Species- and Elevation-Dependent Growth Responses to Climate Warming of Mountain Forests in the Qinling Mountains, Central China
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area and Climate Data
2.2. Tree-Ring Sampling and Chronology Development
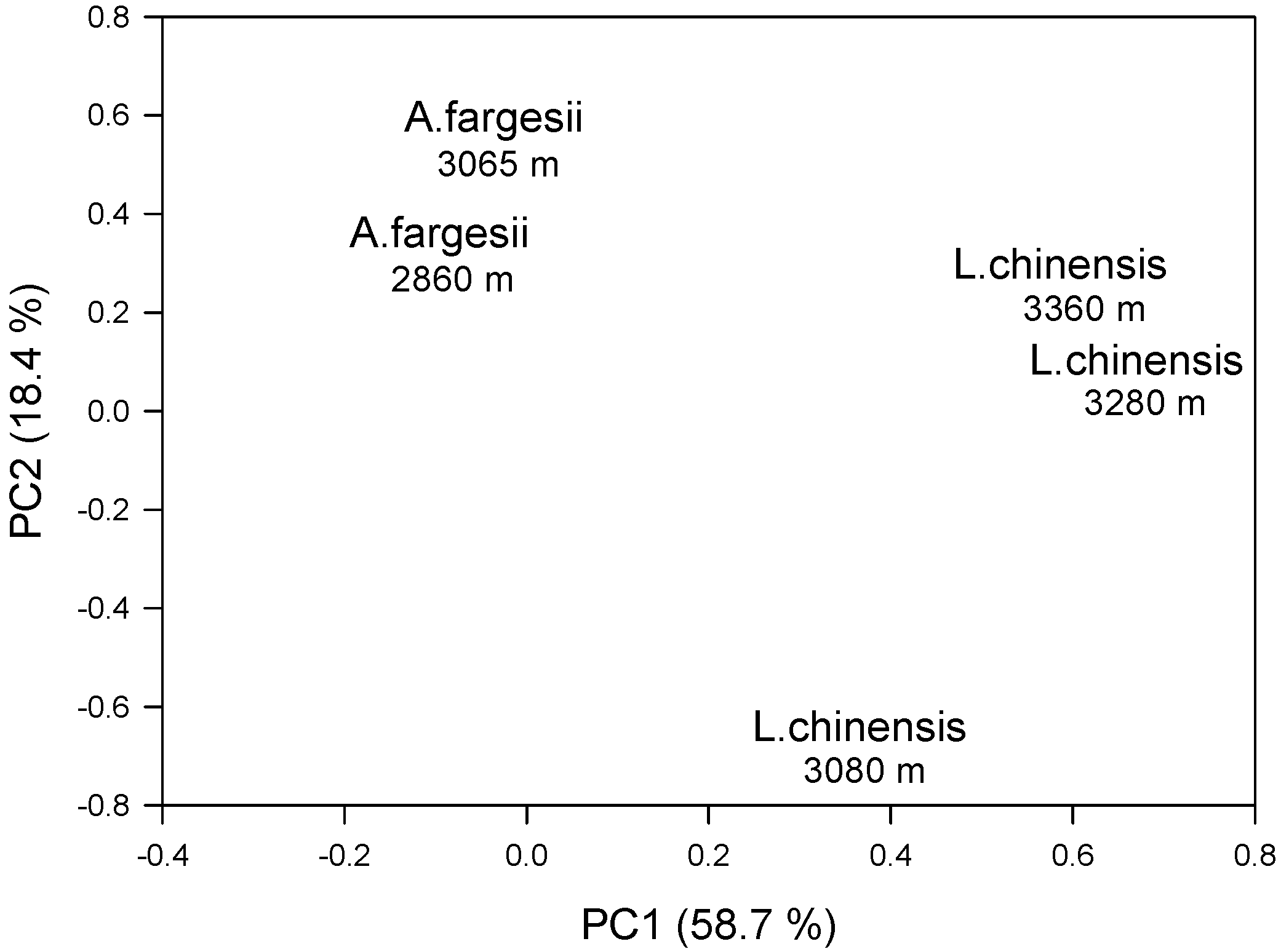
2.3. Spatial- and Species-Specific Analyses of Tree-Ring Width Chronologies
2.4. Relationships between Tree Growth and Climate
3. Results
3.1. Climate Trends
3.2. Characteristics of the Tree-Ring Width Chronologies
3.3. Topographic and Species-Related Influences on Growth Responses to Climate
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Liu, H.; Williams, A.P.; Allen, C.D.; Guo, D.; Wu, X.; Anenkhonov, O.A.; Liang, E.; Sandanov, D.V.; Yin, Y.; Qi, Z.; et al. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Glob. Chang. Biol. 2013, 19, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Fang, O.; Alfaro, R.I.; Zhang, Q.B. Tree rings reveal a major episode of forest mortality in the late 18th century on the Tibetan Plateau. Glob. Planet. Chang. 2018, 163, 44–50. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Evans, M.N.; Lyu, L. Moisture dipole over the Tibetan Plateau during the past five and a half centuries. Nat. Commun. 2015, 6, 8062. [Google Scholar] [CrossRef] [PubMed]
- Liang, E.; Leuschner, C.; Dulamsuren, C.; Wagner, B.; Hauck, M. Global warming-related tree growth decline and mortality on the north-eastern Tibetan Plateau. Clim. Chang. 2016, 134, 163–176. [Google Scholar] [CrossRef]
- Salzer, M.W.; Hughes, M.K.; Bunn, A.G.; Kipfmueller, K.F. Recent unprecedented tree-ring growth in bristlecone pine at the highest elevations and possible causes. Proc. Natl. Acad. Sci. USA 2009, 106, 20348–20353. [Google Scholar] [CrossRef] [PubMed]
- Liang, E.; Shao, X.; Xu, Y. Tree-ring evidence of recent abnormal warming on the southeast Tibetan Plateau. Theor. Appl. Climatol. 2009, 98, 9–18. [Google Scholar] [CrossRef]
- Littell, J.S.; Peterson, D.L.; Tjoelker, M. Douglas-fir growth in mountain ecosystems: Water limits tree growth from stand to region. Ecol. Monogr. 2008, 78, 349–368. [Google Scholar] [CrossRef]
- Chen, D.; Fang, K.; Li, Y.; Dong, Z.; Zhang, Y.; Zhou, F. Response of Pinus taiwanensis growth to climate changes at its southern limit of Daiyun Mountain, mainland China Fujian Province. Sci. China-Earth Sci. 2016, 59, 328–336. [Google Scholar] [CrossRef]
- Camarero, J.J.; Fajardo, A. Poor acclimation to current drier climate of the long-lived tree species Fitzroya cupressoides in the temperate rainforest of southern Chile. Agric. For. Meteorol. 2017, 239, 141–150. [Google Scholar] [CrossRef]
- Ashton, M.S.; Tyrrell, M.L.; Spalding, D.; Gentry, B. Managing Forest Carbon in a Changing Climate; Springer: New York, NY, USA, 2012. [Google Scholar]
- Blois, J.L.; Williams, J.W.; Fitzpatrick, M.C.; Jackson, S.T.; Ferrier, S. Space can substitute for time in predicting climate-change effects on biodiversity. Proc. Natl. Acad. Sci. USA 2013, 110, 9374–9379. [Google Scholar] [CrossRef] [PubMed]
- Elmendorf, S.C.; Henry, G.H.R.; Hollister, R.D.; Fosaa, A.M.; Gould, W.A.; Hermanutz, L.; Hofgaard, A.; Jónsdóttir, I.S.; Jorgenson, J.C.; Lévesque, E.; et al. Experiment, monitoring, and gradient methods used to infer climate change effects on plant communities yield consistent patterns. Proc. Natl. Acad. Sci. USA 2015, 112, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Liu, H.; Wu, X.; Hao, Q. Climate-driven speedup of alpine treeline forest growth in the Tianshan Mountains, northwestern China. Glob. Chang. Biol. 2015, 21, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Bayramzadeh, V.; Zhu, H.; Lu, X.; Attarod, P.; Zhang, H.; Li, X.; Asad, F.; Liang, E. Temperature variability in northern Iran during the past 700 years. Sci. Bull. 2018, 63, 462–464. [Google Scholar] [CrossRef]
- Hartl-Meier, C.; Dittmar, C.; Zang, C.; Rothe, A. Mountain forest growth response to climate change in the Northern Limestone Alps. Trees 2014, 28, 819–829. [Google Scholar] [CrossRef]
- Zimmermann, J.; Hauck, M.; Dulamsuren, C.; Leuschner, C. Climate warming-related growth decline affects Fagus sylvatica, but not other broad-leaved tree species in central European mixed forests. Ecosystems 2015, 18, 560–572. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zhang, Q.; Zeng, X.; Xu, G.; Wu, G.; Wang, W. Species-specific tree growth and intrinsic water-use efficiency of Dahurian larch (Larix gmelinii) and Mongolian pine (Pinus sylvestris var. Mongolica) growing in a boreal permafrost region of the greater Hinggan Mountains, northeastern China. Agric. For. Meteorol. 2018, 248, 145–155. [Google Scholar]
- Dang, H.; Zhang, Y.; Zhang, K.; Jiang, M.; Zhang, Q. Age structure and regeneration of subalpine fir (Abies fargesii) forests across an altitudinal range in the Qinling Mountains, China. For. Ecol. Manag. 2010, 259, 547–554. [Google Scholar] [CrossRef]
- Liu, Y.; Linderholm, H.W.; Song, H.; Cai, Q.; Tian, Q.; Sun, J.; Chen, D.; Simelton, E.; Seftigen, K.; Tian, H.; et al. Temperature variations recorded in Pinus tabulaeformis tree rings from the southern and northern slopes of the central Qinling mountains, central China. Boreas 2009, 38, 285–291. [Google Scholar] [CrossRef]
- Jiang, C.; Mu, X.; Wang, F.; Zhao, G. Analysis of extreme temperature events in the Qinling Mountains and surrounding area during 1960–2012. Quat. Int. 2016, 392, 155–167. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, R.; Wang, H.; Qin, L. Recent climate warming of central China reflected by temperature-sensitive tree growth in the eastern Qinling Mountains and its linkages to the pacific and Atlantic oceans. J. Mt. Sci. 2015, 12, 396–403. [Google Scholar] [CrossRef]
- Cook, E.; Kairiukstis, L. Methods of Dendrochronology: Applications in the Environmental Sciences; Kluwer: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–75. [Google Scholar]
- Cook, E. A time-Series Analysis Approach to Tree Ring Standardization. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1985. [Google Scholar]
- Fritts, H.C. Tree Rings and Climate; Blackburn Press: Caldwell, ID, USA, 2001. [Google Scholar]
- Splechtna, B.E.; Dobry, J.; Klinka, K. Tree-ring characteristics of subalpine fir (Abies lasiocarpa (hook.) nutt.) in relation to elevation and climatic fluctuations. Ann. For. Sci. 2000, 57, 89–100. [Google Scholar] [CrossRef]
- Körner, C. Alpine Treelines: Functional Ecology of the Global High Elevation Tree Limits; Springer: Basel, Switzerland, 2012. [Google Scholar]
- Dang, H.; Jiang, M.; Zhang, Q.; Zhang, Y. Growth responses of subalpine fir (Abies fargesii) to climate variability in the Qinling Mountain, China. For. Ecol. Manag. 2007, 240, 143–150. [Google Scholar] [CrossRef]
- Fan, Z.; Bräuning, A.; Cao, K.; Zhu, S. Growth-climate responses of high-elevation conifers in the central Hengduan Mountains, southwestern China. For. Ecol. Manag. 2009, 258, 306–313. [Google Scholar] [CrossRef]
- Liang, E.; Wang, Y.; Xu, Y.; Liu, B.; Shao, X. Growth variation in Abies georgei var. smithii along altitudinal gradients in the Sygera Mountains, southeastern Tibetan Plateau. Trees 2010, 24, 363–373. [Google Scholar]
- Liu, B.; Wang, Y.; Zhu, H.; Liang, E.; Camarero, J.J. Topography and age mediate the growth responses of Smith fir to climate warming in the southeastern Tibetan Plateau. Int. J. Biometeorol. 2016, 60, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, E.; Sigdel, S.; Liu, B.; Camarero, J.J. The coupling of treeline elevation and temperature is mediated by non-thermal factors on the Tibetan Plateau. Forests 2017, 8, 109. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2007, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, E.; Gričar, J.; Rossi, S.; Čufar, K.; Ellison, A.M. Critical minimum temperature limits xylogenesis and maintains treelines on the southeastern Tibetan Plateau. Sci. Bull. 2017, 62, 804–812. [Google Scholar] [CrossRef]
- Liang, E.; Camarero, J.J. Threshold-dependent and non-linear associations between temperature and tree growth at and below the alpine treeline. Trees 2018, 32, 661–662. [Google Scholar] [CrossRef]
- Ren, P.; Rossi, S.; Camarero, J.J.; Ellison, A.M.; Liang, E.; Peñuelas, J. Critical temperature and precipitation thresholds for the onset of xylogenesis of Juniperus przewalskii in a semi-arid area of the north-eastern Tibetan Plateau. Ann. Bot. 2018, 121, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Ziaco, E.; Truettner, C.; Biondi, F.; Bullock, S. Moisture-driven xylogenesis in Pinus ponderosa from a Mojave Desert mountain reveals high phenological plasticity. Plant Cell Environ. 2018, 41, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Zhao, S.; Jiao, L.; Wen, Y. Relationships between tree age and climate sensitivity of radial growth in different drought conditions of Qilian Mountains, northwestern China. Forests 2018, 9, 135. [Google Scholar] [CrossRef]
- Gao, L.; Gou, X.; Deng, Y.; Liu, W.; Yang, M.; Zhao, Z. Climate–growth analysis of Qilian juniper across an altitudinal gradient in the central Qilian Mountains, northwest China. Trees 2013, 27, 379–388. [Google Scholar] [CrossRef]
- Gurskaya, M.; Shiyatov, S. Distribution of frost injuries in the wood of conifers. Russ. J. Ecol. 2006, 37, 7–12. [Google Scholar] [CrossRef]
- Aussenac, G. Ecology and ecophysiology of circum-Mediterranean firs in the context of climate change. Ann. For. Sci. 2002, 59, 823–832. [Google Scholar] [CrossRef]
- González-Cásares, M.; Pompa-García, M.; Camarero, J.J. Differences in climate–growth relationship indicate diverse drought tolerances among five pine species coexisting in northwestern Mexico. Trees 2017, 31, 531–544. [Google Scholar] [CrossRef]





| Species | Elevation (m) | Aspect | No. Trees | Age at 1.3 m (Years) | MS | Rbar | AC | EPS > 0.85 since. | Period 1950–2015 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean TRW (mm) | Rbar | EPS | SNR | PC1 (%) | |||||||||
| L. chinensis | 3360 | E | 29 | 100 | 0.22 | 0.48 | 0.55 | 1910 | 0.66 | 0.51 | 0.96 | 26.4 | 54.1 |
| 3280 | E | 29 | 130 | 0.24 | 0.31 | 0.58 | 1855 | 0.76 | 0.43 | 0.96 | 21.3 | 46.2 | |
| 3080 | NE | 25 | 135 | 0.17 | 0.38 | 0.52 | 1880 | 0.50 | 0.42 | 0.94 | 15.1 | 45.5 | |
| A. fargesii | 3065 | NE | 21 | 115 | 0.15 | 0.28 | 0.77 | 1950 | 0.63 | 0.35 | 0.90 | 9.16 | 39.4 |
| 2860 | NW | 23 | 90 | 0.14 | 0.28 | 0.73 | 1935 | 1.03 | 0.23 | 0.82 | 4.47 | 29.6 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Liang, E.; Liu, K.; Camarero, J.J. Species- and Elevation-Dependent Growth Responses to Climate Warming of Mountain Forests in the Qinling Mountains, Central China. Forests 2018, 9, 248. https://doi.org/10.3390/f9050248
Liu B, Liang E, Liu K, Camarero JJ. Species- and Elevation-Dependent Growth Responses to Climate Warming of Mountain Forests in the Qinling Mountains, Central China. Forests. 2018; 9(5):248. https://doi.org/10.3390/f9050248
Chicago/Turabian StyleLiu, Bo, Eryuan Liang, Kang Liu, and J. Julio Camarero. 2018. "Species- and Elevation-Dependent Growth Responses to Climate Warming of Mountain Forests in the Qinling Mountains, Central China" Forests 9, no. 5: 248. https://doi.org/10.3390/f9050248






