Relationships between Tree Age and Climate Sensitivity of Radial Growth in Different Drought Conditions of Qilian Mountains, Northwestern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Climate
2.2. Sampling and Chronology Development
2.3. Analytical Method
3. Results
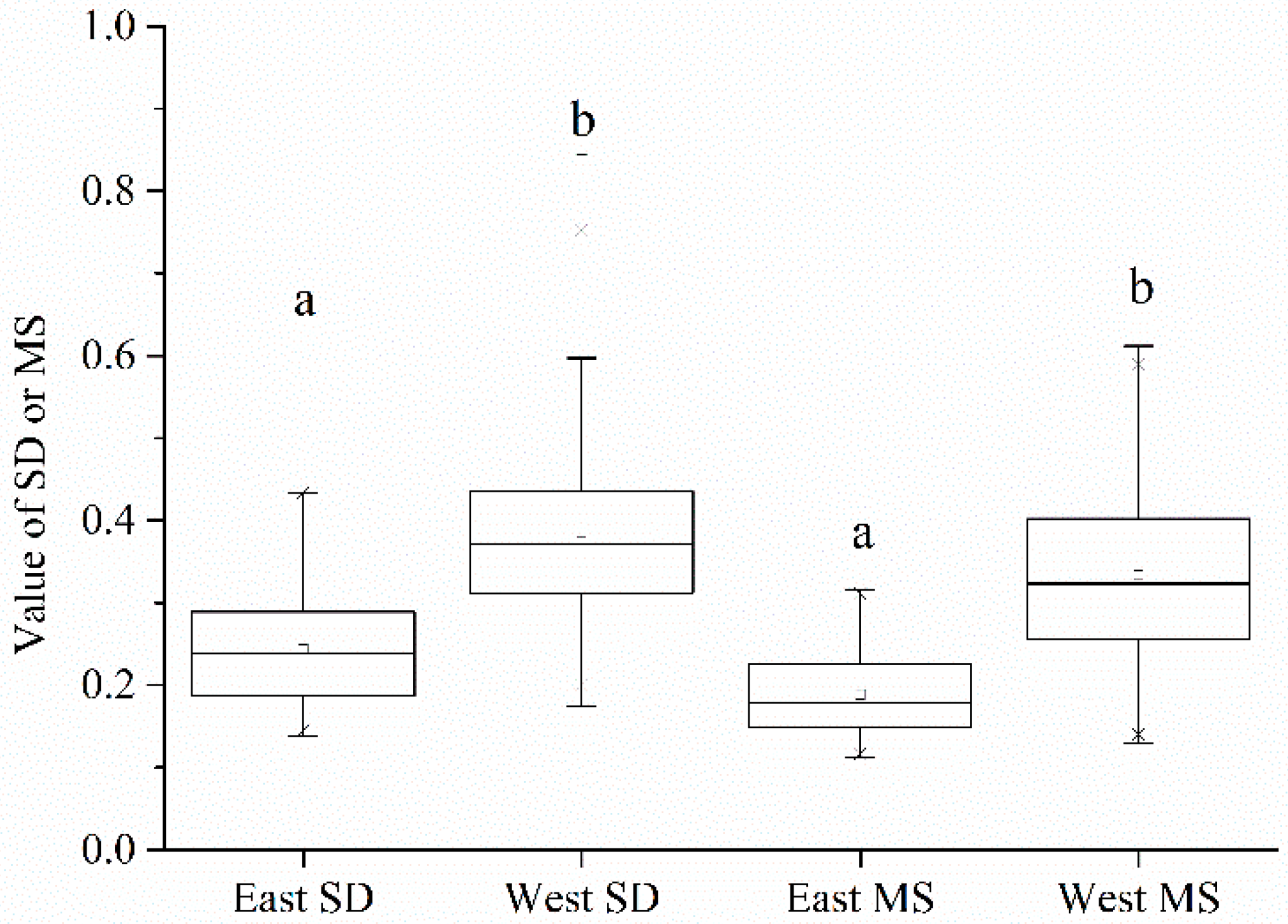
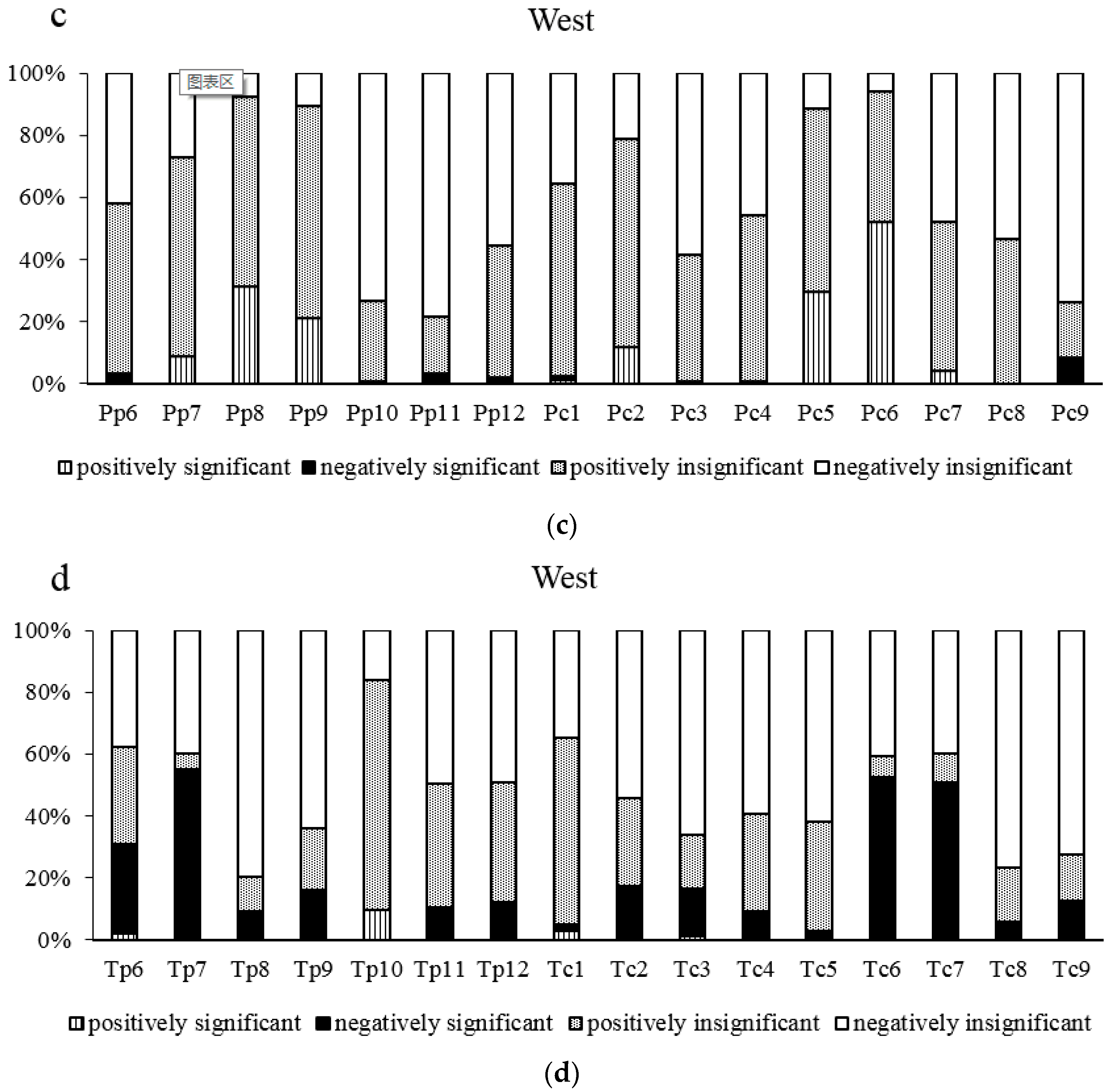
3.1. Chronology Parameters and Their Relationship with the Age of Trees in the East and West
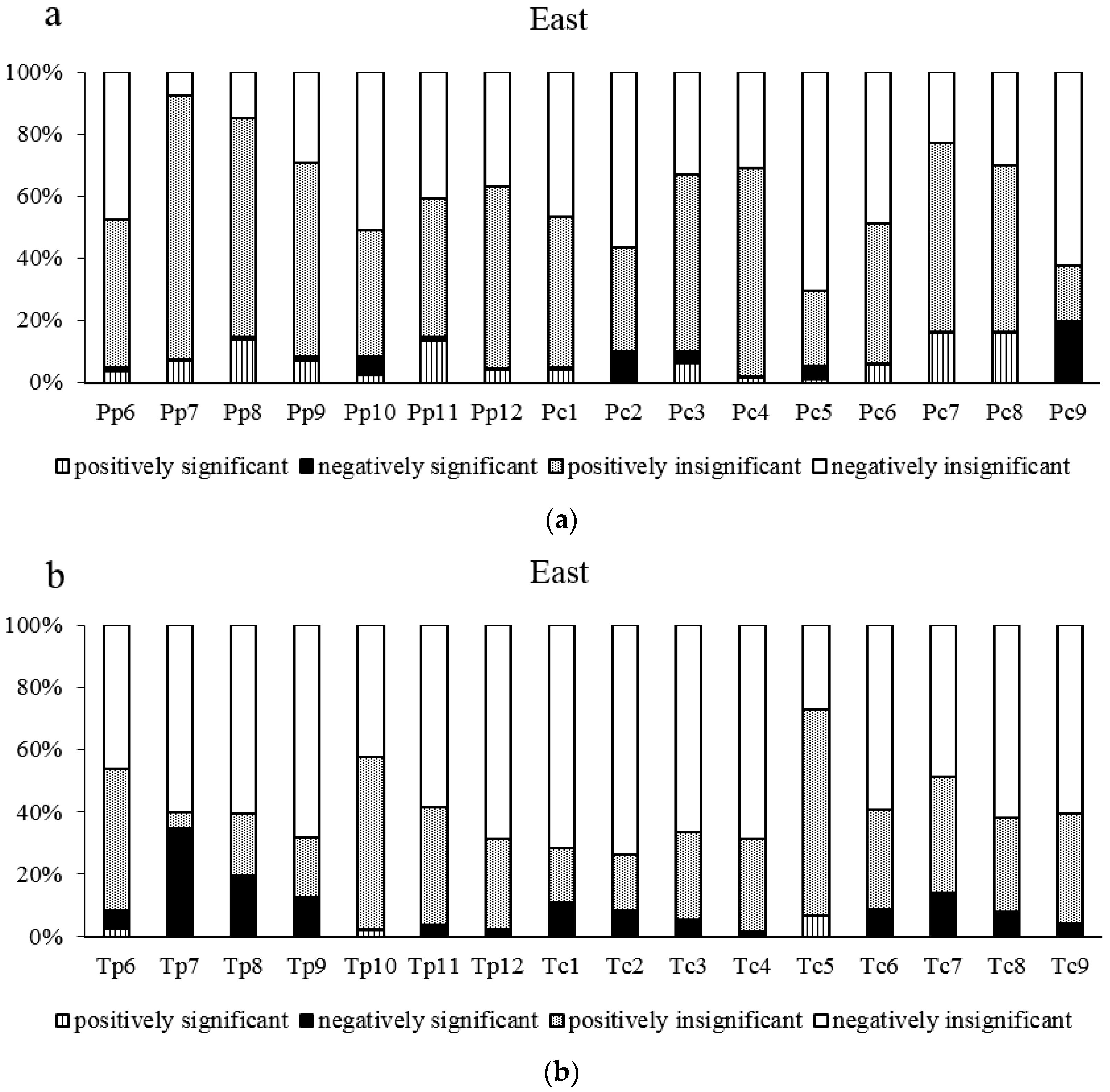
3.2. Analysis of Climate-Radial Growth Relationships in the East and West
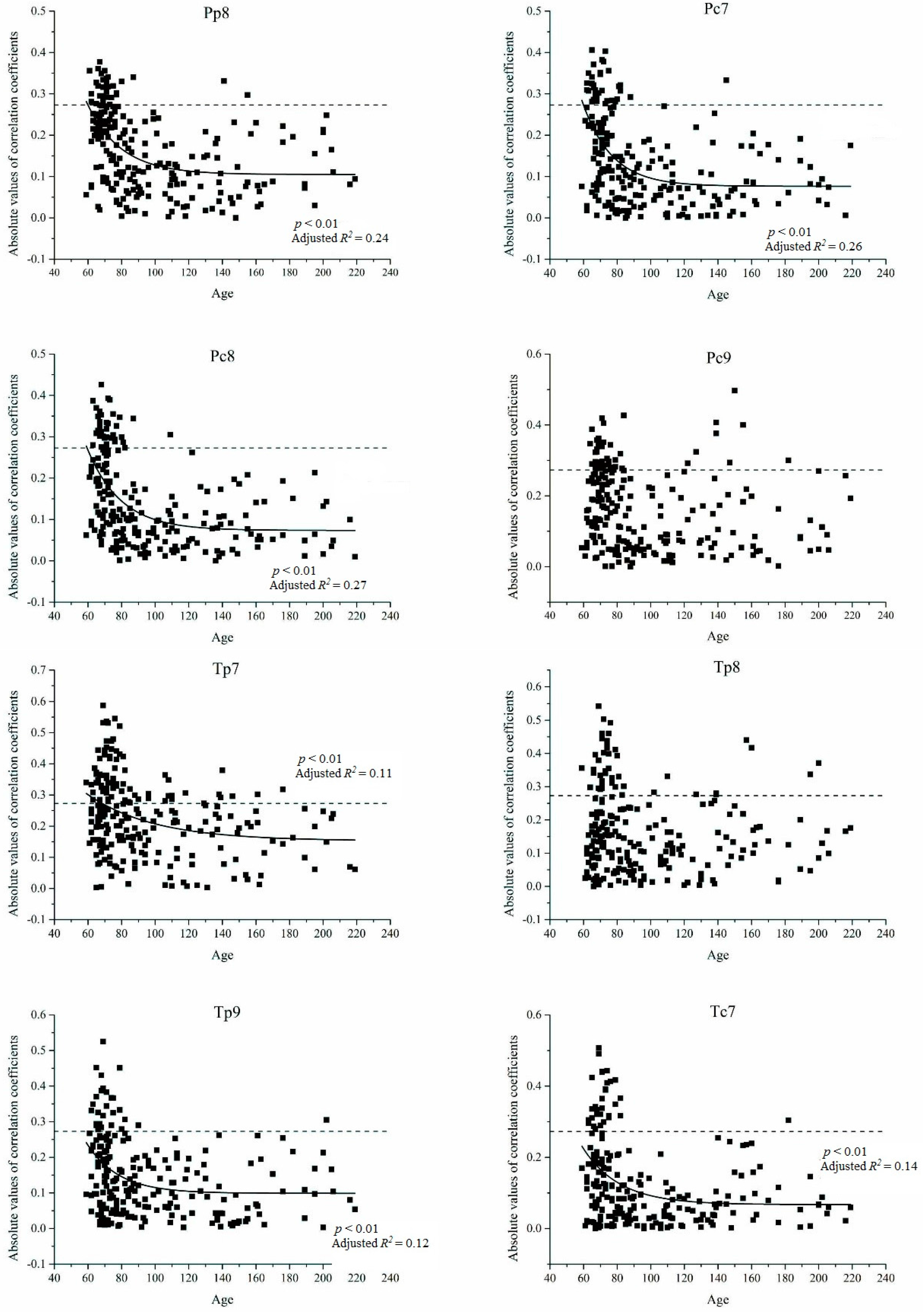
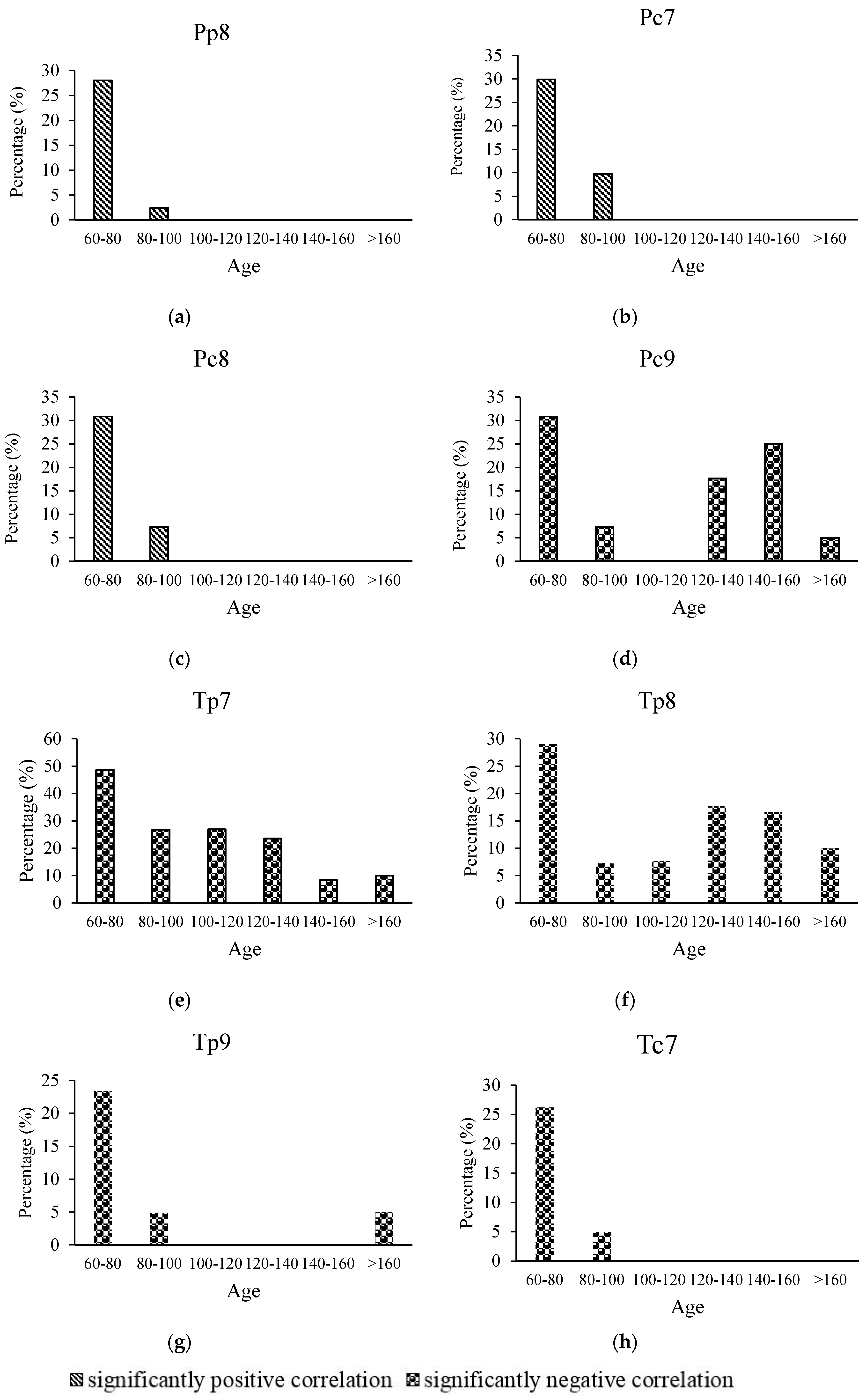
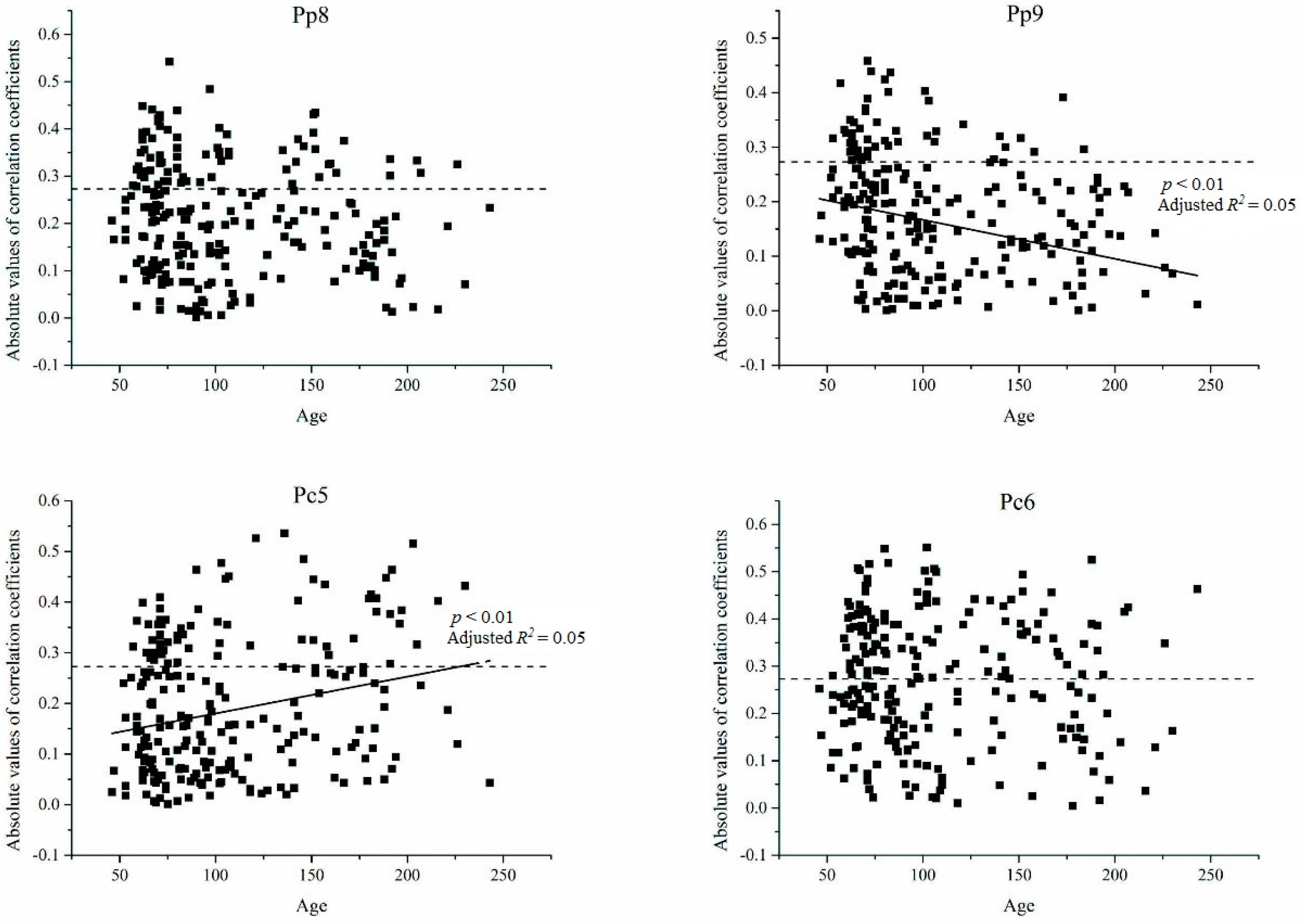
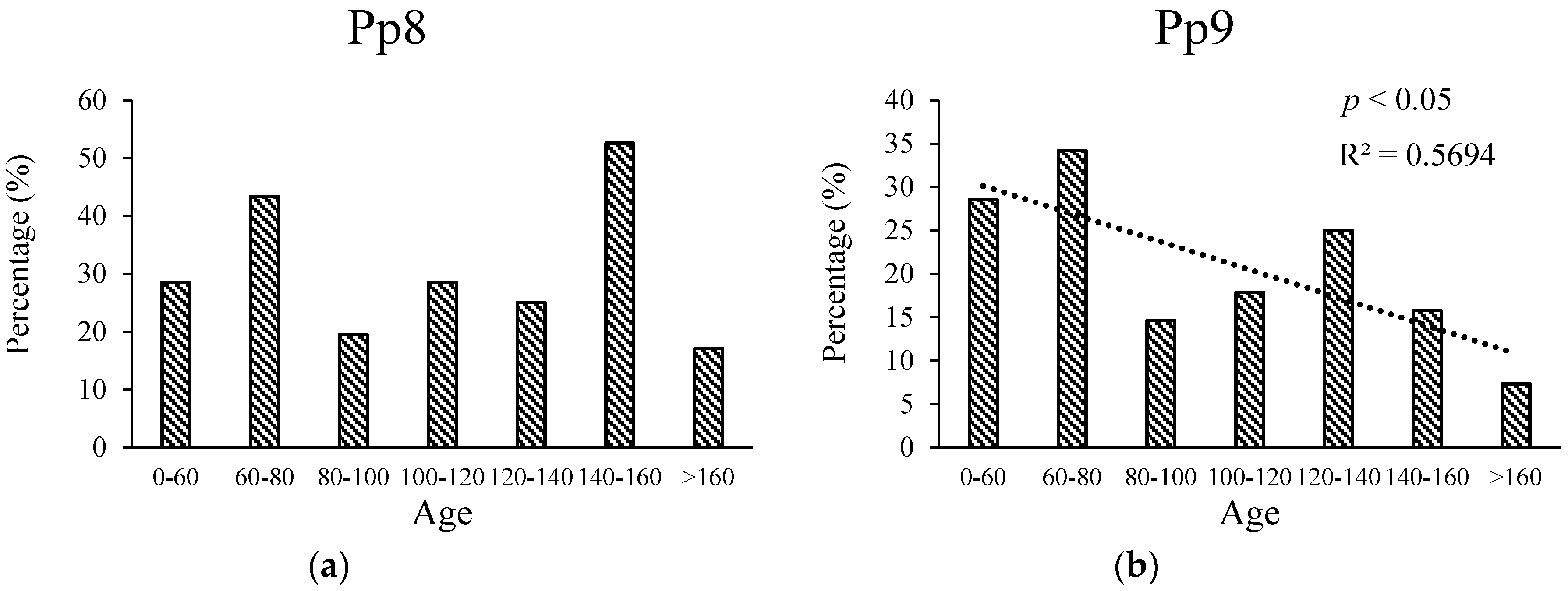
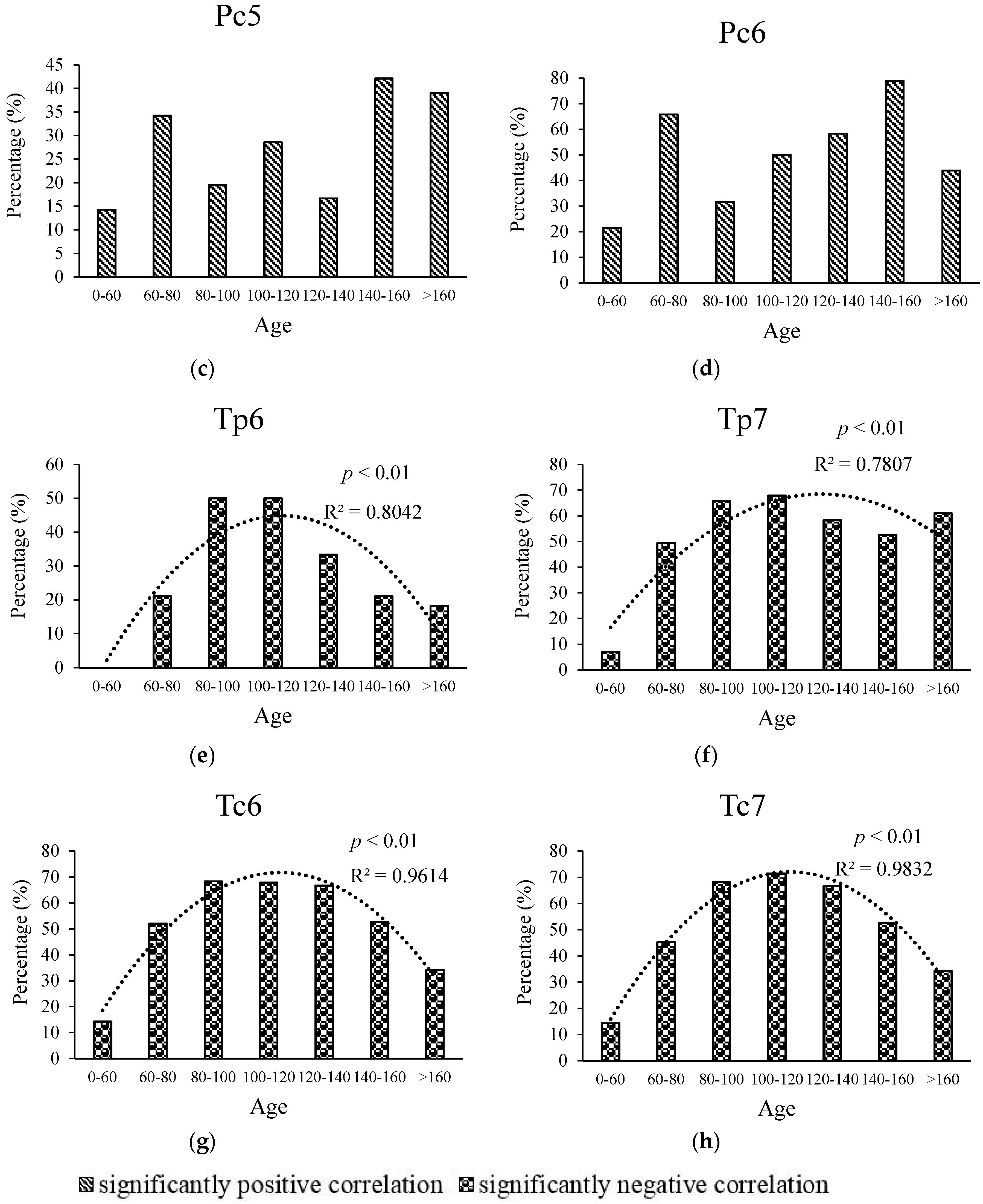
3.3. Relationship between Age and Radial Growth’s Response to Climate in the East and West
4. Discussion
4.1. Different Response of Radial Growth to Climate in the Different Moisture Environments
4.2. Relationship between Age and Climate Sensitivity of Radial Growth in the Wetter Eastern Area
4.3. Relationship between Age and Climate Sensitivity of Radial Growth in the Drier Western Area
4.4. Comparison and Future Perspectives
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Elliott, K.J.; Miniat, C.F.; Pederson, N.; Laseter, S.H. Forest tree growth response to hydroclimate variability in the southern Appalachians. Glob. Chang. Biol. 2015, 21, 4627. [Google Scholar] [CrossRef] [PubMed]
- Griesbauer, H.P.; Green, D.S. Geographic and temporal patterns in white spruce climate-growth relationships in Yukon, Canada. For. Ecol. Manag. 2012, 267, 215–227. [Google Scholar] [CrossRef]
- Suvanto, S.; Nojd, P.; Henttonen, H.M.; Beuker, E.; Makinen, H. Geographical patterns in the radial growth response of Norway spruce provenances to climatic variation. Agric. For. Meteorol. 2016, 222, 10–20. [Google Scholar] [CrossRef]
- Lee, E.H.; Beedlow, P.A.; Waschmann, R.S.; Tingey, D.T.; Wickham, C.; Cline, S.; Bollman, M.; Carlile, C. Douglas-fir displays a range of growth responses to temperature, water, and Swiss needle cast in western Oregon, USA. Agric. For. Meteorol. 2016, 221, 176–188. [Google Scholar] [CrossRef]
- Liang, E.; Shao, X.M.; Eckstein, D.; Liu, X.H. Spatial variability of tree growth along a latitudinal transect in the Qilian Mountains, northeastern Tibetan Plateau. Can. J. For. Res. 2010, 40, 200–211. [Google Scholar] [CrossRef]
- Raney, P.A.; Leopold, D.J.; Dovciak, M.; Beier, C.M. Hydrologic position mediates sensitivity of tree growth to climate: Groundwater subsidies provide a thermal buffer effect in wetlands. For. Ecol. Manag. 2016, 379, 70–80. [Google Scholar] [CrossRef]
- Levesque, M.; Rigling, A.; Bugmann, H.; Weber, P.; Brang, P. Growth response of five co-occurring conifers to drought across a wide climatic gradient in Central Europe. Agric. For. Meteorol. 2014, 197, 1–12. [Google Scholar] [CrossRef]
- Schuster, R.; Oberhuber, W. Age-dependent climate-growth relationships and regeneration of Picea abies in a drought-prone mixed-coniferous forest in the Alps. Can. J. For. Res. 2013, 43, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Voelker, S.L. Age-dependent changes in environmental influences on tree growth and their implications for forest responses to climate change. Tree Physiol. 2011, 4, 455–479. [Google Scholar] [CrossRef]
- Wu, G.J.; Xu, G.B.; Chen, T.; Liu, X.H.; Zhang, Y.F.; An, W.L.; Wang, W.Z.; Fang, Z.A.; Yu, S.L. Age-dependent tree-ring growth responses of Schrenk spruce (Picea schrenkiana) to climate-A case study in the Tianshan Mountain, China. Dendrochronologia 2013, 31, 318–326. [Google Scholar] [CrossRef]
- Copenheaver, C.A.; Crawford, C.J.; Fearer, T.M. Age-specific responses to climate identified in the growth of Quercus alba. Trees-Struct. Funct. 2011, 25, 647–653. [Google Scholar] [CrossRef]
- Li, X.; Liang, E.; Gričar, J.; Prislan, P.; Rossi, S.; Čufar, K. Age dependence of xylogenesis and its climatic sensitivity in Smith fir on the south-eastern Tibetan Plateau. Tree Physiol. 2013, 33, 48–56. [Google Scholar] [CrossRef] [PubMed]
- McMillan, A.M.S.; Winston, G.C.; Goulden, M.L. Age-dependent response of boreal forest to temperature and rainfall variability. Glob. Chang. Biol. 2008, 14, 1904–1916. [Google Scholar] [CrossRef]
- Rozas, V.; DeSoto, L.; Olano, J.M. Sex-specific, age-dependent sensitivity of tree-ring growth to climate in the dioecious tree Juniperus thurifera. New Phytol. 2009, 182, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Gou, X.; Liu, W.; Levia, D.F.; Li, Y. Individual and time-varying tree-ring growth to climate sensitivity of Pinus tabuliformis Carr. and Sabina przewalskii Kom. in the eastern Qilian Mountains, China. Trees-Struct. Funct. 2013, 27, 359–370. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Gao, L. Age-dependent growth responses of Pinus koraiensis to climate in the north slope of Changbai Mountain, North-Eastern China. Acta Ecol. Sin. 2011, 31, 6378–6387. (In Chinese) [Google Scholar]
- Fang, K.Y.; Chen, D.; Gou, X.H.; D’Arrigo, R.; Davi, N. Influence of non-climatic factors on the relationships between tree growth and climate over the Chinese Loess Plateau. Glob. Planet Chang. 2015, 132, 54–63. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhou, P.; Zhang, F.Q.; Liu, X.D.; Chen, G. Spatiotemporal characteristics of dryness/wetness conditions across Qinghai Province, Northwest China. Agric. For. Meteorol. 2013, 182, 101–108. [Google Scholar] [CrossRef]
- Liu, X.C. Picea Crassifolia; Lanzhou University Press: Lanzhou, China, 1992; pp. 13–15. (In Chinese) [Google Scholar]
- He, Z.B.; Zhao, W.Z.; Liu, H.; Zhang, Z.H. Successional process of Picea crassifolia forest after logging disturbance in semiarid mountains: A case study in the Qilian Mountains, northwestern China. For. Ecol. Manag. 2010, 260, 396–402. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wilmking, M. Divergent growth responses and increasing temperature limitation of Qinghai spruce growth along an elevation gradient at the northeast Tibet Plateau. For. Ecol. Manag. 2010, 260, 1076–1082. [Google Scholar] [CrossRef]
- Liang, E.Y.; Leuschner, C.; Dulamsuren, C.; Wagner, B.; Hauck, M. Global warming-related tree growth decline and mortality on the north-eastern Tibetan plateau. Clim. Chang. 2016, 134, 163–176. [Google Scholar] [CrossRef]
- Galván, J.D.; Camarero, J.J.; Gutiérrez, E. Seeing the trees for the forest: Drivers of individual growth responses to climate in Pinus uncinata mountain forests. J. Ecol. 2014, 102, 1244–1257. [Google Scholar] [CrossRef]
- Rozas, V. Individual-based approach as a useful tool to disentangle the relative importance of tree age, size and inter-tree competition in dendroclimatic studies. iForest 2015, 8, 187–194. [Google Scholar] [CrossRef]
- Liang, E.Y.; Shao, X.M.; Eckstein, D.; Huang, L.; Liu, X.H. Topography- and species-dependent growth responses of Sabina przewalskii and Picea crassifolia to climate on the northeast Tibetan Plateau. For. Ecol. Manag. 2006, 236, 268–277. [Google Scholar] [CrossRef]
- Li, D.L.; Liu, D.X. Climate in Gansu; China Meteorological Press: Beijing, China, 2000; pp. 7–9. [Google Scholar]
- Tang, X.; Sun, G.W.; Qian, W.H. Study on the Northernmost Boundary of East-Asian Summer Monsoon; China Meteorological Press: Beijing, China, 2007; pp. 9–11. [Google Scholar]
- Fritts, H. Tree Rings and Climate; Elsevier: Amsterdam, The Netherlands, 2012; pp. 21–25. [Google Scholar]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology: Applications in the Environmental Sciences; Springer: Berlin, Germany, 1990; pp. 18–21. [Google Scholar]
- Rinn, F. TSAPWin: Time Series Analysis and Presentation for Dendrochronology and Related Applications; Rinn Tech: Heidelberg, Germany, 2003. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Cook, E.R. A Time Series Analysis Approach to Tree Ring Standardization. Ph.D. Thesis, The University of Arizona, Tucson, AZ, USA, 1985. [Google Scholar]
- Rahman, M.R.; Lateh, H. Spatio-temporal analysis of warming in Bangladesh using recent observed temperature data and GIS. Clim. Dyn. 2015, 46, 2943–2960. [Google Scholar] [CrossRef]
- Zhang, L.N.; Jiang, Y.; Zhao, S.D.; Kang, X.Y.; Zhang, W.T.; Liu, T. Lingering response of radial growth of Picea crassifolia to climate at different altitudes in the Qilian Mountains, Northwest China. Trees-Struct. Funct. 2016, 31, 455–465. [Google Scholar] [CrossRef]
- Applequist, M.B. A Simple Pith Locator for Use with Off-Center Increment Cores. J. For. Res. 1958, 56, 141. [Google Scholar]
- Bigler, C.; Veblen, T.T. Increased early growth rates decrease longevities of conifers in subalpine forests. Oikos 2009, 118, 1130–1138. [Google Scholar] [CrossRef]
- Fritts, H.C. Growth-rings of trees: Their correlation with climate. Science 1966, 154, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Liang, E.Y.; Shao, X.M.; Liu, X.H. Annual precipitation variation inferred from tree rings since AD 1770 for the western Qilian Mts., northern Tibetan Plateau. Tree-Ring Res. 2009, 65, 95–103. [Google Scholar] [CrossRef]
- Gao, L.L.; Gou, X.H.; Deng, Y.; Yang, M.X.; Zhao, Z.Q.; Cao, Z.Y. Dendroclimatic Response of Picea crassifolia along an Altitudinal Gradient in the Eastern Qilian Mountains, Northwest China. Arct. Antarct. Alp. Res. 2013, 45, 491–499. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.J.; Wei, W.S.; Zhang, R.B.; Yu, S.L.; Shang, H.M.; Zhang, T.W.; Qin, L.; Wang, H.Q.; Chen, F.H. Tree-ring-based annual precipitation reconstruction for the Hexi Corridor, NW China: Consequences for climate history on and beyond the mid-latitude Asian continent. Boreas 2013, 42, 1008–1021. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, L.; Ma, F.; Yao, B.; Liu, J. Altitudinal differences in the leaf fitness of juvenile and mature alpine spruce trees (Picea crassifolia). Tree Physiol. 2008, 28, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zang, R.; Li, C. Physiological and morphological variations of Picea asperata populations originating from different altitudes in the mountains of southwestern China. For. Ecol. Manag. 2006, 221, 285–290. [Google Scholar] [CrossRef]
- Brown, J.M. Photosynthetic Regime of Some Southern Arizona Ponderosa Pine. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1968. [Google Scholar]
- Wang, W.B.; Zhang, F.; Yuan, L.; Wang, Q.T.; Zheng, K.; Zhao, C.Z. Environmental factors effect on stem radial variations of Picea crassifolia in Qilian Mountains, Northwestern China. Forests 2016, 7, 210. [Google Scholar] [CrossRef]
- Schuster, R.; Oberhuber, W. Drought sensitivity of three co-occurring conifers within a dry inner Alpine environment. Trees-Struct. Funct. 2013, 27, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Huang, J.-G.; Stadt, K.J.; Comeau, P.G.; Chen, H.Y.H. Spatial climate-dependent growth response of boreal mixedwood forest in western Canada. Glob. Planet Chang. 2016, 139, 141–150. [Google Scholar] [CrossRef]
- Ashiq, M.W.; Anand, M. Spatial and temporal variability in dendroclimatic growth response of red pine (Pinus resinosa Ait.) to climate in northern Ontario, Canada. For. Ecol. Manag. 2016, 372, 109–119. [Google Scholar] [CrossRef]
- Helama, S.; Laanelaid, A.; Bijak, S.; Jaagus, J. Contrasting tree-ring growth response of Picea abies to climate variability in western and eastern Estonia. Geogr. Ann. A 2016, 98, 155–167. [Google Scholar] [CrossRef]
- Carrer, M.; Urbinati, C. Age-dependent tree-ring growth responses to climate in Larix decidua and Pinus cembra. Ecology 2004, 85, 730–740. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J. Ecol. 2002, 90, 480–494. [Google Scholar] [CrossRef]
- Haavikl, J.; Stahled, W.; Stephenf, M. Temporal aspects of Quercus rubra decline and relationship to climate in the Ozark and Ouachita Mountains, Arkansas. Can. J. For. Res. 2011, 41, 773–781. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Oribe, Y.; Kubo, T.; Funada, R. Cambial sensitivity to rising temperatures by natural condition and artificial heating from late winter to early spring in the evergreen conifer Cryptomeria japonica. Trees-Struct. Funct. 2010, 24, 43–52. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carrer, M. Age-dependent xylogenesis in timberline conifers. New Phytol. 2008, 177, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Deslauriers, A.; Griçar, J.; Seo, J.W.; Rathgeber, C.B.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Moreno-Gutiérrez, C.; Battipaglia, G.; Cherubini, P.; Huertas, A.D.; Querejeta, J.I. Pine afforestation decreases the long-term performance of understorey shrubs in a semi-arid Mediterranean ecosystem: A stable isotope approach. Funct. Ecol. 2014, 29, 19–25. [Google Scholar] [CrossRef]
- Yu, G.; Liu, Y.; Wang, X.; Ma, K. Age-dependent tree-ring growth responses to climate in Qilian juniper (Sabina przewalskii Kom.). Trees-Struct. Funct. 2008, 22, 197–204. [Google Scholar] [CrossRef]
- Primicia, I.; Camarero, J.J.; Janda, P.; Čada, V.; Morrissey, R.C.; Trotsiuk, V.; Bače, R.; Teodosiu, M.; Svoboda, M. Age, competition, disturbance and elevation effects on tree and stand growth response of primary Picea abies forest to climate. For. Ecol. Manag. 2015, 354, 77–86. [Google Scholar] [CrossRef]
- Macdonald, G.M.; Szeicz, J.M. Age dependent tree ring growth response of subarctic white spruce to climate. Can. J. For. Res. 1994, 24, 120–132. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2013: The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
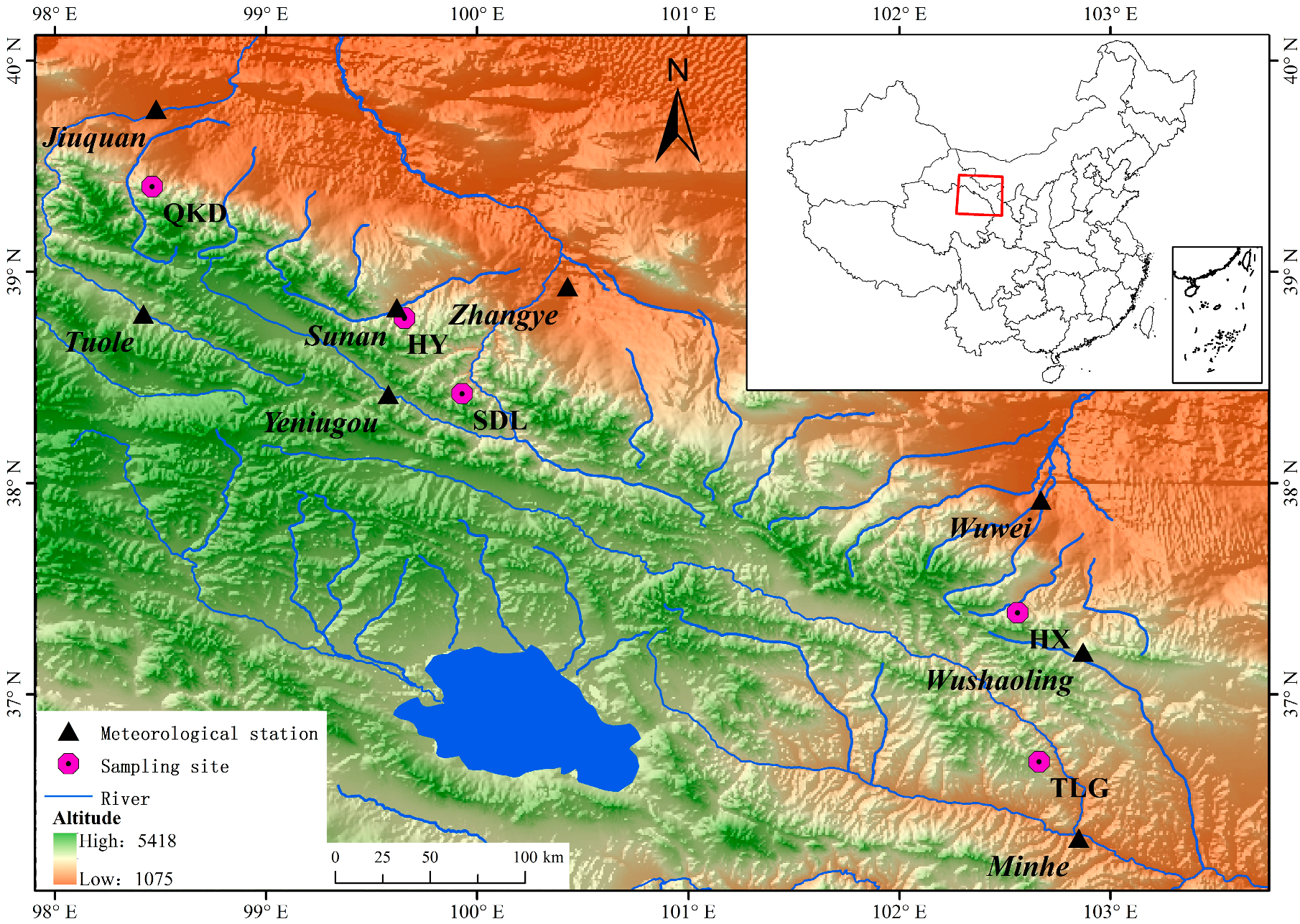
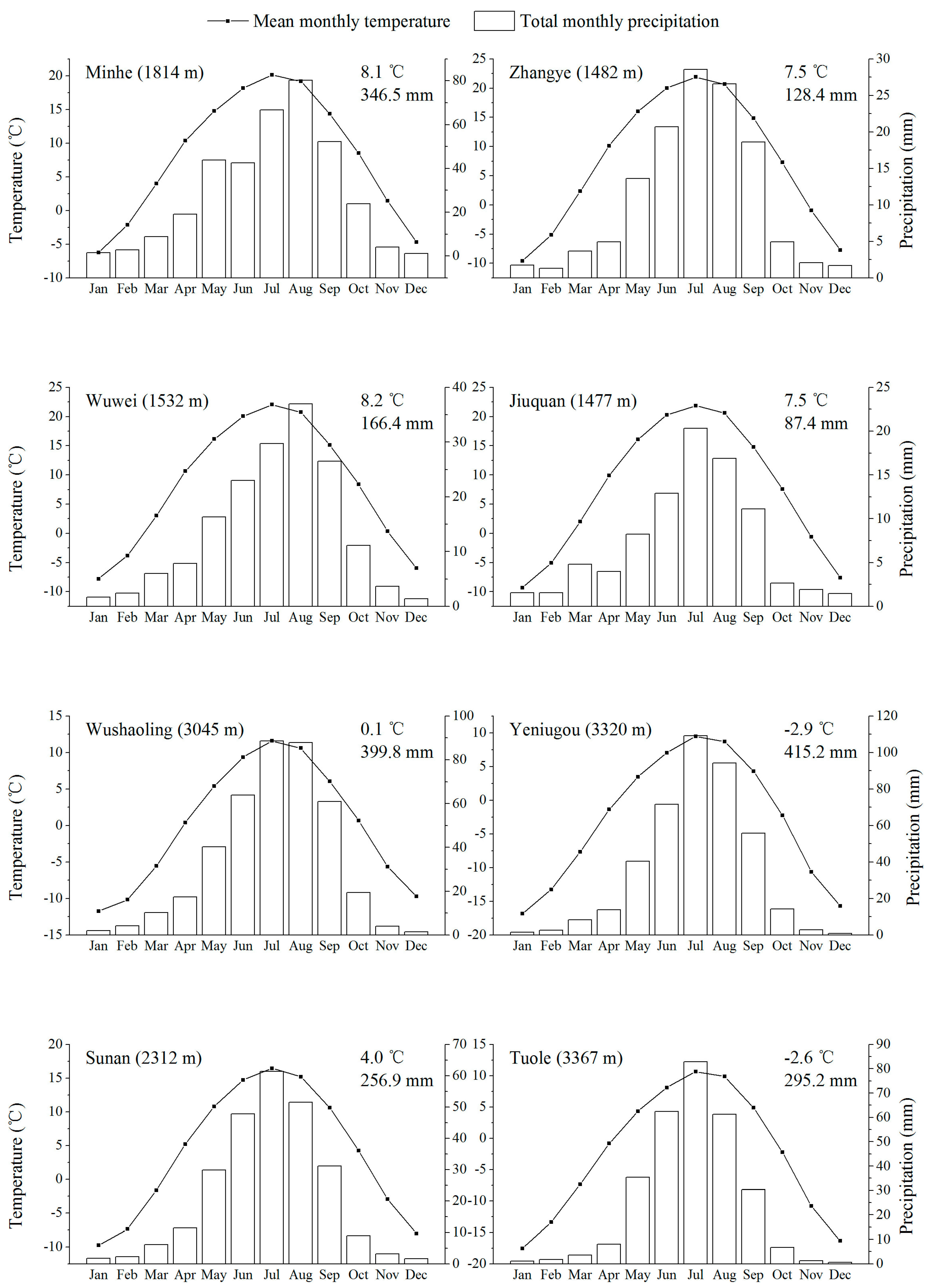


| Site | Altitude (m a.s.l.) | Latitude (°N) | Longitude (°E) | Slope Aspect | Slope | No. of Trees | Climate Data From |
|---|---|---|---|---|---|---|---|
| TLG1 | 2922 | 36.68 | 102.66 | northeast | 30 | 25 | Wushaoling |
| TLG2 | 2802 | 36.68 | 102.66 | northeast | 26 | 25 | Wushaoling |
| HX1 | 2890 | 37.38 | 102.56 | northeast | 33 | 25 | Wuwei, Wushaoling |
| HX2 | 2697 | 37.39 | 102.57 | northeast | 25 | 25 | Wuwei, Wushaoling |
| SDL1 | 3200 | 38.42 | 99.93 | northeast | 31 | 25 | Qilian, Yeniugou |
| SDL2 | 2649 | 38.45 | 99.91 | northeast | 26 | 25 | Qilian, Yeniugou |
| HYG1 | 3086 | 38.78 | 99.65 | northeast | 35 | 25 | Sunan, Yeniugou |
| HYG2 | 2837 | 38.79 | 99.66 | north | 25 | 25 | Sunan, Yeniugou |
| HYG3 | 2654 | 38.79 | 99.67 | north | 27 | 25 | Sunan, Yeniugou |
| QKD | 2896 | 39.40 | 98.46 | north | 28 | 25 | Jiuquan and Tuole |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jiang, Y.; Zhao, S.; Jiao, L.; Wen, Y. Relationships between Tree Age and Climate Sensitivity of Radial Growth in Different Drought Conditions of Qilian Mountains, Northwestern China. Forests 2018, 9, 135. https://doi.org/10.3390/f9030135
Zhang L, Jiang Y, Zhao S, Jiao L, Wen Y. Relationships between Tree Age and Climate Sensitivity of Radial Growth in Different Drought Conditions of Qilian Mountains, Northwestern China. Forests. 2018; 9(3):135. https://doi.org/10.3390/f9030135
Chicago/Turabian StyleZhang, Lingnan, Yuan Jiang, Shoudong Zhao, Liang Jiao, and Yan Wen. 2018. "Relationships between Tree Age and Climate Sensitivity of Radial Growth in Different Drought Conditions of Qilian Mountains, Northwestern China" Forests 9, no. 3: 135. https://doi.org/10.3390/f9030135





