First-Year Vitality of Reforestation Plantings in Response to Herbivore Exclusion on Reclaimed Appalachian Surface-Mined Land
Abstract
:1. Introduction
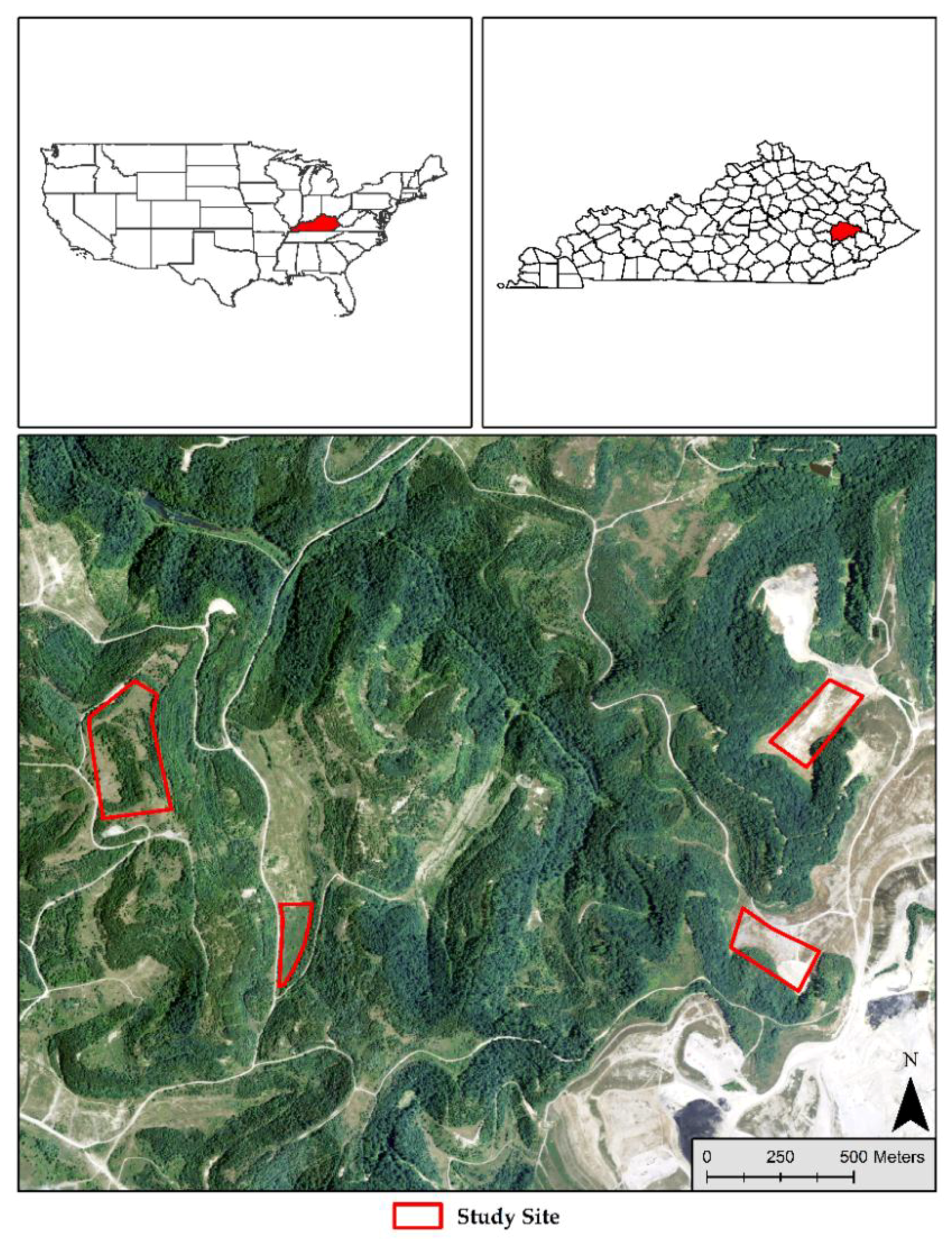
2. Materials and Methods
2.1. Plot Design and Data Collection
2.2. Statistical Analysis
3. Results
3.1. Survival
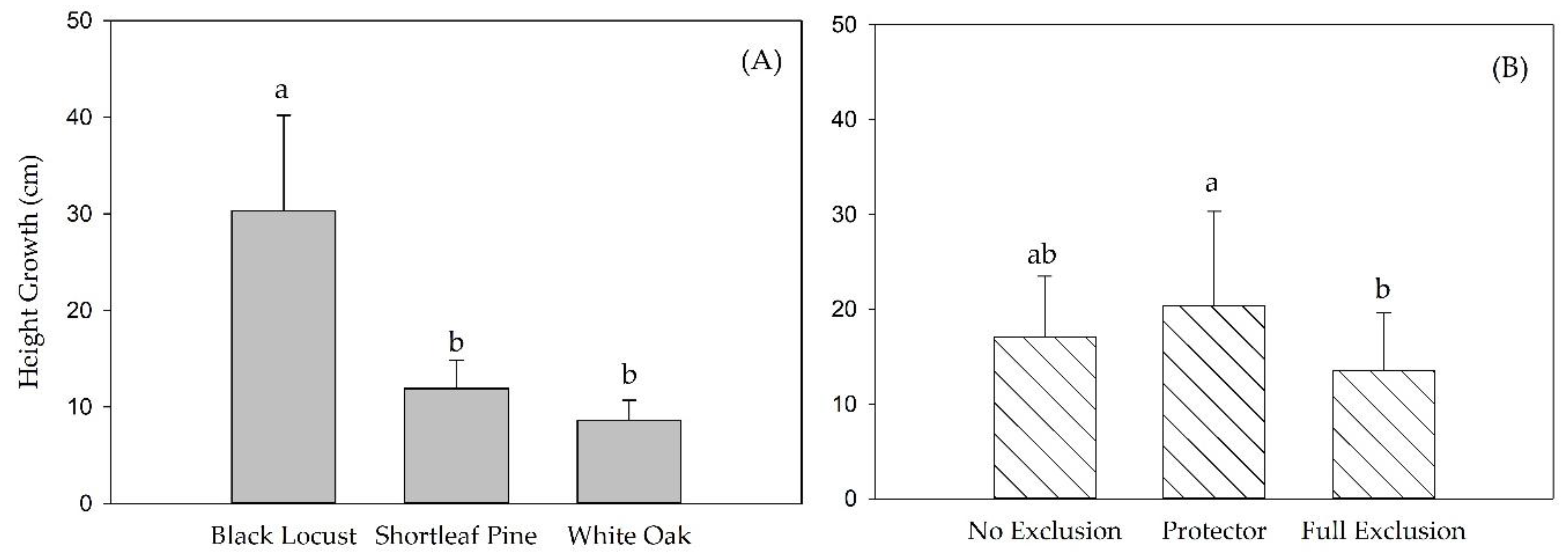
3.2. Height Growth
3.3. Herbivory
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- National Mining Association. Available online: https://nma.org/wp-content/uploads/2017/11/ Mine-Reclamation-2017-2.pdf (accessed on 18 March 2018).
- Wickham, J.D.; Riitters, K.H.; Wade, T.G.; Coan, M.; Homer, C. The effect of Appalachian mountaintop mining on interior forest. Landsc. Ecol. 2007, 22, 179–187. [Google Scholar] [CrossRef]
- Wickham, J.D.; Wood, P.B.; Nicholson, M.C.; Jenkins, W.; Druckenbrod, D.; Suter, G.W.; Strager, M.P.; Mazzarella, C.; Galloway, W.; Amos, J. The overlooked terrestrial impacts of mountaintop mining. Bioscience 2013, 63, 335–348. [Google Scholar] [CrossRef]
- Thurman, N.C.; Sencindiver, J.C. Properties, classification, and interpretations of minesoils at two sites in West Virginia. Soil Sci. Soc. Am. J. 1986, 50, 181–185. [Google Scholar] [CrossRef]
- Thompson, P.J.; Jansen, I.J.; Hooks, C.L. Penetrometer resistance and bulk density as parameters for predicting root system performance in mine soils. Soil Sci. Soc. Am. J. 1987, 51, 1288–1293. [Google Scholar] [CrossRef]
- Chong, S.K.; Cowsart, P.T. Infiltration in reclaimed mined land ameliorated with deep tillage treatments. Soil Tillage Res. 1997, 44, 255–264. [Google Scholar] [CrossRef]
- Conrad, P.W.; Sweigard, R.J.; Graves, D.H.; Ringe, J.M.; Pelkki, M.H. Impacts of spoil conditions on reforestation of surface mined land. Min. Eng. 2002, 54, 39–46. [Google Scholar]
- Evans, D.M.; Zipper, C.E.; Burger, J.A.; Strahm, B.D.; Villamagna, A.M. Reforestation practice for enhancement of ecosystem services on a compacted surface mine: Path toward ecosystem recovery. Ecol. Eng. 2013, 51, 16–23. [Google Scholar] [CrossRef]
- Bohrer, S.L.; Limb, R.F.; Daigh, A.L.; Volk, J.M.; Wick, A.F. Fine and coarse-scale patterns of vegetation diversity on reclaimed surface mine-land over a 40-year chronosequence. Environ. Manag. 2017, 59, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, A.J.; Wynne, R.H.; Zipper, C.E.; Ford, W.M.; Donovan, P.F.; Li, J. Autumn olive (Elaeagnus umbellata) presence and proliferation on former surface coal mines in Eastern USA. Biol. Invasions 2017, 19, 179–195. [Google Scholar] [CrossRef]
- Zipper, C.E.; Burger, J.A.; Skousen, J.G.; Angel, P.N.; Barton, C.D.; Davis, V.; Franklin, J.A. Restoring forests and associated ecosystem services on Appalachian coal surface mines. Environ. Manag. 2011, 47, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Zipper, C.E.; Angel, P.N.; Hall, N.; Skousen, J.G.; Barton, C.D.; Eggerud, S. Establishing Native Trees on Legacy Surface Mines, Forest Reclamation Advisory No. 11; USDOI Office of Surface Mining: Washington, DC, USA, 2013. [Google Scholar]
- Swihart, R.K.; Picone, P.M. Selection of mature growth stages of coniferous browse in temperate forests by white-tailed deer (Odocoileus virginianus). Am. Midl. Nat. 1998, 139, 269–274. [Google Scholar] [CrossRef]
- Cleavitt, N.L.; Berry, E.J.; Hautaniemi, J.; Fahey, T.J. Life stages, demographic rates, and leaf damage for the round-leaved orchids, Platanthera orbiculata (Pursh.) Lindley and P. macrophylla (Goldie) PM Brown in a northern hardwood forest in New Hampshire, USA. Botany 2017, 95, 61–71. [Google Scholar] [CrossRef]
- Dostaler, S.; Ouellet, J.P.; Therrien, J.F.; Cote, S.D. Are feeding preferences of white-tailed deer related to plant constituents? J. Wildl. Manag. 2011, 75, 913–918. [Google Scholar] [CrossRef]
- Burney, O.T.; Jacobs, D.F. Ungulate herbivory of regenerating conifers in relation to foliar nutrition and terpenoid production. For. Ecol. Manag. 2011, 262, 1834–1845. [Google Scholar] [CrossRef]
- Augustine, D.J.; McNaughton, S.J. Ungulate effects on the functional species composition of plant communities: Herbivore selectivity and plant tolerance. J. Wildl. Manag. 1998, 62, 1165–1183. [Google Scholar] [CrossRef]
- Champagne, E.; Perroud, L.; Dumont, A.; Tremblay, J.P.; Cote, S.D. Neighbouring plants and perception of predation risk modulate winter browsing by white-tailed deer (Odocoileus virginianus). Can. J. Zool. 2018, 96, 117–125. [Google Scholar] [CrossRef]
- Rodel, H.G.; Volkl, W.; Kilias, H. Winter browsing of brown hares: Evidence for diet breadth expansion. Mamm. Biol. 2004, 69, 410–419. [Google Scholar] [CrossRef]
- Lehndal, L.; Agren, J. Latitudinal variation in resistance and tolerance to herbivory in the perennial herb Lythrum salicaria is related to intensity of herbivory and plant phenology. J. Evol. Biol. 2015, 28, 576–589. [Google Scholar] [CrossRef] [PubMed]
- White, C.A.; Olmsted, C.E.; Kay, C.E. Aspen, elk, and fire in the Rocky Mountain national parks of North America. Wildl. Soc. Bull. 1998, 26, 449–462. [Google Scholar]
- Ripple, W.J.; Beschta, R.L. Hardwood tree decline following large carnivore loss on the Great Plains, USA. Front. Ecol. Environ. 2007, 5, 241–246. [Google Scholar] [CrossRef]
- Jenkins, L.H.; Murrary, B.D.; Jenkins, M.A.; Webster, C.R. Woody regeneration response to over a decade of deer population reductions in Indiana state parks. J. Torrey Bot. Soc. 2015, 142, 205–219. [Google Scholar] [CrossRef]
- McGraw, J.B.; Furedi, M.A. Deer browsing and population viability of a forest understory plant. Science 2005, 307, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Leege, L.M.; Thompson, J.S.; Parris, D.J. The response of rare and common trilliums (Trillium reliquum, T-cuneatum, and T-maculatum) to deer herbivory and invasive honeysuckle removal. Castanea 2010, 75, 433–443. [Google Scholar] [CrossRef]
- Bradshaw, L.; Waller, D.M. Impacts of white-tailed deer on regional patterns of forest tree recruitment. For. Ecol. Manag. 2016, 375, 1–11. [Google Scholar] [CrossRef]
- Barton, C.D.; University of Kentucky, Lexington, KY, USA. Personal communication, 2016.
- Brinks, J.S.; Lhotka, J.M.; Barton, C.D.; Warner, R.C.; Agouridis, C.T. Effects of fertilization and irrigation on American sycamore and black locust on a reclaimed surface mine in Appalachia. For. Ecol. Manag. 2011, 261, 640–648. [Google Scholar] [CrossRef]
- Agouridis, C.; Barton, C.; Warner, R. Recreating a headwater stream system on a valley fill in the Appalachian coal field. In Spoil to Soil: Mine Site Rehabilitation and Revegetation; Bolan, N., Kirkham, M.B., Ok, Y.S., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2018; pp. 147–174. [Google Scholar]
- Larkin, J.L.; Maehr, D.S.; Krupa, J.J.; Cox, J.J.; Alexy, K.; Under, D.E.; Barton, C.D. Small mammal response to vegetation and spoil conditions on a reclaimed surface mine in eastern Kentucky. Southeast. Nat. 2008, 7, 401–412. [Google Scholar] [CrossRef]
- Thomas, G. Soil pH and soil acidity. In Methods of Soil Analysis Part 3—Chemical Methods; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Soil and Plant Analysis Council. Soil Analysis Handbook of Reference Methods; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Miller, W.; Miller, D. A micro-pipette method for soil mechanical analysis. Commun. Soil Sci. Plant Anal. 1987, 18, 1–15. [Google Scholar] [CrossRef]
- Summer, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D., Bartels, J.M., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Emerson, P.; Skousen, J.; Ziemkiewicz, P. Survival and growth of hardwoods in brown versus gray sandstone on a surface mine in West Virginia. J. Environ. Qual. 2009, 38, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.; Sena, K.L.; Barton, C.D.; French, M. Establishing pine monocultures and mixed pine-hardwood stands on reclaimed surface mined land in eastern Kentucky: Implications for forest resilience in a changing climate. Forests 2017, 8, 375. [Google Scholar] [CrossRef]
- Taylor, M.; Haase, D.L.; Rose, R.L. Fall planting and tree shelters for reforestation in the east Washington Cascades. West. J. Appl. For. 2009, 24, 173–179. [Google Scholar]
- Dick, K.; Alexander, H.D.; Moczygemba, J.D. Use of shelter tubes, grass-specific herbicide, and herbivore exclosures to reduce stressors and improve restoration of semiarid thornscrub forests. Restor. Ecol. 2016, 24, 785–793. [Google Scholar] [CrossRef]
- Piiroinen, T.; Valtonen, A.; Roininen, H. The seed-to-seedling transition is limited by ground vegetation and vertebrate herbivores in a selectively logged rainforest. For. Ecol. Manag. 2017, 384, 137–146. [Google Scholar] [CrossRef]
- Barton, C.; Miller, J.; Sena, K.; Angel, P.; French, M. Evaluating the use of tree shelters for direct seeding of Castanea on a surface mine in Appalachia. Forests 2015, 6, 3514–3527. [Google Scholar] [CrossRef]
- Kelly, D.L. The regeneration of Quercus petraea (sessile oak) in southwest Ireland: A 25-year experimental study. For. Ecol. Manag. 2002, 166, 207–226. [Google Scholar] [CrossRef]
- Drozdowski, S.; Bolibok, L.; Buraczyk, W.; Wisniowski, P. Effect of planting time and method of protection from deer on the growth of oak plantations on the former farmland. Sylwan 2011, 155, 610–621. [Google Scholar]
- Schnurr, J.; Canham, C.D. Linkages among canopy tree neighbourhoods, small mammal herbivores and herbaceous communities in temperate forests. J. Veg. Sci. 2016, 27, 980–998. [Google Scholar] [CrossRef]
- Miller, G.W.; Brose, P.H.; Gottschalk, K.W. Advanced oak seedling development as influenced by shelterwood treatments, competition control, deer fencing, and prescribed fire. J. For. 2017, 115, 179–189. [Google Scholar] [CrossRef]
- Burney, O.T.; Jacobs, D.F. Species selection—A fundamental silvicultural tool to promote forest regeneration under high animal browsing pressure. For. Ecol. Manag. 2018, 408, 67–74. [Google Scholar] [CrossRef]
- Boehm, C.; Quinkenstein, A.; Freese, D. Yield prediction of young black locust (Robinia pseudoacacia L.) plantations for woody biomass production using allometric relations. Ann. For. Res. 2011, 54, 215–227. [Google Scholar]
- Kurokochi, H.; Toyama, K. Invasive tree species Robinia pseudoacacia: A potential biomass resource in Nagano Prefecture, Japan. Small-Scale For. 2015, 14, 205–215. [Google Scholar] [CrossRef]
- Roberts, D.R.; Zimmerman, R.W.; Stringer, J.W.; Carpenter, S.B. The effects of combined nitrogen on growth, nodulation, and nitrogen fixation of black locust seedlings. Can. J. For. Res. 1983, 13, 1251–1254. [Google Scholar] [CrossRef]
- Kim, K.D.; Lee, E.J. Potential tree species for use in the restoration of unsanitary landfills. Environ. Manag. 2005, 36, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brinks, J.; Lhotka, J.; Barton, C. One-year response of American sycamore (Platanus occidentalis L.) and black locust (Robinia pseudoacacia) to granular fertilizer applications on a reclaimed surface mine in eastern Kentucky. Proc. Cent. Hard. For. Conf. 2011, 17, 306–313. [Google Scholar]
- Showalter, J.M.; Burger, J.A.; Zipper, C.E.; Galbraith, J.M.; Donovan, P.F. Influence of mine soil properties on white oak seedling growth: A proposed mine soil classification model. South. J. Appl. For. 2007, 31, 99–107. [Google Scholar]
- Kabrick, J.M.; Knapp, B.O.; Dey, D.C.; Larsen, D.R. Effect of initial seedling size, understory competition, and overstory density on the survival and growth of Pinus echinata seedlings underplanted in hardwood forests for restoration. New For. 2015, 46, 897–918. [Google Scholar] [CrossRef]
- Engeman, R.M.; Anthony, R.M.; Krupa, H.W.; Evans, J. The effects of Vexar® seedling protectors on the growth and development of lodgepole pine roots. Crop Prot. 1997, 16, 57–61. [Google Scholar] [CrossRef]
- Dubois, M.R.; Cappelka, A.H.; Robbins, E.; Somers, G.; Baker, K. Tree shelters and weed control: Effects on protection, survival and growth of cherrybark oak seedlings planted on a cutover site. New For. 2000, 20, 105–118. [Google Scholar] [CrossRef]
- Ward, J.S.; Mervosh, T.L. Strategies to reduce browse damage on eastern white pine (Pinus strobus) in southern New England, USA. For. Ecol. Manag. 2008, 255, 1559–1567. [Google Scholar] [CrossRef]
- Burger, D.W.; Svihra, P.; Harris, R. Treeshelter use in producing container-grown trees. Hortscience 1992, 27, 30–32. [Google Scholar]
- Bellot, J.; Ortiz de Urbina, J.M.; Bonet, A.; Sanchez, J.R. The effects of treeshelters on the growth of Quercus coccifera L. seedlings in a semiarid environment. Forestry 2002, 75, 89–106. [Google Scholar] [CrossRef]
- Andrews, D.M.; Barton, C.D.; Czapka, S.J.; Kolka, R.K.; Sweeney, B.W. Influence of tree shelters on seedling success in an afforested riparian zone. New For. 2010, 39, 157–167. [Google Scholar] [CrossRef]
- Tripler, C.; Canham, C.; Inouye, R.; Schnurr, J. Soil nitrogen availability, plant luxury consumption, and herbivory by white-tailed deer. Oecologia 2002, 133, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Skousen, J.; Gorman, J.; Pena-Yewtukhim, E.; King, J.; Stewart, J.; Emerson, P.; DeLong, C. Hardwood tree survival in heavy ground cover on reclaimed land in West Virginia: Mowing and Ripping Effects. J. Environ. Qual. 2009, 38, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.J. Tales of a repatriated megaherbivore: Challenges and opportunities in the management of reintroduced elk in Appalachia. Proc. Cent. Hard. For. Conf. 2011, 17, 632–642. [Google Scholar]
| Treatment | |||
|---|---|---|---|
| Parameter | No Exclusion | Protector | Full Exclusion |
| Soil pH | 5.60 ± 0.52 | 5.62 ± 0.75 | 6.08 ± 0.69 |
| P (mg/kg) | 5.94 ± 0.91 | 11.19 ± 6.06 | 6.00 ± 1.01 |
| K (mg/kg) | 71.06 ± 17.17 | 65.81 ± 14.32 | 63.94 ± 12.11 |
| Ca (mg/kg) | 580.13 ± 168.38 | 653.44 ± 183.04 | 640.44 ± 163.42 |
| Mg (mg/kg) | 251.19 ± 83.16 | 225.38 ± 75.81 | 269.06 ± 73.44 |
| Zn (mg/kg) | 3.65 ± 1.35 | 3.49 ± 1.49 | 3.49 ± 1.16 |
| Total N (%) | 0.07 ± 0.03 | 0.10 ± 0.06 | 0.08 ± 0.03 |
| Sand (%) | 52.18 ± 10.14 | 56.85 ± 8.48 | 57.25 ± 8.43 |
| Silt (%) | 33.38 ± 7.94 | 29.10 ± 6.31 | 39.33 ± 6.24 |
| Clay (%) | 14.44 ± 2.28 | 14.05 ± 2.37 | 13.42 ± 2.20 |
| CEC * (meq/100 g) | 7.09 ± 1.25 | 6.91 ± 1.40 | 6.65 ± 1.47 |
| Exch † K (meq/100 g) | 0.20 ± 0.05 | 0.18 ± 0.04 | 0.18 ± 0.04 |
| Exch Ca (meq/100 g) | 2.97 ± 1.00 | 3.11 ± 0.93 | 3.49 ± 1.01 |
| Exch Mg (meq/100 g) | 1.94 ± 0.74 | 1.69 ±0.64 | 2.17 ± 0.68 |
| Exch Na (meq/100 g) | 0.02 ± 0.003 | 0.02 ± 0.001 | 0.02 ± 0.004 |
| Treatment | |||
|---|---|---|---|
| Species | No Exclusion | Protector | Full Exclusion |
| Black Locust | 73.1b * ± 10.6 | 80.3a * ± 6.0 | 81.7a * ± 9.9 |
| Shortleaf Pine | 37.8a † ± 10.0 | 36.5a † ± 9.7 | 28.5b † ± 8.7 |
| White Oak | 68.2b * ± 10.4 | 80.5a * ± 5.3 | 80.5a * ± 6.0 |
| Treatment | |||
|---|---|---|---|
| Species | No Exclusion | Protector | Full Exclusion |
| Black Locust | 85.1a * ± 2.7 | 73.8b * ± 6.7 | 3.8c † ± 1.2 |
| Shortleaf Pine | 34.1a ‡ ± 7.0 | 2.9b ‡ ± 1.5 | 0.2c ‡ ± 0.2 |
| White Oak | 72.6a † ± 7.2 | 51.1b † ± 3.9 | 14.8c * ± 3.2 |
| Treatment | Cervid | Rabbit | Small Mammal | Domestic Animal |
|---|---|---|---|---|
| Black locust | ||||
| No Exclusion | 98.8 | 1.3 | 1.7 | - |
| Protector | 99.6 | 0.4 | - | - |
| Full Exclusion | 93.3 | 6.7 | - | - |
| Shortleaf pine | ||||
| No Exclusion | 74.7 | 25.3 | - | - |
| Protector | 50.0 | 50.0 | - | - |
| Full Exclusion | - | 100.0 | - | - |
| White oak | ||||
| No Exclusion | 97.5 | 1.8 | 3.7 | 0.6 |
| Protector | 97.9 | 2.1 | - | - |
| Full Exclusion | 91.7 | 6.7 | 1.7 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hackworth, Z.J.; Lhotka, J.M.; Cox, J.J.; Barton, C.D.; Springer, M.T. First-Year Vitality of Reforestation Plantings in Response to Herbivore Exclusion on Reclaimed Appalachian Surface-Mined Land. Forests 2018, 9, 222. https://doi.org/10.3390/f9040222
Hackworth ZJ, Lhotka JM, Cox JJ, Barton CD, Springer MT. First-Year Vitality of Reforestation Plantings in Response to Herbivore Exclusion on Reclaimed Appalachian Surface-Mined Land. Forests. 2018; 9(4):222. https://doi.org/10.3390/f9040222
Chicago/Turabian StyleHackworth, Zachary J., John M. Lhotka, John J. Cox, Christopher D. Barton, and Matthew T. Springer. 2018. "First-Year Vitality of Reforestation Plantings in Response to Herbivore Exclusion on Reclaimed Appalachian Surface-Mined Land" Forests 9, no. 4: 222. https://doi.org/10.3390/f9040222





