Interstorm Variability in the Biolability of Tree-Derived Dissolved Organic Matter (Tree-DOM) in Throughfall and Stemflow
Abstract
:1. Introduction
2. Materials and Methods
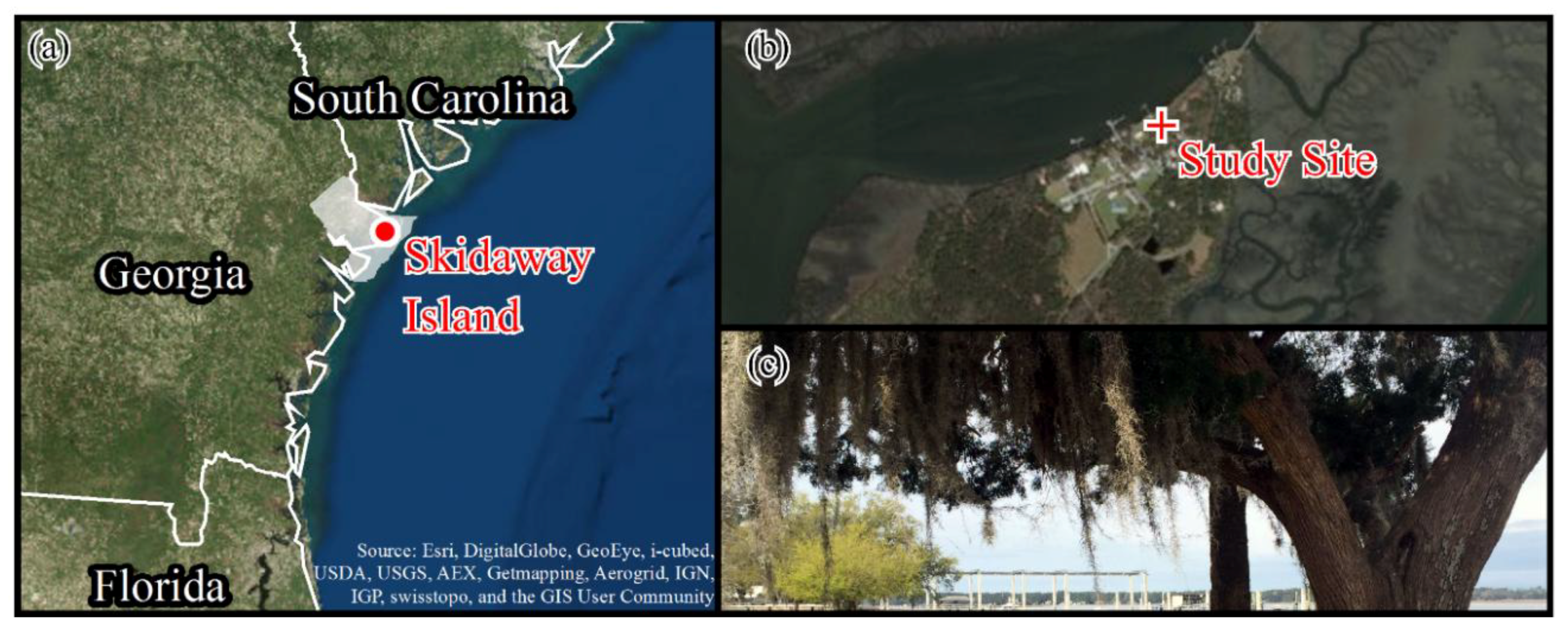
2.1. Study Site Description
2.2. Rainfall, Throughfall and Stemflow Sampling
2.3. Bioincubations
2.4. Dissolved Organic Carbon Concentrations
2.5. Data Analysis
3. Results
3.1. Hydrometeorology for Sampled Storms
3.2. Initial DOM Concentrations
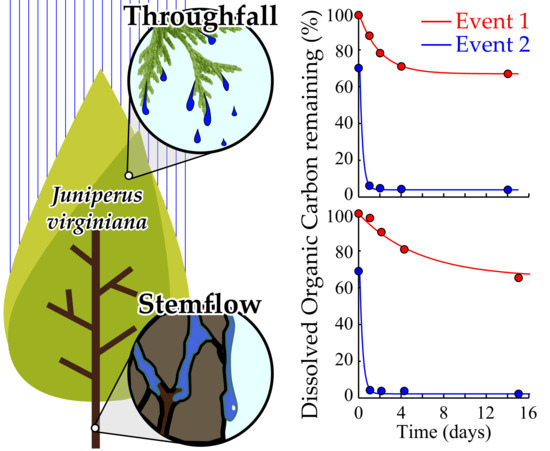
3.3. Interstorm Tree-DOM Biolability
3.4. Tree-BDOM Yield
4. Discussion
4.1. Hydrometeorology
4.2. Tree-DOM Concentration and Biolability
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dawson, H.; Ugolini, F.; Hrutfiord, B.; Zachara, J. Role of soluble organics in the soil processes of a podzol, central cascades, washington. Soil Sci. 1978, 126, 290–296. [Google Scholar] [CrossRef]
- Guggenberger, G.; Kaiser, K. Dissolved organic matter in soil: Challenging the paradigm of sorptive preservation. Geoderma 2003, 113, 293–310. [Google Scholar] [CrossRef]
- Temminghoff, E.J.; Van der Zee, S.E.; de Haan, F.A. Copper mobility in a copper-contaminated sandy soil as affected by ph and solid and dissolved organic matter. Environ. Sci. Technol. 1997, 31, 1109–1115. [Google Scholar] [CrossRef]
- Michalzik, B.; Kalbitz, K.; Park, J.-H.; Solinger, S.; Matzner, E. Fluxes and concentrations of dissolved organic carbon and nitrogen–a synthesis for temperate forests. Biogeochemistry 2001, 52, 173–205. [Google Scholar] [CrossRef]
- Donald, R.G.; Anderson, D.W.; Stewart, J.W. Potential role of dissolved organic carbon in phosphorus transport in forested soils. Soil Sci. Soc. Am. J. 1993, 57, 1611–1618. [Google Scholar] [CrossRef]
- Dittmar, T.; Stubbins, A. 12.6—Dissolved organic matter in aquatic systems. Treatise on Geochemistry, 2nd ed.; Elsevier: Oxford, UK, 2014; pp. 125–156. [Google Scholar]
- Qualls, R.G.; Haines, B.L. Biodegradability of dissolved organic matter in forest throughfall, soil solution, and stream water. Soil Sci. Soc. Am. J. 1992, 56, 578–586. [Google Scholar] [CrossRef]
- Cummins, K.; Klug, J.; Wetzel, R.; Petersen, R.; Suberkropp, K.; Manny, B.; Wuycheck, J.; Howard, F. Organic enrichment with leaf leachate in experimental lotic ecosystems. BioScience 1972, 22, 719–722. [Google Scholar] [CrossRef]
- Hagedorn, F.; Bruderhofer, N.; Ferrari, A.; Niklaus, P.A. Tracking litter-derived dissolved organic matter along a soil chronosequence using 14c imaging: Biodegradation, physico-chemical retention or preferential flow? Soil Biol. Biochem. 2015, 88, 333–343. [Google Scholar] [CrossRef]
- Van Stan, J.T.; Stubbins, A. Tree-dom: Dissolved organic matter in throughfall and stemflow. Limnol. Oceanogr. Lett. 2018. [Google Scholar] [CrossRef]
- Van Stan, J.T.; Wagner, S.; Guillemette, F.; Whitetree, A.; Lewis, J.; Silva, L.; Stubbins, A. Temporal dynamics in the concentration, flux, and optical properties of tree-derived dissolved organic matter in an epiphyte-laden oak-cedar forest. J. Geophys. Res. Biogeosci. 2017, 122, 2982–2997. [Google Scholar] [CrossRef]
- Tan, Z.H.; Zhang, Y.P.; Liang, N.; Song, Q.H.; Liu, Y.H.; You, G.Y.; Li, L.H.; Yu, L.; Wu, C.S.; Lu, Z.Y. Soil respiration in an old-growth subtropical forest: Patterns, components, and controls. J. Geophys. Res. Atmos. 2013, 118, 2981–2990. [Google Scholar] [CrossRef]
- Aitkenhead-Peterson, J.A.; McDowell, W.H.; Neff, J.C. Sources, production, and regulation of allochthonous dissolved organic matter inputs to surface waters. In Aquatic Ecosystems; Elsevier: Cambridge, MA, USA, 2003; pp. 25–70. [Google Scholar]
- Johnson, M.S.; Lehmann, J. Double-funneling of trees: Stemflow and root-induced preferential flow. Ecoscience 2006, 13, 324–333. [Google Scholar] [CrossRef]
- Keenan, R.J.; Reams, G.A.; Achard, F.; de Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of global forest area: Results from the fao global forest resources assessment 2015. For. Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- Briggs, J.M.; Hoch, G.A.; Johnson, L.C. Assessing the rate, mechanisms, and consequences of the conversion of tallgrass prairie to juniperus virginiana forest. Ecosystems 2002, 5, 578–586. [Google Scholar] [CrossRef]
- Elias, R.B.; Dias, E. Gap dynamics and regeneration strategies in juniperus-laurus forests of the azores islands. Plant Ecol. 2009, 200, 179–189. [Google Scholar] [CrossRef]
- Van Stan, J.T., II; Pypker, T.G. A review and evaluation of forest canopy epiphyte roles in the partitioning and chemical alteration of precipitation. Sci. Total Environ. 2015, 536, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Zotz, G.; Bader, M. Epiphytic plants in a changing world-global: Change effects on vascular and non-vascular epiphytes. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2009; pp. 147–170. [Google Scholar]
- Stubbins, A.; Silva, L.M.; Dittmar, T.; Van Stan, J.T. Molecular and optical properties of tree-derived dissolved organic matter in throughfall and stemflow from live oaks and eastern red cedar. Front. Earth Sci. 2017, 5, 22. [Google Scholar] [CrossRef]
- Stubbins, A.; Spencer, R.G.; Chen, H.; Hatcher, P.G.; Mopper, K.; Hernes, P.J.; Mwamba, V.L.; Mangangu, A.M.; Wabakanghanzi, J.N.; Six, J. Illuminated darkness: Molecular signatures of congo river dissolved organic matter and its photochemical alteration as revealed by ultrahigh precision mass spectrometry. Limnol. Oceanogr. 2010, 55, 1467–1477. [Google Scholar] [CrossRef]
- Climate Average from 1948 to 2016 Climate Name GA54.CLI; University of Georgia: Athens, GA, USA, 2012.
- Fellman, J.B.; D’Amore, D.V.; Hood, E.; Boone, R.D. Fluorescence characteristics and biodegradability of dissolved organic matter in forest and wetland soils from coastal temperate watersheds in southeast Alaska. Biogeochemistry 2008, 88, 169–184. [Google Scholar] [CrossRef]
- Bittar, T.B.; Pound, P.; Whitetree, A.; Dean Moore, L.; Van Stan, J.T. Estimation of throughfall and stemflow bacterial flux in a subtropical oak-cedar forest. Geophys. Res. Lett. 2018, 45, 1410–1418. [Google Scholar] [CrossRef]
- Stubbins, A.; Dittmar, T. Low volume quantification of dissolved organic carbon and dissolved nitrogen. Limnol. Oceanogr. Methods 2012, 10, 347–352. [Google Scholar] [CrossRef]
- Crockford, R.; Richardson, D. Partitioning of rainfall into throughfall, stemflow and interception: Effect of forest type, ground cover and climate. Hydrol. Process. 2000, 14, 2903–2920. [Google Scholar] [CrossRef]
- Herwitz, S.R.; Slye, R.E. Three-dimensional modeling of canopy tree interception of wind-driven rainfall. J. Hydrol. 1995, 168, 205–226. [Google Scholar] [CrossRef]
- Owens, M.K.; Lyons, R.K.; Alejandro, C.L. Rainfall partitioning within semiarid juniper communities: Effects of event size and canopy cover. Hydrol. Process. 2006, 20, 3179–3189. [Google Scholar] [CrossRef]
- Zou, C.B.; Caterina, G.L.; Will, R.E.; Stebler, E.; Turton, D. Canopy interception for a tallgrass prairie under juniper encroachment. PLoS ONE 2015, 10, e0141422. [Google Scholar] [CrossRef] [PubMed]
- Metzger, J.C.; Germer, S.; Hildebrandt, A. Factors impacting stemflow generation in a european beech forest: Individual tree versus neighborhood properties. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 23–28 April 2017; p. 8410. [Google Scholar]
- Gay, T.E.; Van Stan, J.T.; Moore, L.D.; Lewis, E.S.; Reichard, J.S. Throughfall alterations by degree of tillandsia usneoides cover in a southeastern us quercus virginiana forest. Can. J. For. Res. 2015, 45, 1688–1698. [Google Scholar] [CrossRef]
- Turner, E.C.; Snaddon, J.L.; Johnson, H.R.; Foster, W.A. The impact of bird’s nest ferns on stemflow nutrient concentration in a primary rain forest, Sabah, Malaysia. J. Trop. Ecol. 2007, 23, 721–724. [Google Scholar] [CrossRef]
- Backnäs, S.; Laine-Kaulio, H.; Kløve, B. Phosphorus forms and related soil chemistry in preferential flowpaths and the soil matrix of a forested podzolic till soil profile. Geoderma 2012, 189, 50–64. [Google Scholar] [CrossRef]
- Spencer, S.; Meerveld, H. Double funnelling in a mature coastal british columbia forest: Spatial patterns of stemflow after infiltration. Hydrol. Process. 2016, 30, 4185–4201. [Google Scholar] [CrossRef]
- Etheridge, J.R.; Birgand, F.; Osborne, J.A.; Osburn, C.L.; Burchell, M.R.; Irving, J. Using in situ ultraviolet-visual spectroscopy to measure nitrogen, carbon, phosphorus, and suspended solids concentrations at a high frequency in a brackish tidal marsh. Limnol. Oceanogr. Methods 2014, 12, 10–22. [Google Scholar] [CrossRef]

| Condition | 14 May 2017 | 3 June 2017 | 30 July 2017 | 28 August 2017 | 23 October 2017 |
|---|---|---|---|---|---|
| Magnitude, mm | 48.3 | 8 | 8.9 | 26.1 | 30.8 |
| Duration, h | 59.3 | 11.5 | 8.5 | 30 | 17.3 |
| Intensity, mm h−1 | 0.8 | 0.7 | 1.0 | 0.9 | 1.8 |
| Throughfall | |||||
| Bare, mm (%) | 26.4 (55%) | 4.7 (58%) | 7.5 (84%) | 19.0 (73%) | 25 (81%) |
| Epiphyte, mm (%) | 28.0 (58%) | 2.1 (26%) | 2.9 (33%) | 13.8 (53%) | 20.0 (65%) |
| Stemflow | |||||
| Bare, mm (%) | 8.0 (17%) | 1.0 (12%) | 1.7 (19%) | 3.9 (15%) | 4.4 (14%) |
| Epiphyte, mm (%) | 4.0 (8%) | 0.2 (3%) | 0.4 (4%) | 0.9 (4%) | 1.6 (5%) |
| Biolabile Yield (mg-C m−2 mm−1 Rainfall) | |||||
|---|---|---|---|---|---|
| 14 May 2017 | 3 June 2017 | 30 July 2017 | 28 August 2017 | 23 October 2017 | |
| TF, bare | 10.3 ± 2.0 | 33.3 ± 15.2 | 62.6 ± 32.5 | 18.9 ± 6.1 | 16.3 ± 3.5 |
| TF, epiphyte | 12.2 ± 1.5 | 32.8 ± 18.8 | 25.5 ± 10.1 | 45.0 ± 17.1 | 28.6 ± 6.6 |
| SF, bare | 5.6 ± 1.8 | 10.6 ± 5.5 | 16.1 ± 8.7 | 7.4 ± 2.5 | 4.9 ± 1.1 |
| SF, epiphyte | 3.9 ± 2.2 | 3.7 ± 1.9 | 5.4 ± 3.0 | 3.3 ± 1.7 | 3.1 ± 0.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howard, D.H.; Stan, J.T.V.; Whitetree, A.; Zhu, L.; Stubbins, A. Interstorm Variability in the Biolability of Tree-Derived Dissolved Organic Matter (Tree-DOM) in Throughfall and Stemflow. Forests 2018, 9, 236. https://doi.org/10.3390/f9050236
Howard DH, Stan JTV, Whitetree A, Zhu L, Stubbins A. Interstorm Variability in the Biolability of Tree-Derived Dissolved Organic Matter (Tree-DOM) in Throughfall and Stemflow. Forests. 2018; 9(5):236. https://doi.org/10.3390/f9050236
Chicago/Turabian StyleHoward, Daniel H., John T. Van Stan, Ansley Whitetree, Lixin Zhu, and Aron Stubbins. 2018. "Interstorm Variability in the Biolability of Tree-Derived Dissolved Organic Matter (Tree-DOM) in Throughfall and Stemflow" Forests 9, no. 5: 236. https://doi.org/10.3390/f9050236
APA StyleHoward, D. H., Stan, J. T. V., Whitetree, A., Zhu, L., & Stubbins, A. (2018). Interstorm Variability in the Biolability of Tree-Derived Dissolved Organic Matter (Tree-DOM) in Throughfall and Stemflow. Forests, 9(5), 236. https://doi.org/10.3390/f9050236