Chainsaw-Carved Cavities Better Mimic the Thermal Properties of Natural Tree Hollows than Nest Boxes and Log Hollows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Sites
2.3. Natural and Artificial Hollows
2.3.1. Glider Cavities
2.3.2. Bat Cavities
2.4. Monitoring Thermal Profiles of Natural and Artificial Hollows
2.5. Statistical Analyses
3. Results
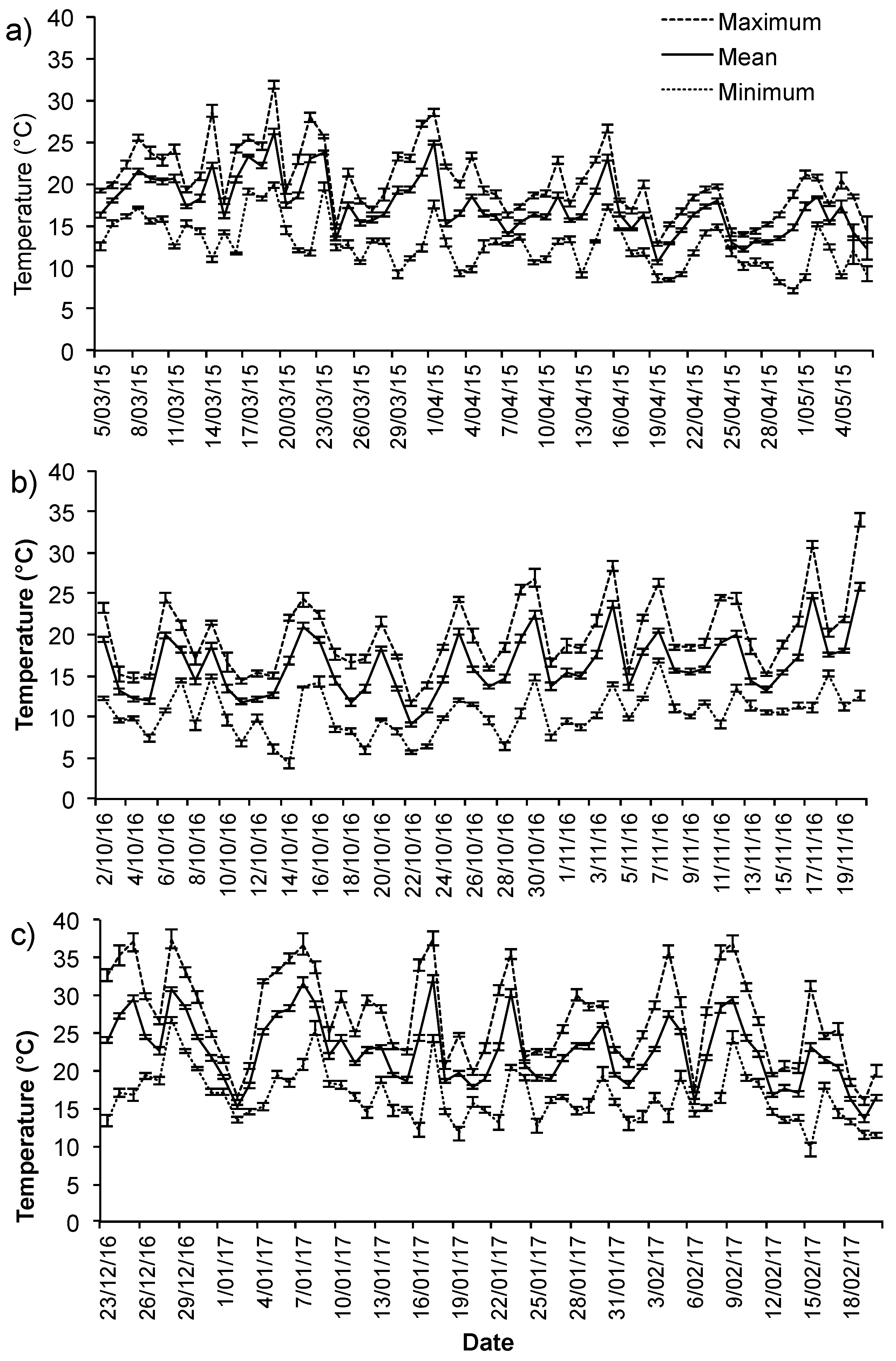
3.1. Ambient Conditions
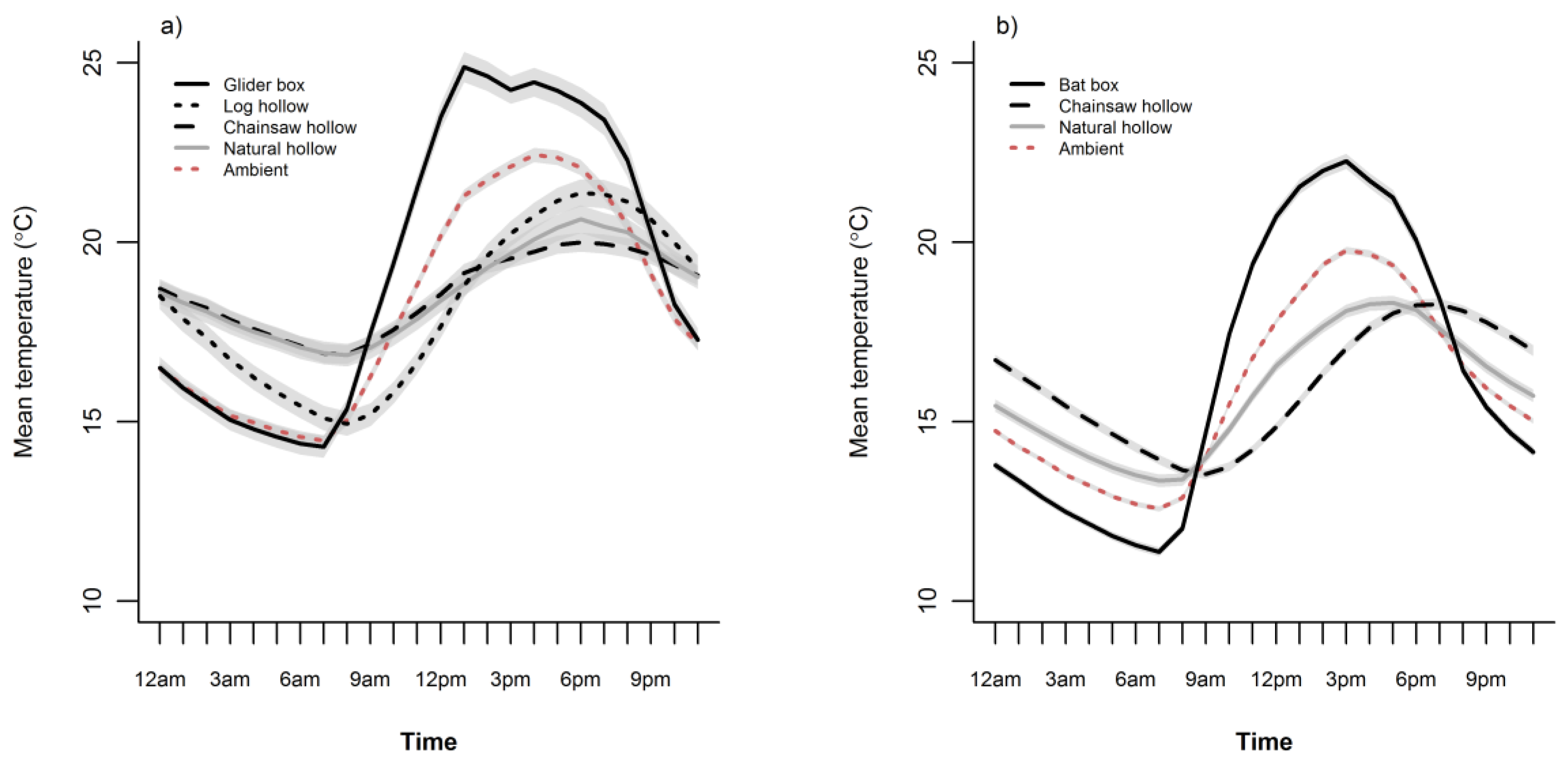
3.2. Cavity Thermal Profiles
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A. Detailed Description of Chainsaw Hollows and Log Hollows
Appendix A.1. Glider Chainsaw Hollows

Appendix A.2. Chainsaw-Carved Glider Log Hollows

Appendix A.3. Bat Chainsaw Hollows
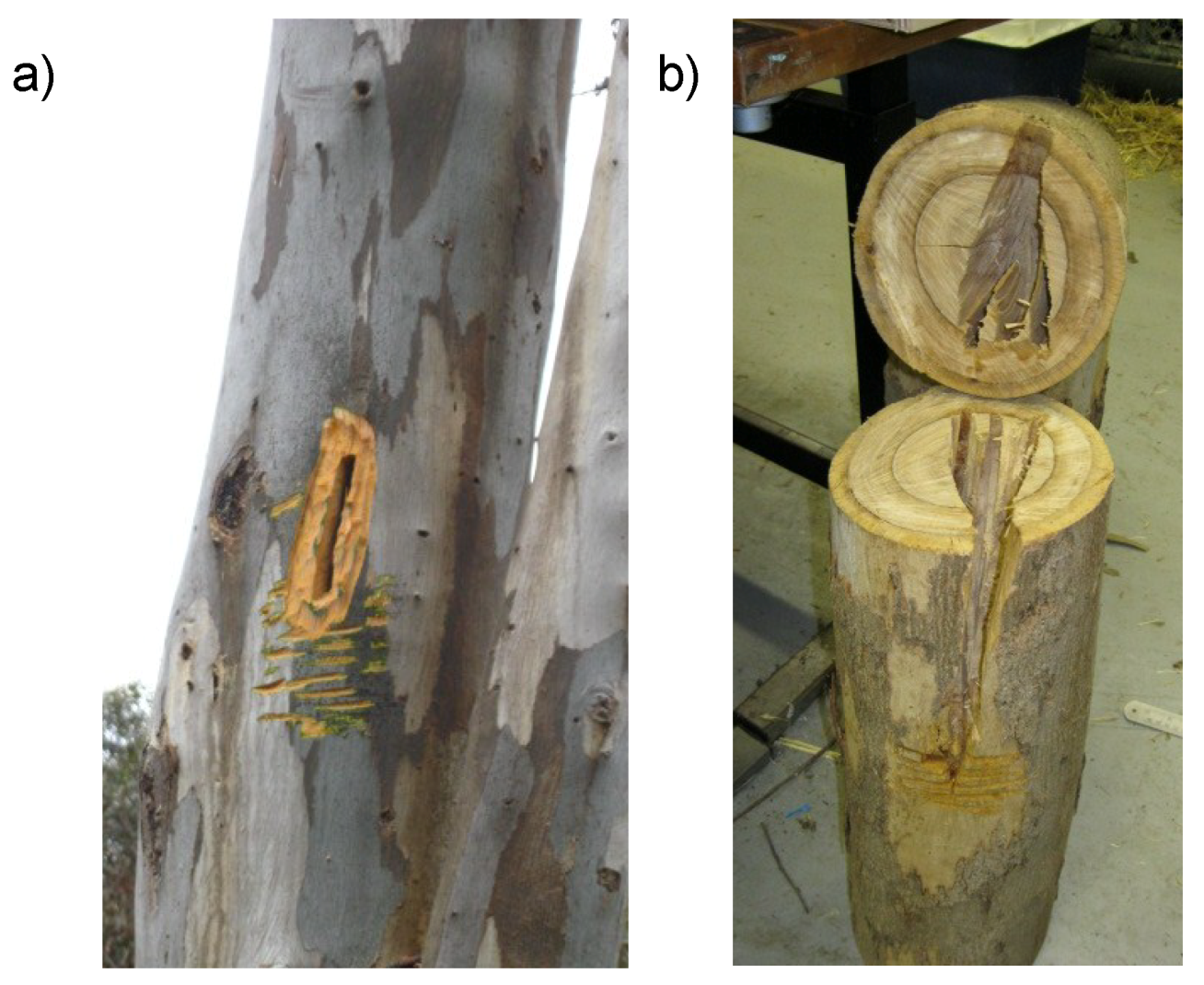
Appendix A.4. Measuring Canopy Cover
Appendix B. Summary of Ambient Conditions during the Study
Appendix C. Table of LMM Parameter Estimates for Mean Cavity Temperatures
| Explanatory Variables | Estimate (±SE) |
|---|---|
| Glider cavities | |
| Intercept: Natural hollow | 7.19 (±0.70) * |
| Type: Chainsaw hollow | 1.77 (±0.31) * |
| Type: Log hollow | −3.36 (±0.32) * |
| Type: Glider box | −8.89 (±0.31) * |
| Ambient temperature | 0.62 (±0.00) * |
| Chainsaw hollow × Ambient temperature | −0.09 (±0.00) * |
| Log hollow × Ambient temperature | 0.19 (±0.00) * |
| Glider box × Ambient temperature | 0.53 (±0.00) * |
| Bat cavities | |
| Intercept: Natural hollow, Trunk, East | 15.75 (±0.17) * |
| Type: Chainsaw hollow | 0.18 (±0.13) |
| Type: Bat box | 0.52 (±0.13) * |
| Ambient temperature 1 | 2.76 (±0.01) * |
| Location: Branch | −0.25 (±0.1) ^ |
| Aspect: North | 0.38 (±0.17) ^ |
| Aspect: South | 0.02 (±0.18) |
| Aspect: West | 0.16 (±0.17) |
| Shade 2 | 0.07 (±0.05) |
| Chainsaw hollow × Ambient temperature | 0.14 (±0.02) * |
| Bat box × Ambient temperature | 2.47 (±0.02) * |
Appendix D. Table of LMM Parameter Estimates for the Maximum, Minimum and Difference in Cavity Temperatures
| Explanatory Variable | Maximum (±SE) | Minimum (±SE) | Difference (±SE) |
|---|---|---|---|
| Glider cavities: day | |||
| Intercept: Natural hollow | 2.53 (±0.88) * | 6.78 (±0.99) * | −1.38 (±0.34) * |
| Type: Chainsaw hollow | 0.23 (±0.30) | 0.30 (±0.96) | −0.79 (±0.44) |
| Type: Log hollow | 1.04 (±0.30) * | −5.03 (±0.97) * | 2.28 (±0.45) * |
| Type: Glider box | 6.24 (±0.31) * | −8.28 (±0.94) * | 8.20 (±0.45) * |
| Ambient temp variable | 0.79 (±0.01) * | 1.01 (±0.01) * | 0.67 (±0.01) * |
| Glider cavities: night | |||
| Intercept: Natural hollow | 3.33 (±0.80) * | 2.34 (±0.25) * | 0.75 (±0.31) ^ |
| Type: Chainsaw hollow | 0.36 (±0.19) | 0.59 (±0.32) | −0.55 (±0.23) ^ |
| Type: Log hollow | 1.31 (±0.20) * | −1.01 (±0.32) * | 1.87 (±0.24) * |
| Type: Glider box | −0.09 (±0.18) | −3.10 (±0.31) * | 2.06 (±0.23) * |
| Ambient temp variable | 0.86 (±0.01) * | 1.03 (±0.00) * | 0.59 (±0.01) * |
| Bat cavities: day | |||
| Intercept: Natural hollow, Trunk, East | 17.08 (±0.63) * | 13.79 (±0.36) * | 3.12 (±0.88) * |
| Type: Chainsaw hollow | −0.58 (±0.42) | 0.28 (±0.28) | −1.05 (±0.66) |
| Type: Bat box | 6.44 (±0.51) * | −1.54 (±0.27) * | 7.49 (±0.65) * |
| Location: Branch | 0.68 (±0.35) | −0.80 (±0.21) * | 1.23 (±0.51) ^ |
| Aspect: North | 2.03 (±0.61) * | −0.30 (±0.34) | 3.34 (±0.86) * |
| Aspect: South | 0.93 (±0.64) | −0.17 (±0.36) | 1.58 (±0.90) |
| Aspect: West | 1.77 (±0.62) * | −0.26 (±0.35) | 2.61 (±0.87) * |
| Ambient temp variable 1 | 3.50 (±0.03) * | 3.27 (±0.01) * | 2.65 (±0.03) * |
| Percent shade 2 | −0.11 (±0.17) | 0.23 (±0.10) ^ | −0.37 (±0.25) |
| Bat cavities: night | |||
| Intercept: Natural hollow, Trunk, East | 18.09 (±0.22) * | 13.26 (±0.44) * | 4.47 (±0.43) * |
| Type: Chainsaw hollow | 0.76 (±0.20) * | 0.44 (±0.41) | −0.29 (±0.33) |
| Type: Bat box | −0.70 (±0.18) * | −2.02 (±0.39) * | 1.24 (±0.31) * |
| Location: Branch | 0.14 (±0.12) | −0.30 (±0.17) | 1.07 (±0.25) * |
| Aspect: North | 0.18 (±0.18) | −0.16 (±0.25) | 0.49 (±0.41) |
| Aspect: South | 0.17 (±0.19) | −0.14 (±0.27) | 0.39 (±0.43) |
| Aspect: West | 0.18 (±0.19) | −0.30 (±0.27) | 0.53 (±0.41) |
| Ambient temp variable 3 | 3.37 (±0.01) * | 2.80 (±0.01) * | 3.12 (±0.02) * |
| Percent shade 2 | 0.05 (±0.06) | 0.14 (±0.09) | −0.23 (±0.12) |
References
- Remm, J.; Lõhmus, A. Tree cavities in forests—The broad distribution pattern of a keystone structure for biodiversity. For. Ecol. Manag. 2011, 262, 579–585. [Google Scholar] [CrossRef]
- Manning, A.D.; Gibbons, P.; Fischer, J.; Oliver, D.L.; Lindenmayer, D.B. Hollow futures? Tree decline, lag effects and hollow-dependent species. Anim. Conserv. 2013, 16, 395–403. [Google Scholar] [CrossRef]
- Le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Blanchard, W.; Manning, A.D.; Gibbons, P. Reduced availability of habitat structures in urban landscapes: Implications for policy and practice. Landsc. Urban Plan. 2014, 125, 57–64. [Google Scholar] [CrossRef]
- López-Baucells, A.; Puig-Montserrat, X.; Torre, I.; Freixas, L.; Mas, M.; Arrizabalaga, A.; Flaquer, C. Bat boxes in urban non-native forests: A popular practice that should be reconsidered. Urban Ecosyst. 2017, 20, 217–225. [Google Scholar] [CrossRef]
- McComb, W.C.; Noble, R.E. Invertebrate use of natural tree cavities and vertebrate nesting boxes. Am. Midl. Nat. 1982, 107, 163–172. [Google Scholar] [CrossRef]
- McComb, W.C.; Noble, R.E. Herpetofaunal use of natural tree cavities and nest boxes. Wildl. Soc. Bull. 1981, 9, 261–267. [Google Scholar]
- Glorioso, B.M.; Waddle, J.H. A review of pipe and bamboo artificial refugia as sampling tools in anuran studies. Herpetol. Conserv. Biol. 2014, 9, 609–625. [Google Scholar]
- Lambrechts, M.M.; Adriaensen, F.; Ardia, D.R.; Artemyev, A.V.; Atienzar, F.; Banbura, J.; Barba, E.; Bouvier, J.-C.; Camprodon, J.; Cooper, C.B.; et al. The design of artificial nestboxes for the study of secondary hole-nesting birds: A review of methodological inconsistencies and potential biases. Acta Ornithol. 2010, 45, 1–26. [Google Scholar] [CrossRef]
- Goldingay, R.L.; Stevens, J.R. Use of artificial tree hollows by Australian birds and bats. Wildl. Res. 2009, 36, 81–97. [Google Scholar] [CrossRef]
- Mering, E.D.; Chambers, C.L. Thinking outside the box: A review of artificial roosts for bats. Wildl. Soc. Bull. 2014, 38, 741–751. [Google Scholar] [CrossRef]
- Rueegger, N. Bat boxes—A review of their use and application, past, present and future. Acta Chiropterol. 2016, 18, 279–299. [Google Scholar] [CrossRef]
- Beyer, G.L.; Goldingay, R.L. The value of nest boxes in the research and management of Australian hollow-using arboreal marsupials. Wildl. Res. 2006, 33, 161–174. [Google Scholar] [CrossRef]
- Newton, I. Population Limitation in Birds; Academic Press: London, UK, 1998. [Google Scholar]
- Newton, I. The role of nest sites in limiting the number of hole nesting birds: A review. Biol. Conserv. 1994, 70, 265–276. [Google Scholar] [CrossRef]
- Goldingay, R.L. Does nest box use reduce the fitness of a tree-cavity dependent mammal? Ecol. Res. 2017, 32, 495–502. [Google Scholar] [CrossRef]
- Goldingay, R.L.; Rueegger, N.N.; Grimson, M.J.; Taylor, B.D. Specific nest box designs can improve habitat restoration for cavity-dependent arboreal mammals. Restor. Ecol. 2015, 23, 482–490. [Google Scholar] [CrossRef]
- Harley, D. An overview of actions to conserve Leadbeater’s Possum (Gymnobelideus leadbeateri). Vic. Nat. 2016, 133, 85–97. [Google Scholar]
- Flaquer, C.; Torre, I.; Ruiz-Jarillo, R. The value of bat-boxes in the conservation of Pipistrellus pygmaeus in wetland rice paddies. Biol. Conserv. 2005, 128, 223–230. [Google Scholar] [CrossRef]
- Berthier, K.; Leippert, F.; Fumagalli, L.; Arlettaz, R. Massive nest-box supplementation boosts fecundity, survival and even immigration without altering mating and reproductive behaviour in a rapidly recovered bird population. PLoS ONE 2012, 7, e36028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brazill-Boast, J.; Pryke, S.R.; Griffith, S.C. Provisioning habitat with custom-designed nest-boxes increases reproductive success in an endangered finch. Austral Ecol. 2013, 38, 405–412. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Crane, M.; Evans, M.C.; Maron, M.; Gibbons, P.; Bekessy, S.; Blanchard, W. The anatomy of a failed offset. Biol. Conserv. 2017, 210 Pt A, 286–292. [Google Scholar] [CrossRef]
- Gibbons, P.; Evans, M.C.; Maron, M.; Gordon, A.; Le Roux, D.S.; von Hase, A.; Lindenmayer, D.B.; Possingham, H.P. A loss-gain calculator for biodiversity offsets and the circumstances in which no net loss is feasible. Conserv. Lett. 2016, 9, 252–259. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature. Biodiversity Offsets Technical Study Paper; IUCN Biodiversity Offsets Technical Study Group: Gland, Switzerland, 2014. [Google Scholar]
- Miller, K.L.; Trezise, J.A.; Kraus, S.; Dripps, K.; Evans, M.C.; Gibbons, P.; Possingham, H.P.; Maron, M. The development of the Australian environmental offsets policy: From theory to practice. Environ. Conserv. 2015, 42, 306–314. [Google Scholar] [CrossRef]
- Le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Bistricer, G.; Manning, A.D.; Gibbons, P. Enriching small trees with artificial nest boxes cannot mimic the value of large trees for hollow-nesting birds. Restor. Ecol. 2015, 24, 252–258. [Google Scholar] [CrossRef]
- Treby, D.L.; Castley, J.G. Distribution and abundance of hollow-bearing trees in urban forest fragments. Urban For. Urban Green. 2015, 14, 655–663. [Google Scholar] [CrossRef]
- Stagoll, K.; Lindenmayer, D.B.; Knight, E.; Fischer, J.; Manning, A.D. Large trees are keystone structures in urban parks. Conserv. Lett. 2012, 5, 115–122. [Google Scholar] [CrossRef]
- Maziarz, M.; Broughton, R.K.; Wesołowski, T. Microclimate in tree cavities and nest-boxes: Implications for hole-nesting birds. For. Ecol. Manag. 2017, 389, 306–313. [Google Scholar] [CrossRef]
- Le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Bistricer, G.; Manning, A.D.; Gibbons, P. Effects of entrance size, tree size and landscape context on nest box occupancy: Considerations for management and biodiversity offsets. For. Ecol. Manag. 2016, 366, 135–142. [Google Scholar] [CrossRef]
- Hurley, V.G.; Harris, G. Simulating Natural Cavities in Slender Cypress Pine (Callitris gracilis murrayensis) for Use by Major Mitchell’s Cockatoo (Lophochroa leadbeateri leadbeateri); Department of Environment and Primary Industries: Melbourne, Australia, 2014.
- Rueegger, N. Artificial tree hollow creation for cavity-using wildlife—Trialling an alternative method to that of nest boxes. For. Ecol. Manag. 2017, 405, 404–412. [Google Scholar] [CrossRef]
- Carey, A.B.; Gill, J.D. Direct habitat improvements—Some recent advances. In Snag Habitat Management: Proceedings of a Symposium. US Forest Service Technical Report RM-99; Davis, J., Goodwin, G., Ockerfells, R., Eds.; US Forest Service: Washington, DC, USA, 1983; pp. 80–87. [Google Scholar]
- Le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Manning, A.D.; Gibbons, P. The future of large old trees in urban landscapes. PLoS ONE 2014, 9, e99403. [Google Scholar] [CrossRef] [PubMed]
- Hurley, V.G.; Harris, G. A Manual of Techniques to Create Simulated Natural Cavities in Slender Cypress Pine (Callitris gracilis murrayensis) for Use by Major Mitchell’s Cockatoo (Lophochroa leadbeateri leadbeateri); Department of Environment, Land, Water and Planning: Mildura, Victoria, Australia, 2015.
- Hooper, R.G.; Taylor, W.E.; Loeb, S.E. Long-term efficacy of artificial cavities for red-cockaded woodpeckers: Lessons learned from Hurricane Hugo. In Red-Cockaded Woodpecker Road to Recovery; Costa, R., Daniels, S.J., Eds.; HancockHouse: Washington, DC, USA, 2004; pp. 430–438. [Google Scholar]
- Saenz, D.; Conner, R.N.; Collins, C.S.; Rudolph, D.C. Initial and long-term use of inserts by red-cockaded woodpeckers. Wildl. Soc. Bull. 2001, 29, 165–170. [Google Scholar]
- Hurley, V.G.; Stark, E.M. Characteristics and Uptake of Simulated Natural Cavities for Major Mitchell’s Cockatoo (Lophochroa leadbeateri leadbeateri) in Slender Cypress-Pine; Department of Environment, Land, Water and Planning: Mildura, Victoria, Australia, 2015.
- The Department of Environment, Land, Water, and Planning. Supporting the Recovery of Leadbeater’s Possum: Progress Report December 2016; The State Government of Victoria: Melbourne, Australia, 2016.
- Griffiths, S.R.; Rowland, J.A.; Briscoe, N.J.; Lentini, P.E.; Handasyde, K.A.; Lumsden, L.F.; Robert, K.A. Surface reflectance drives nest box temperature profiles and thermal suitability for target wildlife. PLoS ONE 2017, 12, e0176951. [Google Scholar] [CrossRef] [PubMed]
- Rowland, J.A.; Briscoe, N.J.; Handasyde, K.A. Comparing the thermal suitability of nest-boxes and tree-hollows for the conservation-management of arboreal marsupials. Biol. Conserv. 2017, 209, 341–348. [Google Scholar] [CrossRef]
- Dawson, R.D.; Lawrie, C.C.; O’Brien, E.L. The importance of microclimate variation in determining size, growth and survival of avian offspring: Experimental evidence from a cavity nesting passerine. Oecologia 2005, 144, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Huey, R.B. Physiological consequences of habitat selection. Am. Nat. 1991, 137, S91–S115. [Google Scholar] [CrossRef]
- Porter, W.P.; Kearney, M. Size, shape, and the thermal niche of endotherms. Proc. Natl. Acad. Sci. USA 2009, 106 (Suppl. 2), 19666–19672. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.J. Temperature regulation and evaporative water loss in the brush-tailed possum Trichosurus vulpecula. Comp. Biochem. Physiol. 1969, 28, 401–407. [Google Scholar] [CrossRef]
- Visser, H.G. Development of temperature regulation. In Avian Growth and Development; Starck, J.M., Rickles, R.E., Eds.; Oxford University Press: Oxford, UK, 1998; pp. 117–156. [Google Scholar]
- Geiser, F.; Brigham, R.M. Torpor, thermal biology, and energetics in Australian long-eared bats (Nyctophilus). J. Comp. Physiol. B 2000, 170, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Sedgeley, J.A. Quality of cavity microclimate as a factor influencing selection of maternity roosts by a tree-dwelling bat, Chalinolobus tuberculatus, in New Zealand. J. Appl. Ecol. 2001, 38, 425–438. [Google Scholar] [CrossRef]
- Willis, C.K.R.; Brigham, R.M. Social thermoregulation exerts more influence than microclimate on forest roost preferences by a cavity-dwelling bat. Behav. Ecol. Sociobiol. 2007, 62, 97–108. [Google Scholar] [CrossRef]
- Kerth, G.; Weissmann, K.; Konig, B. Day roost selection in female Bechstein’s bats (Myotis bechsteinii): A field experiment to determine the influence of roost temperature. Oecologia 2001, 126, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McComb, W.C.; Noble, R.E. Microclimates of nest boxes and natural cavities in bottomland hardwoods. J. Wildl. Manag. 1981, 45, 284–289. [Google Scholar] [CrossRef]
- Bartonicka, T.; Rehak, Z. Influence of the microclimate of bat boxes on their occupation by the soprano pipistrelle Pipistrellus pygmaeus: Possible cause of roost switching. Acta Chiropterol. 2007, 9, 517–526. [Google Scholar] [CrossRef]
- Isaac, J.L.; Parsons, M.; Goodman, B.A. How hot do nest boxes get in the tropics? A study of nest boxes for the endangered mahogany glider. Wildl. Res. 2008, 35, 441–445. [Google Scholar] [CrossRef]
- Amat-Valero, M.; Calero-Torralbo, M.A.; Vaclav, R.; Valera, F. Cavity types and microclimate: Implications for ecological, evolutionary, and conservation studies. Int. J. Biometeorol. 2014, 58, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Purcell, K.L.; Verner, J.; Oring, L.W. A comparison of the breeding ecology of birds nesting in boxes and tree cavities. Auk 1997, 114, 646–656. [Google Scholar] [CrossRef]
- Bortolotti, G.R. Effect of nest-box size on nest-site preference and reproduction in American Kestrels. J. Raptor Res. 1994, 28, 127–133. [Google Scholar]
- Miller, K.E. Nesting success of the great crested flycatcher in nest boxes and in tree cavities: Are nest boxes safer from nest predation? Wilson Bull. 2002, 14, 179–185. [Google Scholar] [CrossRef]
- Singh, A.; Bhatt, D.; Sethi, V.K.; Dadwal, N. Nesting success of the oriental magpie robin Copsychus saularis in nest boxes and tree cavities. Wildl. Biol. 2016, 22, 277–283. [Google Scholar] [CrossRef]
- Gehlbach, F.R. Nest-box versus natural-cavity nests of the eastern screech-owl: An exploratory study. J. Raptor Res. 1994, 28, 154–157. [Google Scholar]
- Czeszczewik, D.; Walankiewicz, W.; Mitrus, C.; Nowakowski, W. Nest-box data of Pied Flycatcher Ficedula hypoleuca may lead to erroneous generalizations. Vogelwelt 1999, 120, 361–366. [Google Scholar]
- Mänd, R.; Tilgar, V.; Lõhmus, A.; Leivits, A. Providing nest boxes for hole-nesting birds—Does habitat matter? Biodivers. Conserv. 2005, 14, 1823–1840. [Google Scholar] [CrossRef]
- Wesołowski, T. Reports from nestbox atudies: A review of inadequacies. Acta Ornithol. 2011, 46, 13–17. [Google Scholar] [CrossRef]
- Møller, A.P. Parasites, predators and nest boxes: Facts and artefacts in nest box studies of birds? Oikos 1989, 56, 421–423. [Google Scholar] [CrossRef]
- Isaac, J.L.; De Gabriel, J.L.; Goodman, B.A. Microclimate of daytime den sites in a tropical possum: Implications for the conservation of tropical arboreal marsupials. Anim. Conserv. 2008, 11, 281–287. [Google Scholar] [CrossRef]
- Catry, I.; Franco, A.M.A.; Sutherland, W.J. Adapting conservation efforts to face climate change: Modifying nest-site provisioning for lesser kestrels. Biol. Conserv. 2011, 144, 1111–1119. [Google Scholar] [CrossRef]
- Goldingay, R.L. Temperature variation in nest boxes in eastern Australia. Aust. Mammal. 2015, 37, 225–233. [Google Scholar] [CrossRef]
- Gibbons, P.; Lindenmayer, D.B. Tree Hollows and Wildlife Conservation in Australia; CSIRO Publishing: Melbourne, Australia, 2002. [Google Scholar]
- Vesk, P.A.; Nolan, R.; Thomson, J.R.; Dorrough, J.W.; Mac Nally, R. Time lags in provision of habitat resources through revegetation. Biol. Conserv. 2008, 141, 174–186. [Google Scholar] [CrossRef]
- Australian Bureau of Meteorology. Climate Data Online. Available online: http://www.bom.gov.au/climate/data/ (accessed on 7 July 2017).
- Dare, A.J.; McDonald, P.G.; Clarke, M.F. The ecological context and consequences of colonisation of a site by bell miners (Manorina melanophrys). Wildl. Res. 2007, 34, 616–623. [Google Scholar] [CrossRef]
- Bircanin, L.; Short, A. Glimpses of the Past: Mont Park, Larundel, Plenty; North Eastern Metropolitan Psychiatric Service: Melbourne, Australia, 1995.
- Griffiths, S.R.; Bender, R.; Godinho, L.N.; Lentini, P.E.; Lumsden, L.F.; Robert, K.A. Bat boxes are not a silver bullet conservation tool. Mamm. Rev. 2017, 47, 261–265. [Google Scholar] [CrossRef]
- Goldingay, R.L. Characteristics of tree hollows used by Australian arboreal and scansorial mammals. Aust. J. Zool. 2011, 59, 277–294. [Google Scholar] [CrossRef]
- Beyer, G.L.; Goldingay, R.L.; Sharpe, D.J. The characteristics of squirrel glider (Petaurus norfolcensis) den trees in subtropical Australia. Aust. J. Zool. 2008, 56, 13–21. [Google Scholar] [CrossRef]
- Traill, B.J.; Lill, A. Use of tree hollows by two sympatric gliding possums, the squirrel glider, Petaurus norfolcensis and the sugar glider, P. breviceps. Aust. Mammal. 1997, 20, 79–88. [Google Scholar]
- Smiley, T.E.; Fraedrich, B.R. Determining strength loss from decay. J. Aboricult. 1992, 18, 201–204. [Google Scholar]
- Kunz, T.H.; Lumsden, L.F. Ecology of cavity and foliage roosting bats. In Bat Ecology; Kunz, T.H., Fenton, M.B., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 3–89. [Google Scholar]
- Dunster, J.A. Tree Risk Assessment Manual, 1st ed.; International Society of Arboriculture: Champaign, IL, USA, 2013. [Google Scholar]
- Anderson, D.L.; Koomjian, W.; French, B.; Altenhoff, S.R.; Luce, J. Review of rope-based access methods for the forest canopy: Safe and unsafe practices in published information sources and a summary of current methods. Methods Ecol. Evol. 2015, 6, 865–872. [Google Scholar] [CrossRef]
- Tuttle, M.D.; Kiser, M.; Kiser, S. The Bat House Builder’s Hand-Book; Bat Conservation International: Austin, TX, USA, 2013. [Google Scholar]
- Maxim Integrated Products Inc. DS1922L/ DS1922T: Temperature Logger iButton with 8KB Data-Log Memory—19-4990, Rev 13; Maxim Integrated Products Inc.: San Jose, CA, USA, 2015. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Savliev, A.A.; Smith, G. Chapter 4—Dealing with Heterogeneity. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; pp. 71–100. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Savliev, A.A.; Smith, G. Chapter 6—Violation of Independence Part I. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; pp. 143–160. [Google Scholar]
- Ardia, D.R.; Pérez, J.H.; Clotfelter, E.D. Nest box orientation affects internal temperature and nest site selection by Tree Swallows. J. Field Ornithol. 2006, 77, 339–344. [Google Scholar] [CrossRef]
- Derby, R.W.; Gates, D.M. Temperature of tree trunks-calculated and observed. Am. J. Bot. 1966, 53, 580–587. [Google Scholar] [CrossRef]
- Coombs, A.B.; Bowman, J.; Garroway, C.J. Thermal properties of tree cavities during winter in a northern hardwood forest. J. Wildl. Manag. 2010, 74, 1875–1881. [Google Scholar] [CrossRef]
- Briscoe, N.J.; Handasyde, K.A.; Griffiths, S.R.; Porter, W.P.; Krockenberger, A.; Kearney, M.R. Tree-hugging koalas demonstrate a novel thermoregulatory mechanism for arboreal mammals. Biol. Lett. 2014, 10, 20140235. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.R.; Green, J.A.; Pichegru, L. Extreme microclimate conditions in artificial nests for endangered African Penguins. Bird Conserv. Int. 2014, 24, 201–213. [Google Scholar] [CrossRef]
- Havera, S.P. Temperature variation in a Fox Squirrel nest box. J. Wildl. Manag. 1979, 43, 251–253. [Google Scholar] [CrossRef]
- Speakman, J.R.; Lumsden, L.F.; Hays, G.C. Predation rates on bats released to fly during daylight in south-eastern Australia. J. Zool. 1994, 233, 318–321. [Google Scholar] [CrossRef]
- Webb, D.R. Thermal tolerance of avian embryos: A review. Condor 1987, 89, 874–898. [Google Scholar] [CrossRef]
- Coumou, D.; Rahmstorf, S. A decade of weather extremes. Nat. Clim. Chang. 2012, 2, 491–496. [Google Scholar] [CrossRef]
- Charter, M.; Izhaki, I.; Mocha, Y.B.; Kark, S. Nest-site competition between invasive and native cavity nesting birds and its implication for conservation. J. Environ. Manag. 2016, 181, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Grarock, K.; Lindenmayer, D.B.; Wood, J.T.; Tidemann, C.R. Does human-induced habitat modification influence the impact of introduced species? A case study on cavity-nesting by the introduced common myna (Acridotheres tristis) and two Australian native parrots. Environ. Manag. 2013, 52, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.J.; McCarthy, M.A.; van der Ree, R. The use of nest boxes in urban natural vegetation remnants by vertebrate fauna. Wildl. Res. 2005, 32, 509–516. [Google Scholar] [CrossRef]
- Durant, R.; Luck, G.W.; Matthews, A. Nest-box use by arboreal mammals in a peri-urban landscape. Wildl. Res. 2009, 36, 565–573. [Google Scholar] [CrossRef]
- Agnelli, P.; Maltagliati, G.; Ducci, L.; Cannicci, S. Artificial roosts for bats: Education and research. The “be a bat’s friend” project of The Natural History Museum of The University of Florence. Hystrix-Ital. J. Mammal. 2011, 22, 215–223. [Google Scholar] [CrossRef]
- Quin, B.R.; Baker-Gabb, D.J. Conservation and Management of the Turquoise Parrot Neophema pulchella in North-East Victoria; Arthur Rylah Institute Technical Report Series No. 125; Victorian Department of Conservation and Environment: Melbourne, Australia, 1993.
- Emison, M.R. Use of supplementary nest hollows by an endangered subspecies of Red-tailed Black-cockatoo. Vic. Nat. 1996, 113, 262–263. [Google Scholar]
- Suckling, G.C.; Macfarlane, M.A. Introduction of the sugar glider, Petaurus breviceps, into re-established forest of the Tower-Hill State Game Reserve. Vic. Aust. Wildl. Res. 1983, 10, 249–258. [Google Scholar] [CrossRef]
- Irvine, R.; Bender, R. Introduction of the sugar glider Petaurus breviceps into re-established forest of the Organ Pipes National Park, Victoria. Vic. Nat. 1997, 114, 230–239. [Google Scholar]
- Roads and Traffic Authority of New South Wales. Biodiversity Guidelines: Protecting and Managing Biodiversity on RTA Projects; Roads and Traffic Authority New South Wales: Sydney, Australia, 2011.
- Carey, A.B.; Sanderson, H.R. Routing to accelerate tree-cavity formation. Wildl. Soc. Bull. 1981, 9, 14–21. [Google Scholar]
- Gano, R.D.; Mosher, JA. Artificial cavity construction: An alternative to nest boxes. Wildl. Soc. Bull. 1983, 11, 74–76. [Google Scholar]
- Cox, J.A.; McCormick, J.K. New insights from an attempt to reintroduce Red-cockaded Woodpeckers in northern Florida. J. Field Ornithol. 2016, 87, 360–370. [Google Scholar] [CrossRef]
- Standards Australia Committee EV-018. Australian Standard AS 4373—2007—Pruning of Amenity Trees; Standards Australia: Sydney, Australia, 2007. [Google Scholar]
- Copeyon, C.K. A technique for constructing cavities for the red-cockaded woodpecker. Wildl. Soc. Bull. 1990, 18, 303–311. [Google Scholar]
- Lindenmayer, D.B.; Welsh, A.; Donnelly, C.; Crane, M.; Michael, D.; Macgregor, C.; McBurney, L.; Montague-Drake, R.; Gibbons, P. Are nest boxes a viable alternative source of cavities for hollow-dependent animals? Long-term monitoring of nest box occupancy, pest use and attrition. Biol. Conserv. 2009, 142, 33–42. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Wood, J.; McBurney, L.; Michael, D.; Crane, M.; Macgregor, C.; Montague-Drake, R.; Gibbons, P.; Banks, S.C. Cross-sectional vs. longitudinal research: A case study of trees with hollows and marsupials in Australian forests. Ecol. Monogr. 2011, 81, 557–580. [Google Scholar] [CrossRef]
- McClure, C.J.W.; Pauli, B.P.; Heath, J.A. Simulations reveal the power and peril of artificial breeding sites for monitoring and managing animals. Ecol. Appl. 2017, 27, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Kane, B.C.P.; Ryan, H.D.P. Examining formulas that assess strength loss due to decay in trees: Woundwood toughness improvement in red maple (Acer rubrum). J. Arboric. 2003, 29, 209–217. [Google Scholar]
- Beckschafer, P.; Seidel, D.; Kleinn, C.; Xu, J.C. On the exposure of hemispherical photographs in forests. iForest-Biogeosci. For. 2013, 6, 228–237. [Google Scholar] [CrossRef]
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analyzer (GLA), Version 2.0: Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Fisheye Photographs, Users Manual and Program Documentation; Simon Fraser University: New York, NY, USA, 1999. [Google Scholar]





| Cavity Type | Survey Period | |
|---|---|---|
| Bat cavities | Autumn | |
| Bat box | 35 | |
| Chainsaw hollow | 35 | |
| Natural tree hollow | 23 | |
| Glider cavities | Spring | Summer |
| Glider box | 10 | 10 |
| Chainsaw hollow | 10 | 9 |
| Log hollow | 9 | 7 |
| Natural tree hollow | 7 | 10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griffiths, S.R.; Lentini, P.E.; Semmens, K.; Watson, S.J.; Lumsden, L.F.; Robert, K.A. Chainsaw-Carved Cavities Better Mimic the Thermal Properties of Natural Tree Hollows than Nest Boxes and Log Hollows. Forests 2018, 9, 235. https://doi.org/10.3390/f9050235
Griffiths SR, Lentini PE, Semmens K, Watson SJ, Lumsden LF, Robert KA. Chainsaw-Carved Cavities Better Mimic the Thermal Properties of Natural Tree Hollows than Nest Boxes and Log Hollows. Forests. 2018; 9(5):235. https://doi.org/10.3390/f9050235
Chicago/Turabian StyleGriffiths, Stephen R., Pia E. Lentini, Kristin Semmens, Simon J. Watson, Linda F. Lumsden, and Kylie A. Robert. 2018. "Chainsaw-Carved Cavities Better Mimic the Thermal Properties of Natural Tree Hollows than Nest Boxes and Log Hollows" Forests 9, no. 5: 235. https://doi.org/10.3390/f9050235





