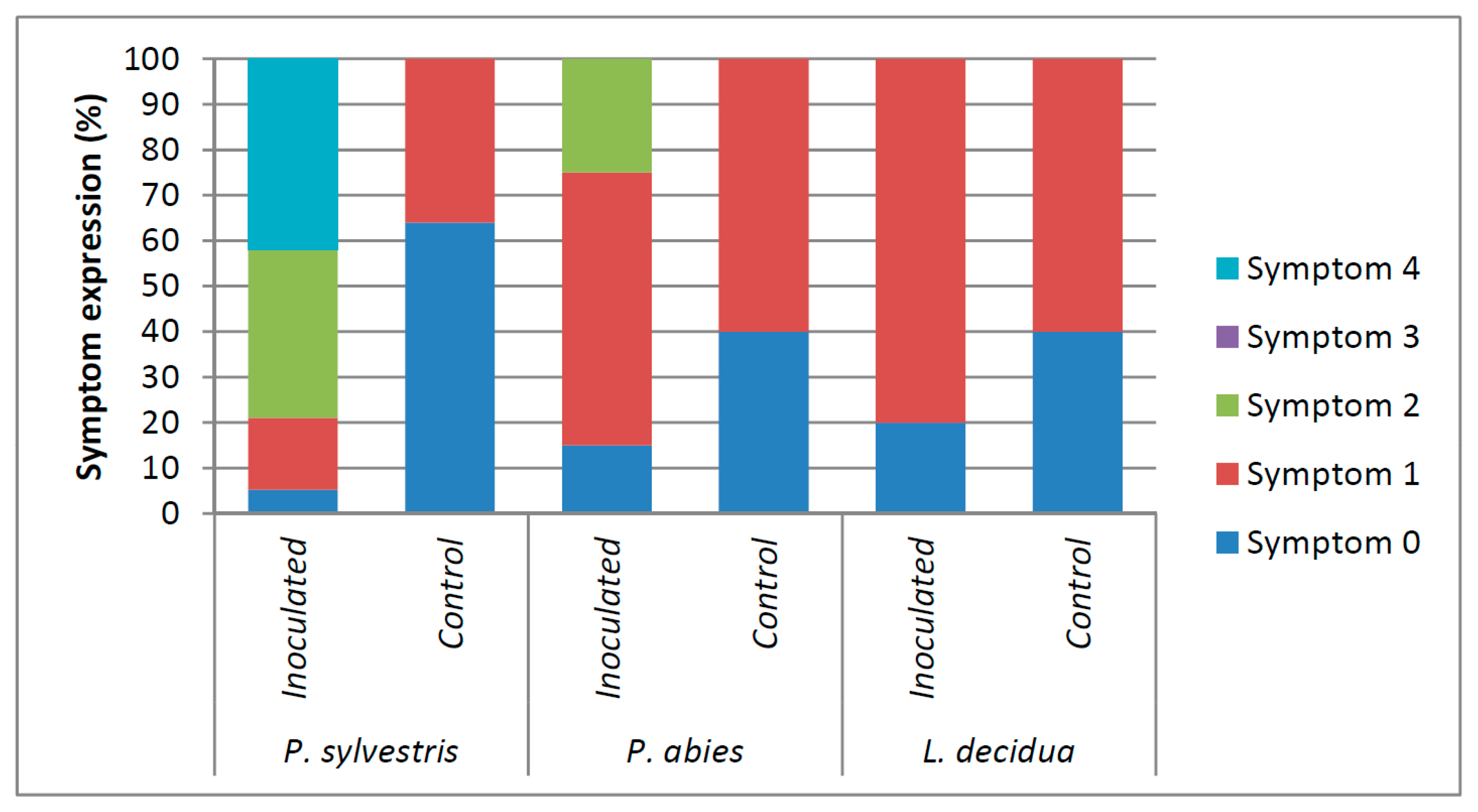
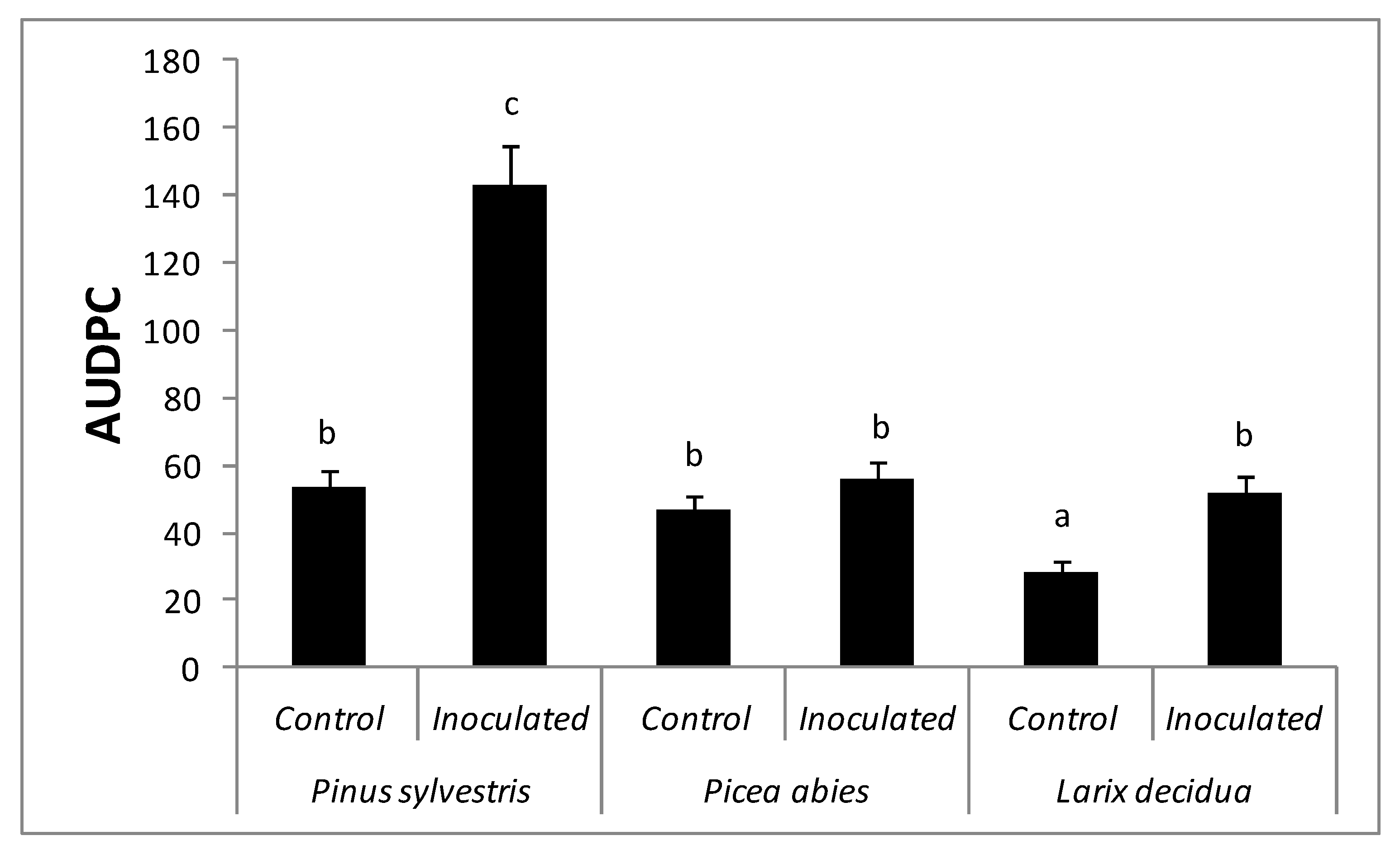
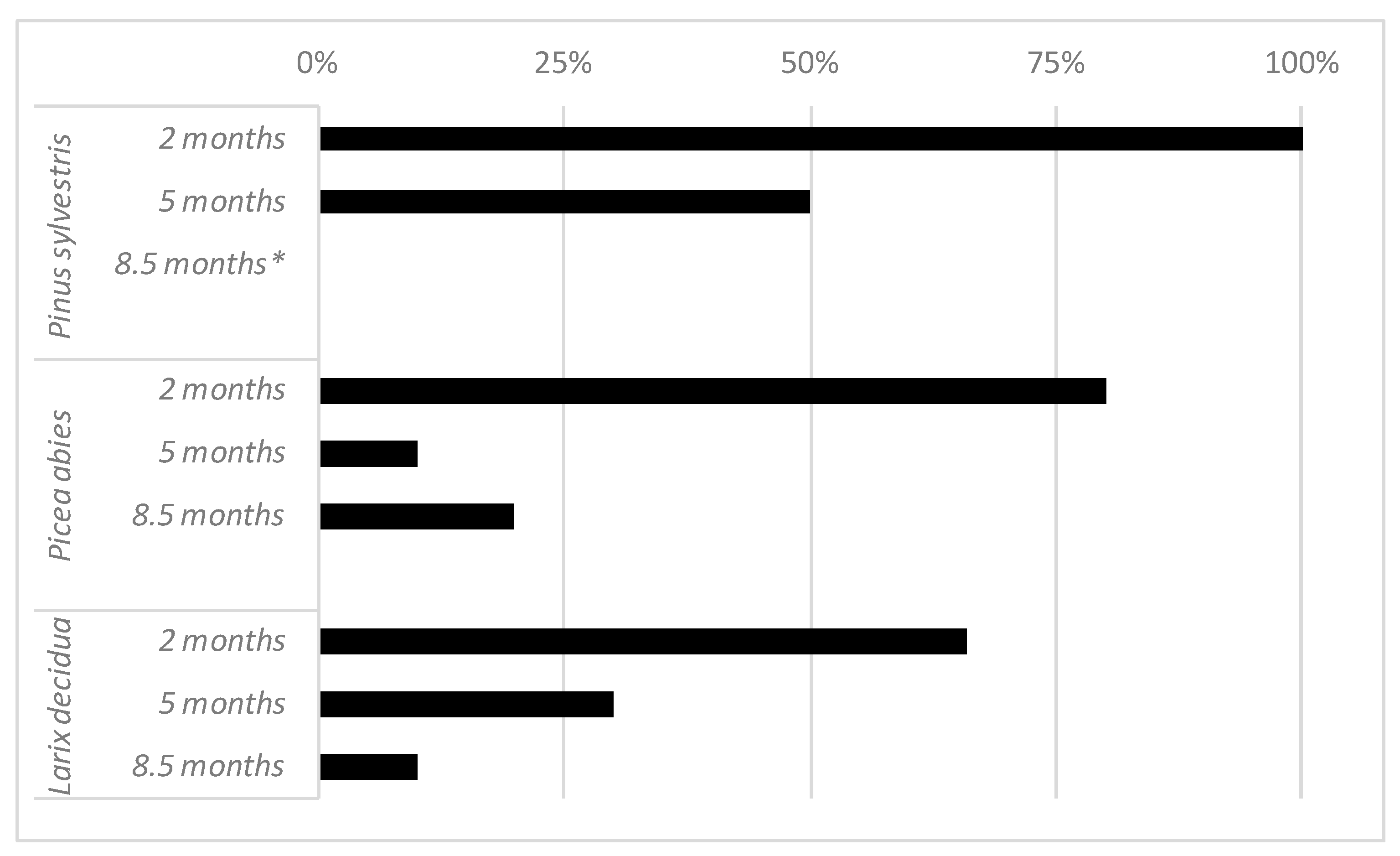
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ministry of Agriculture of the Czech Republic. Information on Forests and Forestry in the Czech Republic by 2016; Ministry of Agriculture of the Czech Republic: Prague, Czech Republic, 2017.
- Stenlid, J.; Oliva, J.; Boberg, J.B.; Hopkins, A.J.M. Emerging diseases in European forest ecosystems and responses in society. Forests 2011, 2, 486–504. [Google Scholar] [CrossRef]
- Santini, A.; Ghelardini, L.; De Pace, C.; Desprez-Loustau, M.L.; Capretti, P.; Chandelier, A.; Cech, T.; Chira, D.; Diamandis, S.; Gaitniekis, T.; et al. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 2013, 197, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Ramsfield, T.D.; Bentz, B.J.; Faccoli, M.; Jactel, H.; Brockerhoff, E.G. Forest health in a changing world: Effects of globalization and climate change on forest insect and pathogen impacts. Forestry 2016, 89, 245–252. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization (EPPO). Data sheets on quarantine pests: Gibberella circinata. EPPO Bull. 2005, 35, 383–386. [Google Scholar] [CrossRef]
- Hepting, G.H.; Roth, E.R. Pitch canker, a new disease of some Southern pines. J. For. 1946, 44, 742–744. [Google Scholar]
- Hepting, G.H.; Roth, E.R. Host relations and spread of the pine pitch canker disease. Phytopathology 1953, 43, 475. [Google Scholar]
- Viljoen, A.; Wingfield, M.J.; Marasas, W.F.O. First report of Fusarium subglutinans f. sp. pini on pine seedlings in South Africa. Plant Dis. 1994, 78, 309–312. [Google Scholar] [CrossRef]
- Guerra-Santos, J.J. Pitch canker on Monterey pine in Mexico. In Current and Potential Impacts of Pitch Canker in Radiata Pine, Proceedings of the IMPACT Monterey Workshop, Monterey, CA, USA, 30 November–3 December 1998; Devey, M., Matheson, C., Gordon, T., Eds.; CSIRO Forestry & Forest Products: Canberra, Australia, 1999; Volume 112, pp. 58–61. [Google Scholar]
- Wingfield, M.J.; Jacobs, A.; Coutinho, T.A.; Ahumada, R.; Wingfield, B.D. First report of the pitch canker fungus, Fusarium circinatum, on pines in Chile. Plant Pathol. 2002, 51, 397. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, S.-H.; Yang, S.-I.; Lee, Y.-W. First report of Pitch Canker disease on Pinus rigida in Korea. Plant Pathol. J. 2000, 16, 52–54. [Google Scholar]
- Kobayashi, T.; Muramoto, M. Pitch canker of Pinus luchuensis, a new disease of Japanese forests. For. Pests 1989, 40, 169–173. [Google Scholar]
- Alonso, R.; Bettucci, L. First report of the pitch canker fungus Fusarium circinatum affecting Pinus taeda seedlings in Uruguay. Australas. Plant Dis. Notes 2009, 4, 91–92. [Google Scholar] [CrossRef]
- Steenkamp, E.T.; Rodas, C.A.; Kvas, M.; Wingfield, M.J. Fusarium circinatum and pitch canker of Pinus in Colombia. Australas. Plant Pathol. 2012, 41, 483–491. [Google Scholar] [CrossRef]
- Pfenning, L.H.; da Silva Costa, S.; de Melo, M.P.; Costa, H.; Ventura, J.A.; Auer, C.G.; dos Santos, Á.F. First report and characterization of Fusarium circinatum, the causal agent of pitch canker in Brazil. Trop. Plant Pathol. 2014, 39, 210–216. [Google Scholar] [CrossRef]
- Dwinell, D. Global Distribution of the Pitch Canker Fungus. In Current and Potential Impacts of Pitch Canker in Radiata Pine, Proceedings of the IMPACT Monterey Workshop, Monterey, CA, USA, 30 November–3 December 1998; CSIRO Forestry & Forest Products: Canberra, Australia, 1999; pp. 54–57. [Google Scholar]
- Landeras, E.; García, P.; Fernández, M.; Braña, M.; Pérez-Sierra, A.; León, M.; Abad-Campos, P.; Armengol, J. Outbreak of Pitch Canker Caused by Fusarium circinatum on Pinus spp. in Northern Spain. Plant Dis. 2005, 89, 1015. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization (EPPO). First Report of Gibberella circinata in France; EPPO: Paris, France, 2006; Volume 5. [Google Scholar]
- Carlucci, A.; Colatruglio, L.; Frisullo, S. First Report of Pitch Canker Caused by Fusarium circinatum on Pinus halepensis and P. pinea in Apulia (Southern Italy). Plant Dis. 2007, 91, 1683. [Google Scholar] [CrossRef]
- Bragança, H.; Diogo, E.; Moniz, F.; Amaro, P. First Report of Pitch Canker on Pines Caused by Fusarium circinatum. Plant Dis. 2009, 93, 1079. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Risk assessment of Gibberella circinata for the EU territory and identification and evaluation of risk management options. Eur. Food Saf. Auth. 2010, 8, 1620. [Google Scholar] [CrossRef]
- Watt, M.S.; Ganley, R.J.; Kriticos, D.J.; Manning, L.K. Dothistroma needle blight and pitch canker: The current and future potential distribution of two important diseases of Pinus species. Can. J. For. Res. 2011, 41, 412–424. [Google Scholar] [CrossRef]
- Ganley, R.J.; Watt, M.S.; Manning, L.; Iturritxa, E. A global climatic risk assessment of pitch canker disease. Can. J. For. Res. 2009, 39, 2246–2256. [Google Scholar] [CrossRef]
- Bezos, D.; Martínez-Álvarez, P.; Fernández, M.; Diez, J.J. Epidemiology and Management of Pine Pitch Canker Disease in Europe—A Review. Balt. For. 2017, 23, 279–293. [Google Scholar]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D.; Coutinho, T.A. Pitch canker caused by Fusarium circinatum—A growing threat to pine plantations and forests worldwide. Australas. Plant Pathol. 2008, 37, 319–334. [Google Scholar] [CrossRef]
- Gordon, T.R.; Okamoto, D.; Storer, A.J.; Wood, D.L. Susceptibility of five landscape pines to pitch canker disease, caused by Fusarium subglutinans f. sp. pini. Hortscience 1998, 33, 868–871. [Google Scholar]
- Enebak, B.S.A.; Stanosz, G.R. Responses of conifer species of the Great Lakes region of North America to inoculation with the pitch canker pathogen Fusarium circinatum. For. Pathol. 2003, 33, 333–338. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P.; Pando, V.; Diez, J.J. Alternative species to replace Monterey pine plantations affected by pitch canker caused by Fusarium circinatum in northern Spain. Plant Pathol. 2014, 63, 1086–1094. [Google Scholar] [CrossRef]
- Iturritxa, E.; Mesanza, N.; Elvira-Recuenco, M.; Serrano, Y.; Quintana, E.; Raposo, R. Evaluation of genetic resistance in Pinus to pitch canker in Spain. Australas. Plant Pathol. 2012, 41, 601–607. [Google Scholar] [CrossRef]
- Iturritxa, E.; Ganley, R.J.; Raposo, R.; García-Serna, I.; Mesanza, N.; Kirkpatrick, S.C.; Gordon, T.R. Resistance levels of Spanish conifers against Fusarium circinatum and Diplodia pinea. For. Pathol. 2013, 43, 488–495. [Google Scholar] [CrossRef]
- Gordon, T.R. Pitch canker disease of pines. Phytopathology 2006, 96, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Matheson, A.C.; Devey, M.E.; Gordon, T.L.; Werner, W.; Vogler, D.R.; Balocchi, C.; Carson, M.J. Heritability of response to inoculation by pine pitch canker of seedlings of radiata pine. Aust. For. 2006, 69, 101–106. [Google Scholar] [CrossRef]
- Hodge, G.R.; Dvorak, W.S. Variation in pitch canker resistance among provenances of Pinus patula and Pinus tecunumanii from Mexico and Central America. New For. 2007, 33, 193–206. [Google Scholar] [CrossRef]
- Mitchell, R.; Wingfield, M.; Steenkamp, E.; Coutinho, T. Tolerance of Pinus patula full-sib families to Fusarium circinatum in a greenhouse study. South. For. J. For. Sci. 2012, 74, 247–252. [Google Scholar] [CrossRef]
- Elvira-Recuenco, M.; Iturritxa, E.; Majada, J.; Alia, R.; Raposo, R. Adaptive potential of maritime pine (Pinus pinaster) populations to the emerging pitch canker pathogen, Fusarium circinatum. PLoS ONE 2014, 9, e114971. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.; Cavers, S.; Cottrell, J.E.; Ennos, R.A. Genetic variation for needle traits in Scots pine (Pinus sylvestris L.). Tree Genet. Genomes 2016, 12, 40. [Google Scholar] [CrossRef]
- Belletti, P.; Ferrazzini, D.; Piotti, A.; Monteleone, I.; Ducci, F. Genetic variation and divergence in Scots pine (Pinus sylvestris L.) within its natural range in Italy. Eur. J. For. Res. 2012, 131, 1127–1138. [Google Scholar] [CrossRef]
- Wójkiewicz, B.; Cavers, S.; Wachowiak, W. Current Approaches and Perspectives in Population Genetics of Scots Pine (Pinus sylvestris L.). For. Sci. 2016, 62, 343–354. [Google Scholar] [CrossRef]
- Lagercrantz, U.; Ryman, N. Genetic Structure of Norway Spruce (Picea abies): Concordance of Morphological and Allozymic Variation. Evolution 1990, 44, 38–53. [Google Scholar] [PubMed]
- Maier, J. Genetic variation in European larch (Larix decidua Mill). Ann. For. Sci. 1992, 49, 39–47. [Google Scholar] [CrossRef]
- Lewandowski, A.; Mejnartowicz, L. Levels and patterns of allozyme variation in some European larch (Larix decidua) populations. Hereditas 1991, 115, 221–226. [Google Scholar] [CrossRef]
- Storer, A.J.; Gordon, T.R.; Clark, S.L. Association of the pitch canker fungus, Fusarium subglutinans f. sp. pini, with Monterey pine seeds and seedlings in California. Plant Pathol. 1998, 47, 649–656. [Google Scholar]
- Mitchell, R.G.; Zwolinski, J.; Jones, N.; Coutinho, T. The effect of applying prophylactic measures on the post-planting survival of Pinus patula in South Africa. S. Afr. For. J. 2004, 200, 51–58. [Google Scholar]
- Kim, Y.-S.; Woo, K.-S.; Koo, Y.-B.; Yeo, J.-K. Variation in susceptibility of six pine species and hybrids to pitch canker caused by Fusarium circinatum. For. Pathol. 2008, 38, 419–428. [Google Scholar] [CrossRef]
- Vivas, M.; Zas, R.; Solla, A. Screening of Maritime pine (Pinus pinaster) for resistance to Fusarium circinatum, the causal agent of Pitch Canker disease. Forestry 2012, 85, 185–192. [Google Scholar] [CrossRef]
- Elvira-Recuenco, M.; Iturritxa, E.; Raposo, R. Impact of seed transmission on the infection and development of pitch canker disease in Pinus radiata. Forests 2015, 6, 3353–3368. [Google Scholar] [CrossRef]
- Swett, C.L.; Kirkpatrick, S.C.; Gordon, T.R. Evidence for a Hemibiotrophic Association of the Pitch Canker Pathogen Fusarium circinatum with Pinus radiata. Plant Dis. 2016, 100, 79–84. [Google Scholar] [CrossRef]
- Martínez-Alvarez, P.; Alves-Santos, F.M.; Diez, J.J. In Vitro and In Vivo Interactions between Trichoderma viride and Fusarium circinatum. Silva Fenn. 2012, 46, 303–316. [Google Scholar] [CrossRef]
- Cerqueira, A.; Alves, A.; Berenguer, H.; Correia, B.; Gómez-Cadenas, A.; Diez, J.J.; Monteiro, P.; Pinto, G. Phosphite shifts physiological and hormonal profile of Monterey pine and delays Fusarium circinatum progression. Plant Physiol. Biochem. 2017, 114, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Martín-García, J.; Paraschiv, M.; Flores-Pacheco, J.A.; Chira, D.; Diez, J.J.; Fernández, M. Susceptibility of several northeastern conifers to Fusarium circinatum and strategies for biocontrol. Forests 2017, 8, 318. [Google Scholar] [CrossRef]
- Plíva, A. Typologický Klasifikační Systém ÚHÚL; ÚHÚL (Ústav pro hospodářskou úpravu lesů): Brandýs nad Labem, Czech Republic, 1987. [Google Scholar]
- Martínez-Álvarez, P.; Fernández-González, R.A.; Sanz-Ros, A.V.; Pando, V.; Diez, J.J. Two fungal endophytes reduce the severity of pitch canker disease in Pinus radiata seedlings. Biol. Control 2016, 94, 1–10. [Google Scholar] [CrossRef]
- Correll, J.C.; Gordon, T.R.; Mccain, A.H.; Fox, J.W.; Koehler, C.S.; Wood, D.L.; Schultz, M.E. Pitch canker disease in California: Pathogenicity, distribution, and canker development on Monterey pine (Pinus radiata). Plant Dis. 1991, 75, 676–682. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P. Environmentally Friendly Methods for the Integrated Management of Pine Pitch Canker (PPC) Disease. Ph.D. Thesis, University of Valladolid, Palencia, Spain, 2015. [Google Scholar]
- Leslie, J.; Summerell, B. The Fusarium Laboratory Manual; Blackwell Publishing Professional: Ames, IA, USA, 2006. [Google Scholar]
- Prieto-Recio, C.; Martín-García, J.; Diez, J.J. Pathogenicity of Spanish isolates of Heterobasidion annosum s. s. in Pinus pinaster seedlings. For. Pathol. 2014, 44, 163–165. [Google Scholar] [CrossRef]
- Zamora-Ballesteros, C.; Haque, M.M.U.; Diez, J.J.; Martín-García, J. Pathogenicity of Phytophthora alni complex and P. plurivora in Alnus glutinosa seedlings. For. Pathol. 2016. [Google Scholar] [CrossRef]
- García-Pérez, A. Métodos Avanzados de Estadística Aplicada. Métodos Robustos y de Remuestreo; García-Pérez, A., Ed.; UNED Universidad Nacional a Distancia: Madrid, Spain, 2010. [Google Scholar]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Therneau, T. A Package for Survival Analysis in S. R Package Version 2.41-3. Available online: https://CRAN.R-project.org/package=survival (accessed on 18 December 2017).
- Pérez-Sierra, A.; Landeras, E.; León, M.; Berbegal, M.; García-Jiménez, J.; Armengol, J. Characterization of Fusarium circinatum from Pinus spp. in northern Spain. Mycol. Res. 2007, 111, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Adalia, E.J.; Flores-Pacheco, J.A.; Martínez-Álvarez, P.; Martín-García, J.; Fernández, M.; Diez, J.J. Effect of mycoviruses on the virulence of Fusarium circinatum and laccase activity. Physiol. Mol. Plant Pathol. 2016, 94, 8–15. [Google Scholar] [CrossRef]
- Mullett, M.; Pérez-Sierra, A.; Armengol, J.; Berbegal, M. Phenotypical and Molecular Characterisation of Fusarium circinatum: Correlation with Virulence and Fungicide Sensitivity. Forests 2017, 8, 458. [Google Scholar] [CrossRef]
- Dvorak, W.S.; Hodge, G.R.; Kietzka, J.E. Genetic variation in survival, growth, and stem form of Pinus leiophylla in Brazil and South Africa and provenance resistance to pitch canker. South. Hemisph. For. J. 2007, 69, 125–135. [Google Scholar] [CrossRef]
- Dvorak, W.S.; Potter, K.M.; Hipkins, V.D.; Hodge, G.R. Genetic Diversity and Gene Exchange in Pinus oocarpa, a Mesoamerican Pine with Resistance to the Pitch Canker Fungus (Fusarium circinatum). Int. J. Plant Sci. 2009, 170, 609–626. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Wingfield, M.J.; Hodge, G.R.; Steenkamp, E.T.; Coutinho, T.A. Selection of Pinus spp. in South Africa for tolerance to infection by the pitch canker fungus. New For. 2012, 43, 473–489. [Google Scholar] [CrossRef]
- Cheddadi, R.; Vendramin, G.G.; Litt, T.; Francois, L.; Kageyama, M.; Lorentz, S.; Laurent, J.; De Beaulieu, J.; Sadori, L.; Jost, A.; et al. Imprints of glacial refugia in the modern genetic diversity of Pinus sylvestris. Glob. Ecol. Biogeogr. 2006, 15, 271–282. [Google Scholar] [CrossRef]
- Prus-Glowacki, W.; Stephan, B.R. Genetic Variation of Pinus sylvestris from Spain in Relation to Other European Populations. Silva Fenn. 1994, 43, 7–14. [Google Scholar]
- CAB International (The Centre for Agriculture and Bioscience International). Gibberella circinata (Pitch Canker)—Pinus; Crop Protection Compendium; CAB International: Wallingford, UK, 2007. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-García, J.; Lukačevičová, A.; Flores-Pacheco, J.A.; Diez, J.J.; Dvořák, M. Evaluation of the Susceptibility of Several Czech Conifer Provenances to Fusarium circinatum. Forests 2018, 9, 72. https://doi.org/10.3390/f9020072
Martín-García J, Lukačevičová A, Flores-Pacheco JA, Diez JJ, Dvořák M. Evaluation of the Susceptibility of Several Czech Conifer Provenances to Fusarium circinatum. Forests. 2018; 9(2):72. https://doi.org/10.3390/f9020072
Chicago/Turabian StyleMartín-García, Jorge, Aneta Lukačevičová, Juan Asdrúbal Flores-Pacheco, Julio Javier Diez, and Miloň Dvořák. 2018. "Evaluation of the Susceptibility of Several Czech Conifer Provenances to Fusarium circinatum" Forests 9, no. 2: 72. https://doi.org/10.3390/f9020072




