Leaf Venation Variation and Phenotypic Plasticity in Response to Environmental Heterogeneity in Parrotia subaequalis (H. T. Chang) R. M. Hao et H. T. Wei, An Endemic and Endangered Tree Species from China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Leaf Sample Collection
2.2. Leaf Venation
2.3. Vein Density Measurements
2.4. Data Analysis
3. Results
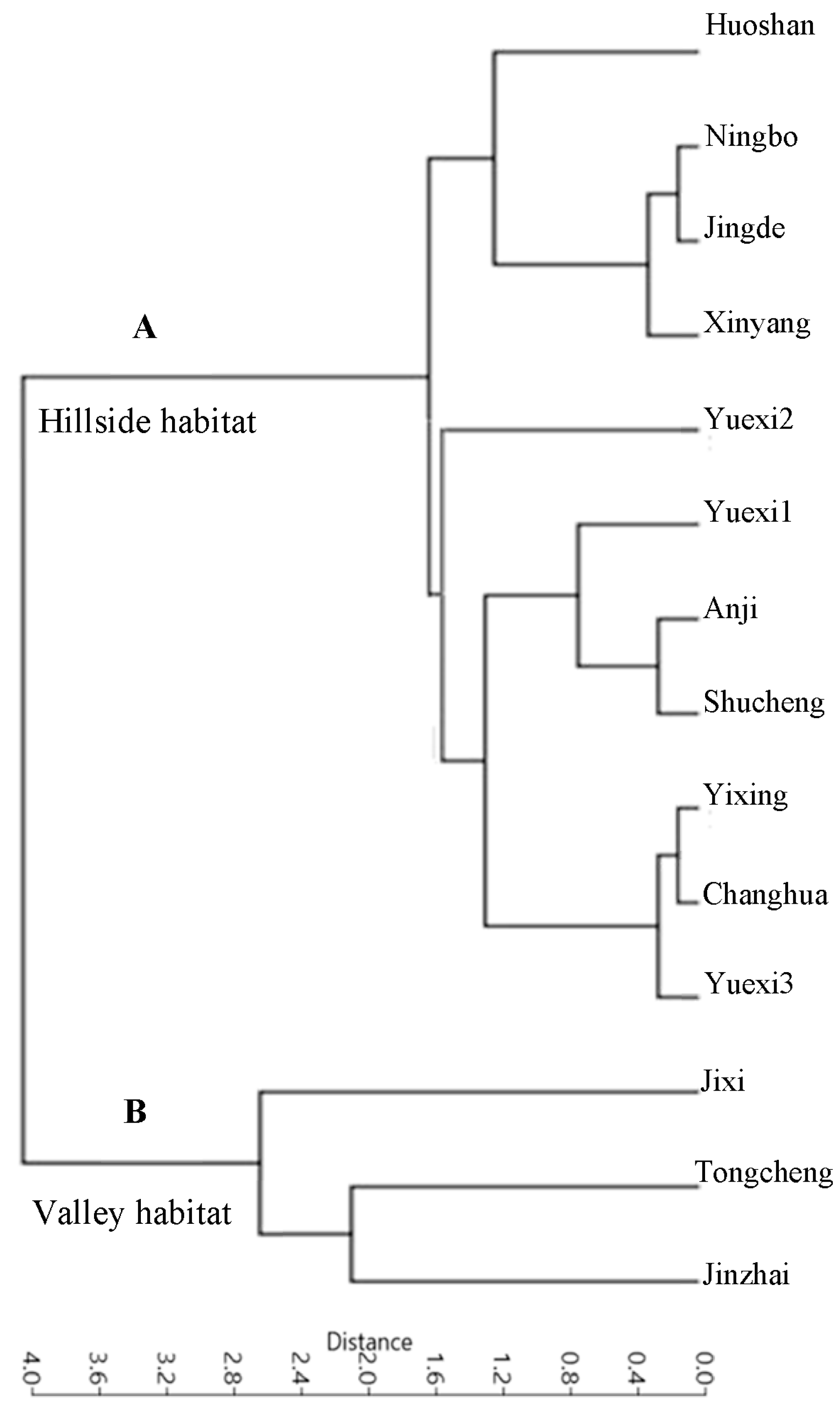
3.1. Leaf Venation Clustering Analysis of P. Subaequalis
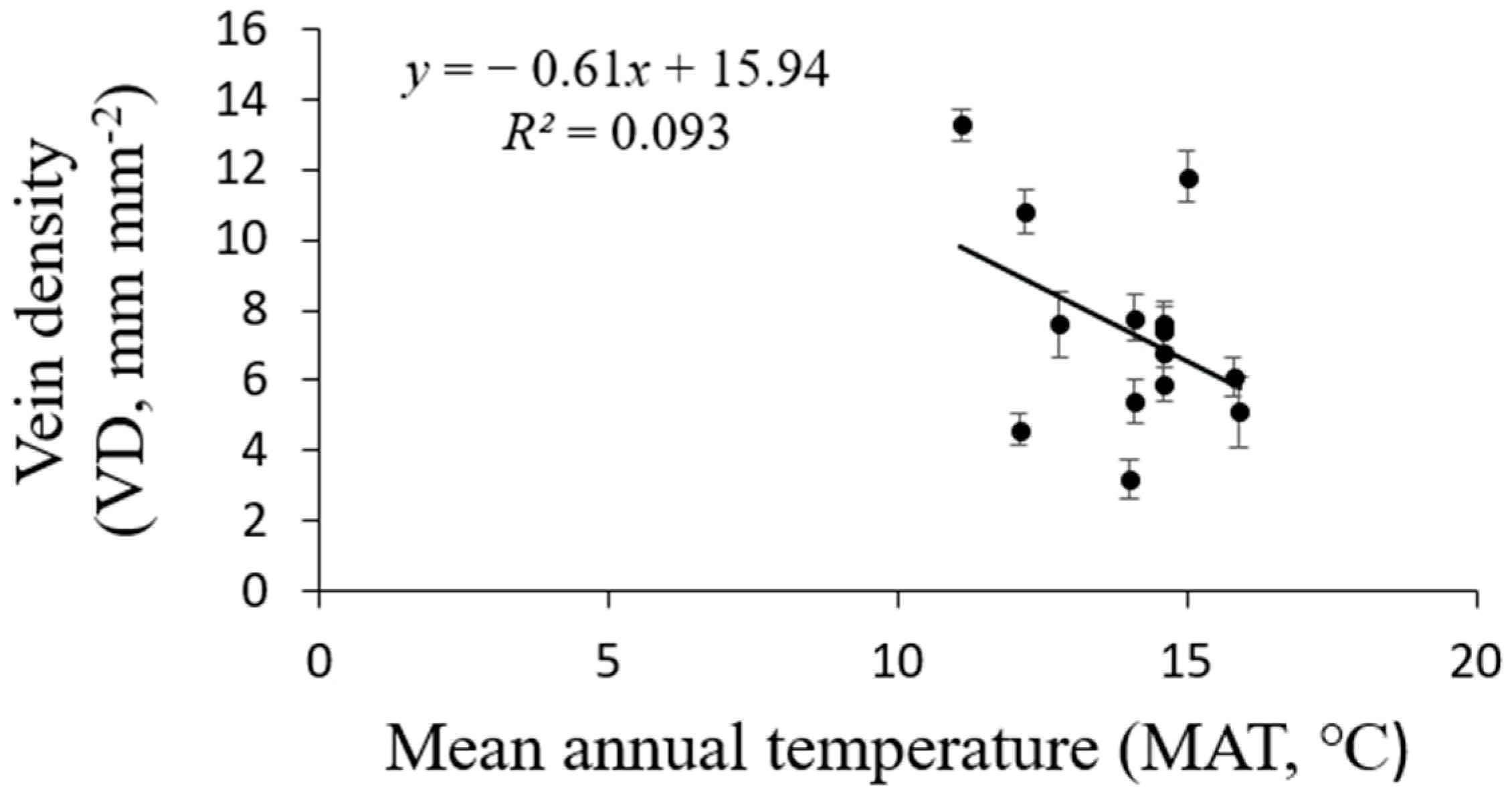
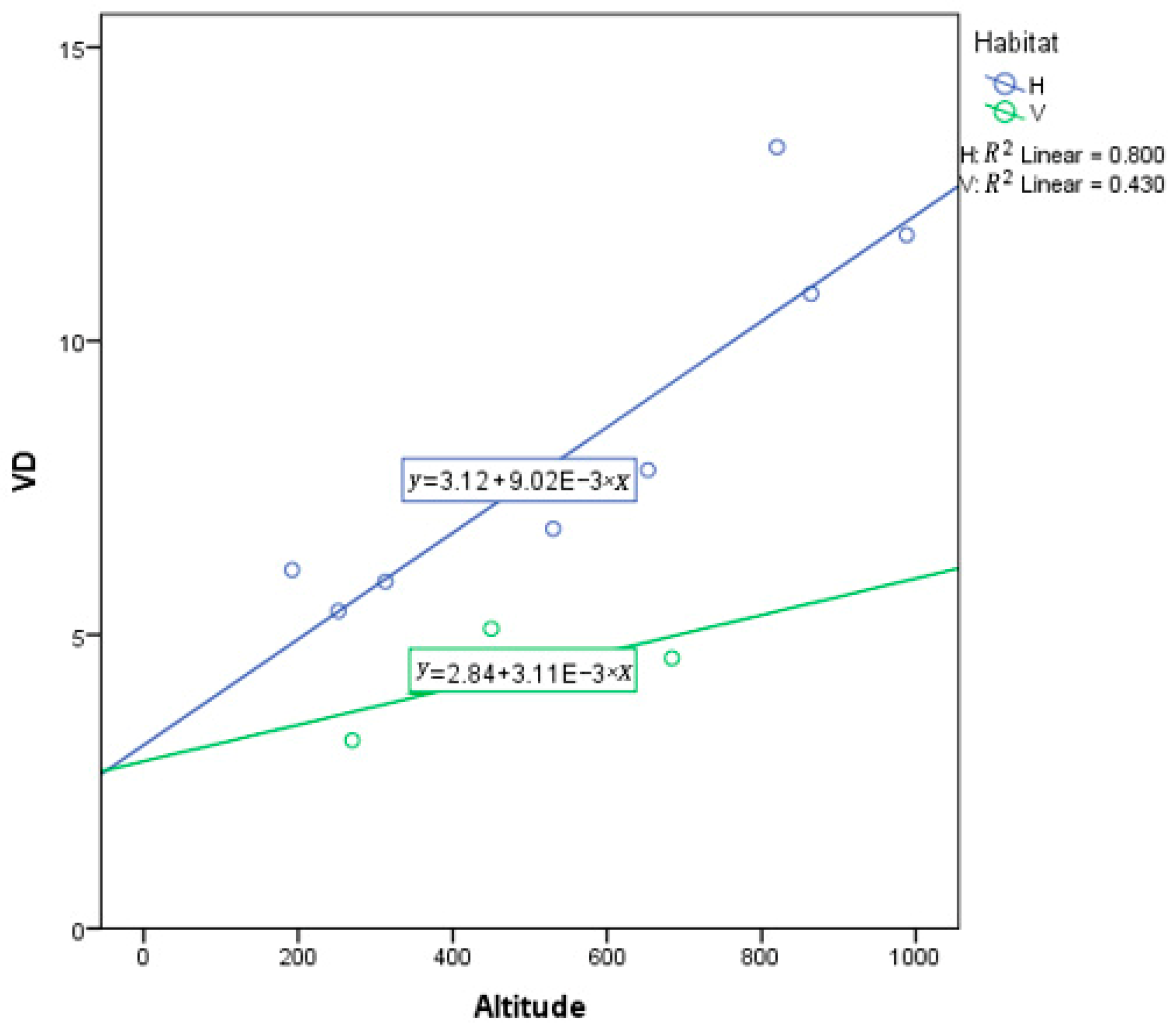
3.2. Effects of Elevation and Climatic Factors on VD
4. Discussion
Correlation between Vein Trait and Climatic Conditions
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Population | Laminar Shape | Base Shape | Base Angle | Primary Veins Size | The Pair of Major Secondary Veins | The Angle between Major Secondary Veins and Primary Veins | Intersecondary Veins | Intercostal Tertiary Veins Angle Varability | Variation of Major Secondary Angle to Tertiary | Tertiary Veins | Higher Level Veins | Veinlets | Areole | Areolation | Tooth |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Changhua (CH) | 0 | 0 | 2 | 1 | 5.7 | 0 | 1 | 0 | 3 | 0 | 0 | 2 | 2 | 2 | 1 |
| Anji (AJ) | 0 | 0 | 2 | 1 | 5.6 | 0 | 1 | 0 | 3 | 0 | 0 | 3 | 2 | 2 | 1 |
| Ningbo (NB) | 0 | 0 | 2 | 1 | 5.7 | 1 | 1 | 0 | 3 | 0 | 0 | 3 | 2 | 2 | 1 |
| Yixing (YX) | 0 | 0 | 2 | 1 | 5.7 | 1 | 1 | 0 | 3 | 0 | 0 | 3 | 2 | 2 | 1 |
| Jixi (JX) | 1 | 0 | 1 | 1 | 5.1 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 1 |
| Jingde (JD) | 0 | 0 | 2 | 1 | 5.8 | 1 | 1 | 0 | 3 | 0 | 0 | 3 | 2 | 2 | 1 |
| Tongcheng (TC) | 0 | 1 | 1 | 1 | 6.3 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 1 |
| Huoshan (HS) | 1 | 0 | 2 | 1 | 6 | 1 | 1 | 0 | 3 | 0 | 0 | 3 | 2 | 2 | 1 |
| Shucheng (SC) | 0 | 0 | 2 | 1 | 5.55 | 0 | 1 | 0 | 3 | 0 | 0 | 2 | 2 | 2 | 1 |
| Yuexi3 (YX3) | 0 | 0 | 2 | 1 | 5.8 | 0 | 1 | 0 | 3 | 0 | 0 | 3 | 2 | 2 | 1 |
| Yuexi2 (YX2) | 1 | 0 | 2 | 1 | 5.3 | 0 | 1 | 0 | 3 | 0 | 0 | 3 | 2 | 2 | 1 |
| Yuexi1 (YX1) | 0 | 0 | 2 | 1 | 6.3 | 0 | 1 | 0 | 3 | 0 | 0 | 3 | 2 | 2 | 1 |
| Jinzhai (JZ) | 0 | 0 | 1 | 1 | 6.2 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 1 |
| Xinyang (XY) | 0 | 0 | 2 | 1 | 6 | 1 | 1 | 0 | 3 | 0 | 0 | 3 | 2 | 2 | 1 |
| Tests of Between-Subjects Effects | ||||||
|---|---|---|---|---|---|---|
| Dependent Variable: VD | ||||||
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. | Partial Eta Squaed |
| Corrected Model | 87.946 a | 2 | 43.973 | 28.301 | 0.000 | 0.837 |
| Intercept | 8.209 | 1 | 8.209 | 5.284 | 0.042 | 0.324 |
| Altitude | 51.591 | 1 | 51.591 | 33.204 | 0.000 | 0.751 |
| Habitat | 22.112 | 1 | 22.112 | 14.231 | 0.003 | 0.564 |
| Error | 17.091 | 11 | 1.554 | |||
| Total | 868.72 | 14 | ||||
| Corrected Total | 105.037 | 13 | ||||
| Tests of Between-Subjects Effects | ||||||
|---|---|---|---|---|---|---|
| Dependent Variable: VD | ||||||
| Source | Type Ⅲ Sum of Squares | df | Mean Square | F | Sig. | Partial Eta Squaed |
| Corrected Model | 45.596 a | 2 | 22.798 | 4.219 | 0.044 | 0.434 |
| Intercept | 27.954 | 1 | 27.954 | 5.173 | 0.044 | 0.320 |
| MAT | 9.241 | 1 | 9.241 | 1.71 | 0.218 | 0.135 |
| Habitat | 35.849 | 1 | 35.849 | 6.634 | 0.026 | 0.376 |
| Error | 59.441 | 11 | 5.404 | |||
| Total | 868.72 | 14 | ||||
| Corrected Total | 105.037 | 13 | ||||
| Tests of Between-Subjects Effects | ||||||
|---|---|---|---|---|---|---|
| Dependent Variable: VD | ||||||
| Source | Type Ⅲ Sum of Squares | df | Mean Square | F | Sig. | Partial Eta Squaed |
| Corrected Model | 63.194 a | 2 | 31.597 | 8.306 | 0.006 | 0.602 |
| Intercept | 43.208 | 1 | 43.208 | 11.359 | 0.006 | 0.508 |
| MAP | 26.838 | 1 | 26.838 | 7.055 | 0.022 | 0.391 |
| Habitat | 8.702 | 1 | 8.702 | 2.288 | 0.159 | 0.172 |
| Error | 41.843 | 11 | 3.804 | |||
| Total | 868.72 | 14 | ||||
| Corrected Total | 105.037 | 13 | ||||
References
- McELwain, J.C.; Yiotis, C.; Lawson, T. Using modern plant trait relationships between observed and theoretical maximum stomatal conductance and vein density to examine patterns of plant macroevolution. New Phytol. 2016, 209, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Y.; Zhang, Z.X.; College of Nature Conservation; Beijing Forestry University. Leaf venation and its taxonomical significance in Sect. Denticulatae (Salix) of China. Bull. Bot. Res. 2017, 37, 481–491. [Google Scholar]
- Sack, L.; Scoffoni, C.; Mckown, A.D.; Frole, K.; Rawls, M.; Havran, J.C.; Tran, H.; Tran, T. Developmentally based scaling of leaf venation architecture explains global patterns. Nat. Commun. 2012, 3, 837. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Wang, Z.X.; Li, R.Y.; Deng, P.; Ma, L.; Sun, B.-N. Vein density of angiosperms as a paleoclimate proxy: A case study using fossil leaves of Zelkova, and Machilus. Palaeoworld 2016, 25, 60–66. [Google Scholar] [CrossRef]
- Krober, W.; Heklau, H.; Bruelheide, H. Leaf morphology of 40 evergreen and deciduous broad leaved subtropical tree species and relationships to functional ecophysiological traits. Plant Biol. 2015, 17, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Zhang, Z.; Li, X. Influences of environmental factors on leaf morphology of Chinese jujubes. PLoS ONE 2015, 10, e0127825. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Scoffoni, C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Dunbar-Co, S.; Sporck, M.J.; Sack, L. Leaf trait diversification and design in seven rare taxa of the Hawaiian Plantago radiation. Int. J. Plant Sci. 2009, 170, 61–75. [Google Scholar]
- Sellin, A.; Õunapuu, E.; Kaurilind, E.; Alber, M. Size-dependent variability of leaf and shoot hydraulic conductance in silver birch. Trees 2012, 26, 821–831. [Google Scholar] [CrossRef]
- Xie, S.P.; Sun, B.N.; Zhao, J.Y. Leaf venation density of Ginkgo biloba L. as a palaeoclimaticproxy. In Proceedings of the 26th Annual Seminar of China Ancient Society (ASCAS 2011), Guizhou, China, 18–20 October 2011; p. 166. [Google Scholar]
- Brodribb, T.J.; Feild, T.S.; Sack, L. Viewing leaf structure and evolution from a hydraulic perspective. Funct. Plant Biol. 2010, 37, 488–498. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, J. Plant Morphology, Structure and Environment; Lanzhou University Press: Lanzhou, China, 1989. [Google Scholar]
- Zhu, Y.; Kang, H.; Xie, Q.; Wang, Z.; Yin, S.; Liu, C. Pattern of leaf vein density and climate relationship of Quercus variabilis populations remains unchanged with environmental changes. Trees 2012, 26, 597–607. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Xu, X.; Niu, H. Understanding the wide geographic range of a clonal perennial grass: Plasticity versus local adaptation. AoB Plants 2016, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Roth-Nebelsick, A.; Uhl, D.; Mosbrugger, V.; Kerp, H. Evolution and function of leaf venation architecture: A review. Ann. Bot. 2001, 87, 553–566. [Google Scholar] [CrossRef]
- Carins Murphy, M.R.; Jordan, G.J.; Brodribb, T.J. Cell expansion not cell differentiation predominantly coordinates veins and stomata within and among herbs and woody angiosperms grown under sun and shade. Ann. Bot. 2016, 118, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Carins Murphy, M.R.; Jordan, G.J.; Brodribb, T.J. Acclimation to humidity modifies the link between leaf size and the density of veins and stomata. Plant Cell Environ. 2014, 37, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.L.; Chen, Y.J.; Brodribb, T.J.; Cao, K.-F. Weak coordination between vein and stomatal densities in 105 angiosperm tree species along altitudinal gradients in Southwest China. Funct. Plant Biol. 2016, 43. [Google Scholar] [CrossRef]
- Price, C.A.; Munro, P.R.; Weitz, J.S. Estimates of leaf vein density are scale dependent. Plant Physiol. 2014, 164, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Caringella, M.A.; Bongers, F.J.; Sack, L. Leaf hydraulic conductance varies with vein anatomy across Arabidopsis thaliana wild-type and leaf vein mutants. Plant Cell Environ. 2015, 38, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.M.; Wei, H.T. A new combination of Hamamelidaceae. Acta Phytotaxon. Sin. 1998, 36, 80. [Google Scholar]
- Chang, H.D. Hamamelidaceae. In Flora Republicae Popularis Sinica; Chang, H.D., Ed.; Science Press: Beijing, China, 1997; Volume 35, pp. 73–74. [Google Scholar]
- Ellie, M.W.; Jayanthi, N.; Yang, X.Y.; Ballesteros, D.; Sun, W.; Pritchard, H.W. Plant species with extremely small populations (PSESP) in China: A seed and spore biology perspective. Plant. Divers. 2016, 38, 209–220. [Google Scholar]
- Li, W.; Zhang, G.F. Population structure and spatial pattern of the endemic and endangered subtropical tree Parrotia subaequalis (Hamamelidaceae). Flora 2015, 212, 10–18. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y. China Species Red List; Higher Education Press: Beijing, China, 2004; Volume 1. [Google Scholar]
- Schneider, J.V.; Negraschis, V.; Habersetzer, J.; Rabenstein, R.; Wesenberg, J.; Wesche, K.; Zizka, G. Taxonomic diversity masks leaf vein–climate relationships: Lessons from herbarium collections across a latitudinal rainfall gradient in West Africa. Bot. Lett. 2018. [Google Scholar] [CrossRef]
- Yin, M.Y.; Jiang, Z.M.; Zhu, X.C.; Bao, W.-Q.; Zhao, H.; Wuyun, T. High-level phenotypic variations in populations of Armeniaca sibirica in Nei Mongol, China. Chin. J. Plant Ecol. 2016, 40, 1090–1099. [Google Scholar]
- Liu, J.Q.; Yin, M.Y.; Zuo, S.Y.; Yang, S.-B.; Tana, W. Phenotypic variations in natural populations of Amygdalus pedunculate. Chin. J. Plant Ecol. 2017, 41, 1091–1102. [Google Scholar]
- China Meteorological Data Service Center(CMDC). Available online: www. cdc.cma.gov.cn (accessed on 3 May 2018).
- Dizeo, D.; Strittmatter, C.G. Nueva técnica de diafanización. Bol. Soc. Argent. Bot. 1973, 15, 126–129. [Google Scholar]
- Zhang, Y.; Yang, S.J.; Sun, M.; Cao, K.-F. Stomatal traits are evolutionarily associated with vein density in basal angiosperms. Plant Sci. J. 2014, 32, 320–328. [Google Scholar]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Jaleel, C.A.; Manivannan, P.; Wallid, A.; Farooq, M.; Jasim Al-Juburi, H.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Crifo, C.; Currano, E.D.; Baresch, A.; Jaramillo, C. Variations in angiosperm leaf vein density have implications for interpreting life form in the fossil record. Geology 2014, 42, 919–922. [Google Scholar] [CrossRef]
- Mario, P.; Paolo, S. Leaf vein density and photosynthetic rate in Rosa: Is there a correlation? Bol. Soc. Argent. Bot. 2016, 51, 683–687. [Google Scholar]
- Blonder, B.; Enquist, B.J. Inferring climate from angiosperm leaf venation networks. New Phytol. 2014, 204, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Jordan, G.J.; Carpenter, R.J. Unified changes in cell size permit coordinated leaf evolution. New Phytol. 2013, 199, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, L.G.; Pias, B. Convergence in leaf phenology traits of two understory ferns in the northwestern Iberian Peninsula. Jour. Plant Ecol. 2018, 11, 92–102. [Google Scholar]
- Geekiyanage, N.; Goodale, U.M.; Cao, K.; Kitajima, K. Leaf trait variation associated with habitat affinity of tropical karst tree species. Ecol. Evol. 2018, 8, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Holbrook, N.M. Declining hydraulic efficiency as transpiring leaves desiccate: Two types of response. Plant Cell Environ. 2006, 29, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.; Daily, D.C.; Hickey, L.J.; Mitchell, J.V.; Johnson, K.R.; Wilf, P.; Wing, S.L. Manual of Leaf Architecture; Cornell University Press: New York, NY, USA, 2009. [Google Scholar]
- Scoffoni, C.; Rawls, M.; McKown, A.; Cochard, H.; Sack, L. Decline of leaf hydraulic conductance with dehydration: Relationship to leaf size and venation architecture. Plant Physiol. 2011, 156, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Ballian, D.; Faruk, B.; Azra, Č.; Saša, P.; Jozo, F. Population differentiation in the wild cherry (Prunus avium L.) in Bosnia and Herzegovina. Period. Biol. 2012, 114, 43–54. [Google Scholar]
- Boyce, C.K.; Brodribb, T.J.; Field, T.S.; Zwieniecki, M.A. Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc. Biol. Sci. 2009, 276, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Guan, Z.J.; Sun, M.; Zhang, J.J.; Cao, K.F.; Hu, H. Evolutionary association of stomatal traits with leaf vein density in Paphiopedilum, Orchidaceae. PLoS ONE 2012, 7, e40080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q. Floristic analysis on Anhui Wanfo Provincial Nature Reserve. Anhui For. Sci. Technol. 2014, 40, 56–58. [Google Scholar]
- Murphy, M.R.C.; Jordan, G.J.; Brodribb, T.J. Differential leaf expansion can enable hydraulic acclimation o sun and shade. Plant Cell Environ. 2012, 35, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Zwieniecki, M.A.; Boyce, C.K.; Holbrook, N.M. Hydraulic limitations imposed by crown placement determine final size and shape of Quercus rubra L. leaves. Plant Cell Environ. 2004, 27, 357–365. [Google Scholar] [CrossRef]
- McAdam, S.A.M.; Eléouët, M.P.; Best, M.; Brodribb, T.J.; Murphy, M.R.C.; Cook, S.D.; Dalmais, M.; Dimitriou, T.; Gelinas-Marion, A.; Gill, W.M.; et al. Linking auxin with photosynthetic rate via leaf venation. Plant Physiol. 2017, 175, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.Q.; Yan, J.F.; Weng, Q. Population ecological status quo and protection study on Shaniondendron subaequalis MB Deng, HT Wei in Longchi Mountion Reserve in Yixing. J. Jiangsu For. Sci. Technol. 2004, 31, 4–5. [Google Scholar]
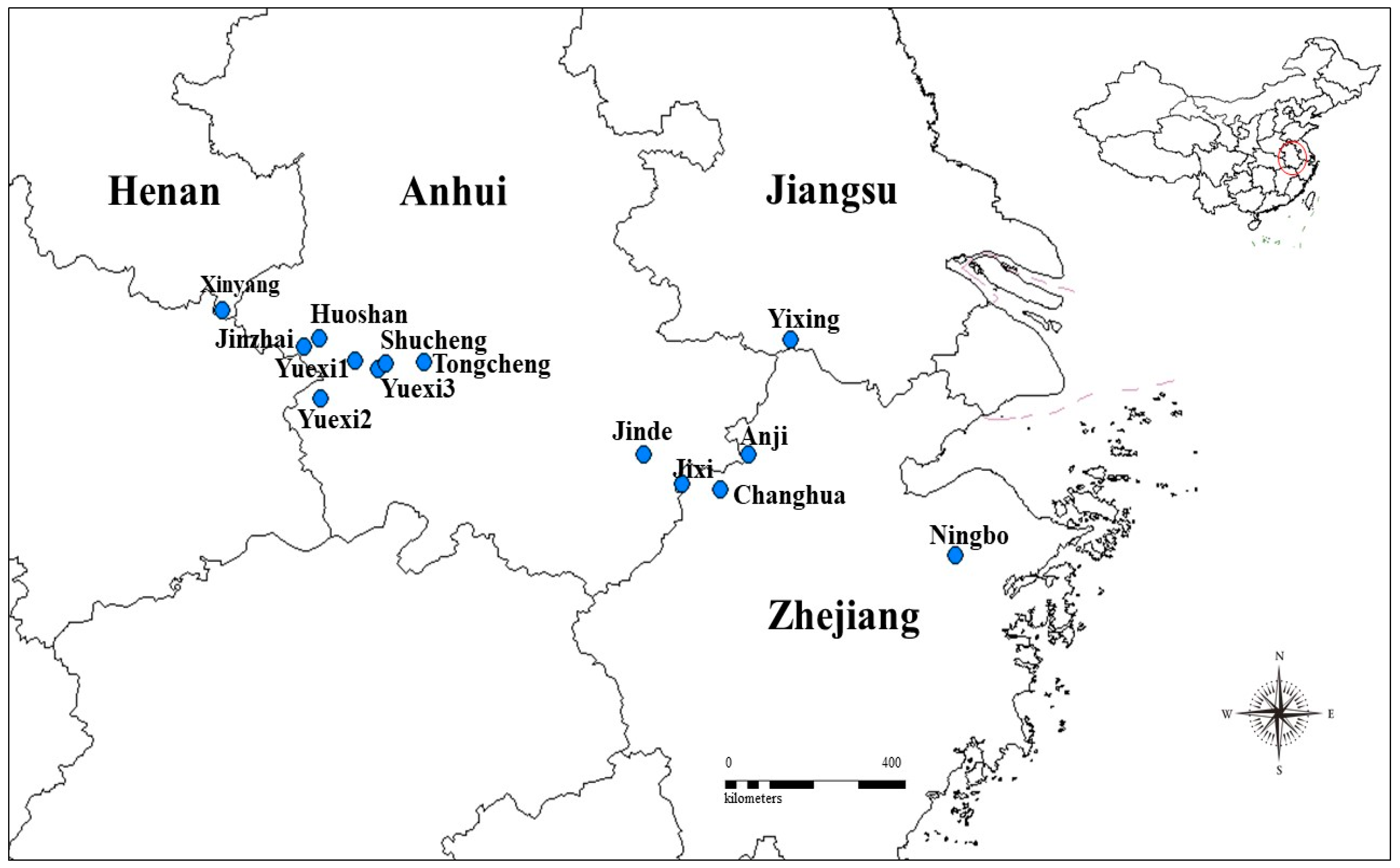

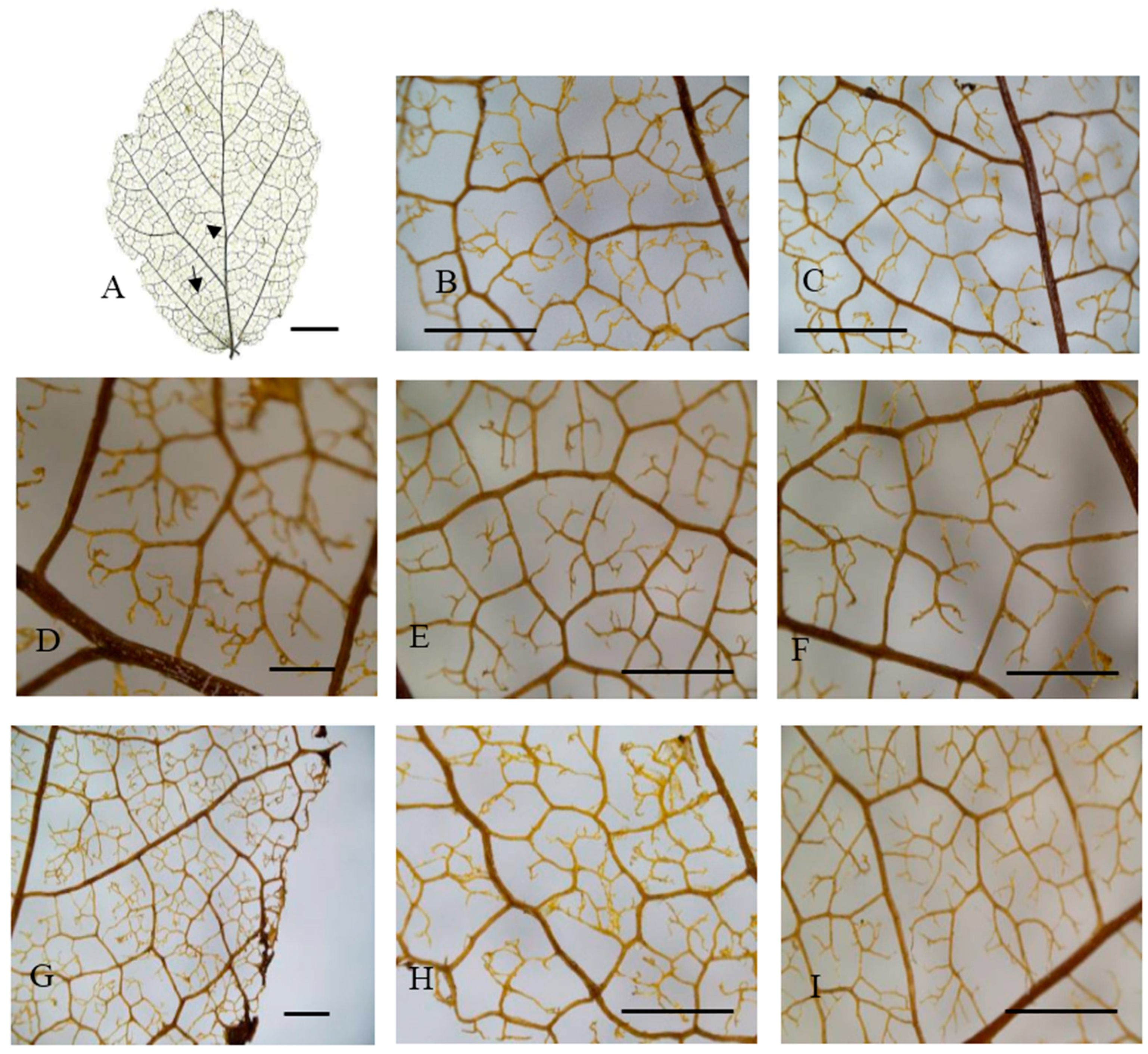
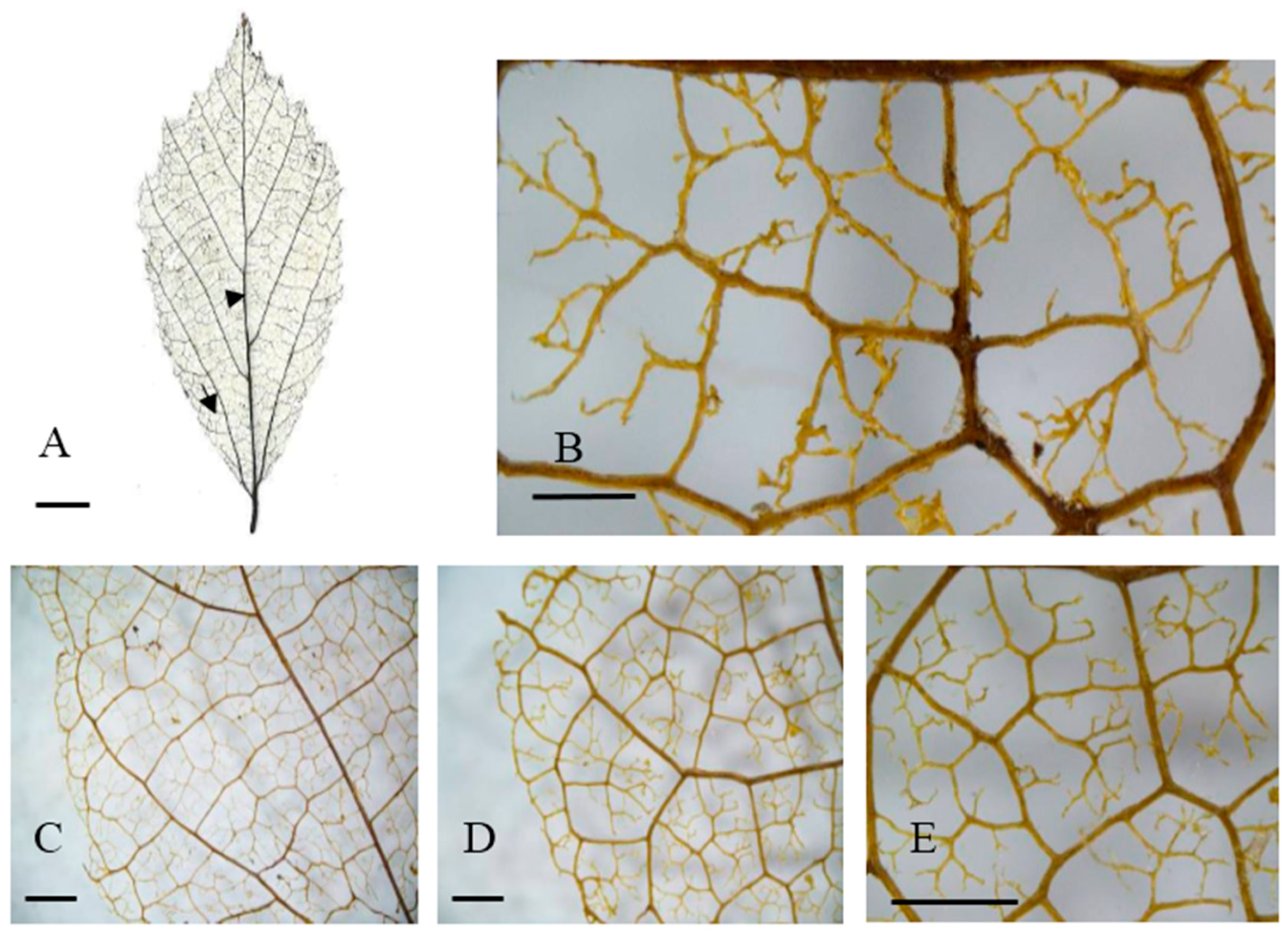




| Population No. and Code | Sample Location | Altitude (m) | Latitude (N) | Longitude (E) | Habitat | Sample Size | Mean Soil Depth (cm) | Average Annual Precipitation (mm) | Average Annual Air Temperature (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 1. CH | Changhua, Zhejiang | 864 | 30°10′ | 119°11′ | Hillside | 10 | 25.5 | 1123.6 | 12.2 |
| 2. AJ | Anji, Zhejiang | 820 | 30°23′ | 119°24′ | Hillside | 10 | 11 | 1220.1 | 15 |
| 3. NB | Ningbo, Zhejiang | 988 | 29°41′ | 121°02′ | Hillside | 10 | 20 | 1110.4 | 11.1 |
| 4. YX | Yixing, Jiangsu | 252 | 31°14′ | 119°44′ | Hillside | 10 | 27 | 1294.6 | 14.1 |
| 5. JX | Jixi, Anhui | 684 | 30°12′ | 118°53′ | Valley | 10 | 39 | 1357.2 | 12.1 |
| 6. JD | Jingde, Anhui | 653 | 30°25′ | 118°35′ | Hillside | 10 | 21 | 1286.1 | 14.1 |
| 7. TC | Tongcheng, Anhui | 270 | 31°05′ | 116°51′ | Valley | 10 | 34 | 1290.5 | 14 |
| 8. HS | Huoshan, Anhui | 530 | 31°15′ | 116°01′ | Hillside | 10 | 12 | 1351.3 | 14.6 |
| 9. SC | Shucheng, Anhui | 584 | 31°4′ | 116°33′ | Hillside | 10 | 28 | 1171.8 | 12.8 |
| 10. JZ | Jinzhai, Anhui | 450 | 31°12′ | 115°54′ | Valley | 10 | 42 | 1419.9 | 15.9 |
| 11. YX3 | Yuexi, Anhui | 583 | 31°02′ | 116°29′ | Hillside | 10 | 26 | 1296.4 | 14.6 |
| 12. YX2 | Yuexi, Anhui | 313 | 30°49′ | 116°02′ | Hillside | 10 | 24 | 1290.4 | 14.6 |
| 13. YX1 | Yuexi, Anhui | 449 | 31°06′ | 116°19′ | Hillside | 10 | 25 | 1290.4 | 14.6 |
| 14. XY | Xinyang, Henan | 192 | 31°27′ | 115°16′ | Hillside | 10 | 26 | 1380.5 | 15.8 |
| Code | Characteristics | Character States |
|---|---|---|
| 1 | Laminar Shape | (0) Broadly Obovate; (1) Oblong |
| 2 | Base Shape | (0) Rounded; (1) Concave |
| 3 | Base Angle | (0) Acute; (1) Right Angle; (2) Obtuse |
| 4 | Primary Veins Size | (0) vw/lw * × 100% = 1.25–2%; (1) vw/lw × 100% < 1.25% |
| 5 | The Pair of Major Secondary Veins | Take the Average Value |
| 6 | The Angle between Major Secondary Veins and Primary Veins | (0) Narrow Acute (<45°); (1) Moderate (45°–65°); (2) Wide Acute (65°–80°) |
| 7 | Intersecondary Veins | (0) Simple; (1) Compound; (2) Mixed Simple and Compound |
| 8 | Intercostal Tertiary Veins Angle Varability | (0) Consistent; (1) Increasing proximally; (2) Decreasing proximally |
| 9 | Variation of Major Secondary Angle to Tertiary | (0) Acute; (1) Acute-Obtuse; (2) Acute-Right Angle; (3) Right Angle; (4) Right Angle-Obtuse; (5) Obtuse |
| 10 | Tertiary Veins | (0) Reticulate; (1) Percurrent; (2) Mixed Reticulate and Percurrent |
| 11 | Higher Level Veins | (0) Reticulate; (1) Percurrent; (2) Mixed Reticulate and Percurrent |
| 12 | Veinlets | (0) Absent; (1) 1-branched; (2) 2-branched; (3) 3-branched |
| 13 | Areole | (0) Mostly Absent (1) Moderate development; (2) Good development |
| 14 | Areolation | (0) Good development; (1) Moderate development; (2) Poor development; (3) Lacking |
| 15 | Tooth | (0) Entire; (1) Glandular |
| Population | Laminar Shape | Baes Angle | Intercostal Tertiary Vein Fabric | Areole Development | Free Ending Veinlet (FEV) Branching | Areole Shape |
|---|---|---|---|---|---|---|
| Changhua (CH) | Broadly Obovate | Obtuse | R | Moderate | two branched | Q and P, rarely T |
| Anji (AJ) | Broadly Obovate | Obtuse | R | Moderate | three branched | Q and P, rarely T |
| Ningbo (NB) | Broadly Obovate | Obtuse | R | Moderate | two or three branched | Q and T |
| Yixing (YX) | Broadly Obovate | Obtuse | R | Moderate | two branched | Q and P, rarely T |
| Jixi (JX) | Oblong | Right Angle | R | Moderate | two branched | Q and P, rarely T |
| Jingde (JD) | Broadly Obovate | Obtuse | R | Moderate | three branched | variable |
| Tongcheng (TC) | Broadly Obovate | Right Angle | R | Moderate | two branched | variable |
| Huoshan (HS) | Oblong | Obtuse | R | Moderate | three branched | Q and P, rarely T |
| Shucheng (SC) | Broadly Obovate | Obtuse | R | Moderate | three branched | Q and P, rarely T |
| Yuexi3 (YX3) | Broadly Obovate | Obtuse | R | Moderate | two branched | Q and P, rarely T |
| Yuexi2 (YX2) | Oblong | Obtuse | R | Moderate | three branched | Q and P, rarely T |
| Yuexi1 (YX1) | Broadly Obovate | Obtuse | R | Moderate | three branched | Q and P, rarely T |
| Jinzhai (JZ) | Broadly Obovate | Right Angle | R | Moderate | two branched | variable |
| Xinyang (XY) | Broadly Obovate | Obtuse | R | Moderate | three branched | Q and P, rarely T |
| No. Population, Province | Altitude (m) | Habitat | Vein Density (mm mm−2) |
|---|---|---|---|
| VD (Mean ± SD) | |||
| 1. CH, Zhejiang | 864 | Hillside | 10.8 ± 0.88 |
| 2. AJ, Zhejiang | 820 | Hillside | 13.3 ± 1.02 |
| 3. NB, Zhejiang | 988 | Hillside | 11.8 ± 0.92 |
| 4. YX, Jiangsu | 252 | Hillside | 5.4 ± 0.61 |
| 5. JX, Anhui | 684 | Valley | 4.6 ± 0.45 |
| 6. JD, Anhui | 653 | Hillside | 7.8 ± 0.64 |
| 7. TC, Anhui | 270 | Valley | 3.2 ± 0.55 |
| 8. HS, Anhui | 530 | Hillside | 6.8 ± 0.64 |
| 9. SC, Anhui | 584 | Hillside | 7.6 ± 0.73 |
| 10. JZ, Anhui | 450 | Valley | 5.1 ± 0.45 |
| 11. YX3, Anhui | 583 | Hillside | 7.6 ± 0.68 |
| 12. YX2, Anhui | 313 | Hillside | 5.9 ± 0.51 |
| 13. YX1, Anhui | 449 | Hillside | 7.4 ± 0.68 |
| 14. XY, Henan | 192 | Hillside | 6.1 ± 0.54 |
| Min–max | 3.2–13.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yang, J.; Huang, Y.; Jia, Z.; Fang, Y. Leaf Venation Variation and Phenotypic Plasticity in Response to Environmental Heterogeneity in Parrotia subaequalis (H. T. Chang) R. M. Hao et H. T. Wei, An Endemic and Endangered Tree Species from China. Forests 2018, 9, 247. https://doi.org/10.3390/f9050247
Zhang L, Yang J, Huang Y, Jia Z, Fang Y. Leaf Venation Variation and Phenotypic Plasticity in Response to Environmental Heterogeneity in Parrotia subaequalis (H. T. Chang) R. M. Hao et H. T. Wei, An Endemic and Endangered Tree Species from China. Forests. 2018; 9(5):247. https://doi.org/10.3390/f9050247
Chicago/Turabian StyleZhang, Lifang, Jing Yang, Yang Huang, Zhiyi Jia, and Yanming Fang. 2018. "Leaf Venation Variation and Phenotypic Plasticity in Response to Environmental Heterogeneity in Parrotia subaequalis (H. T. Chang) R. M. Hao et H. T. Wei, An Endemic and Endangered Tree Species from China" Forests 9, no. 5: 247. https://doi.org/10.3390/f9050247
APA StyleZhang, L., Yang, J., Huang, Y., Jia, Z., & Fang, Y. (2018). Leaf Venation Variation and Phenotypic Plasticity in Response to Environmental Heterogeneity in Parrotia subaequalis (H. T. Chang) R. M. Hao et H. T. Wei, An Endemic and Endangered Tree Species from China. Forests, 9(5), 247. https://doi.org/10.3390/f9050247




