Leaf Temperature Fluctuations of Typical Psammophytic Plants and Their Application to Stomatal Conductance Estimation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Methods
2.2. Data Analysis
2.3. The Framework of the CWSI and IG Models
2.3.1. CWSI Model
2.3.2. IG Model
3. Results
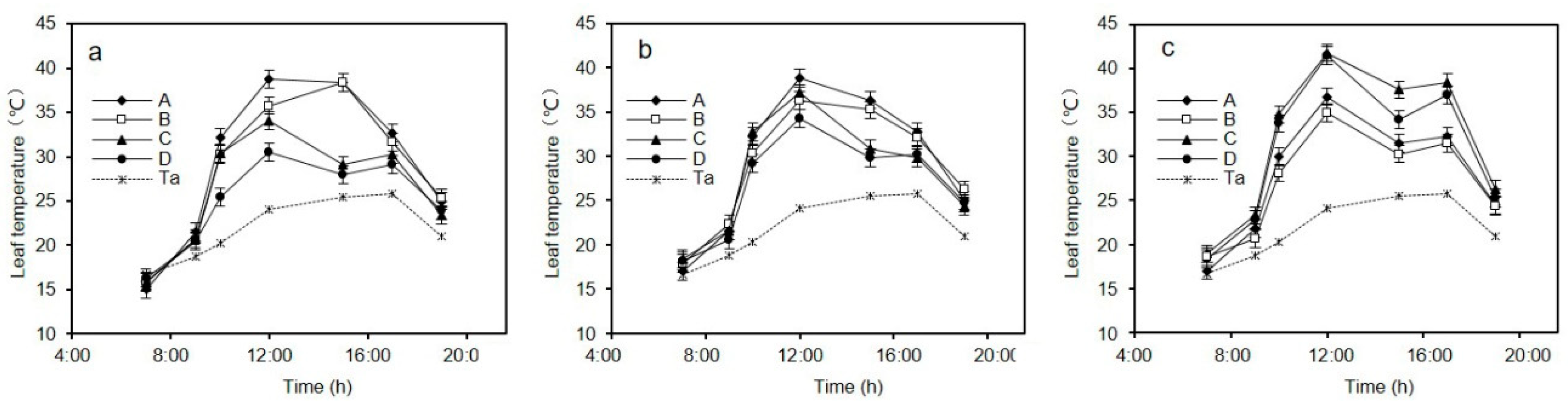
3.1. Diurnal Variation in Leaf Temperature under Varying Soil Moisture
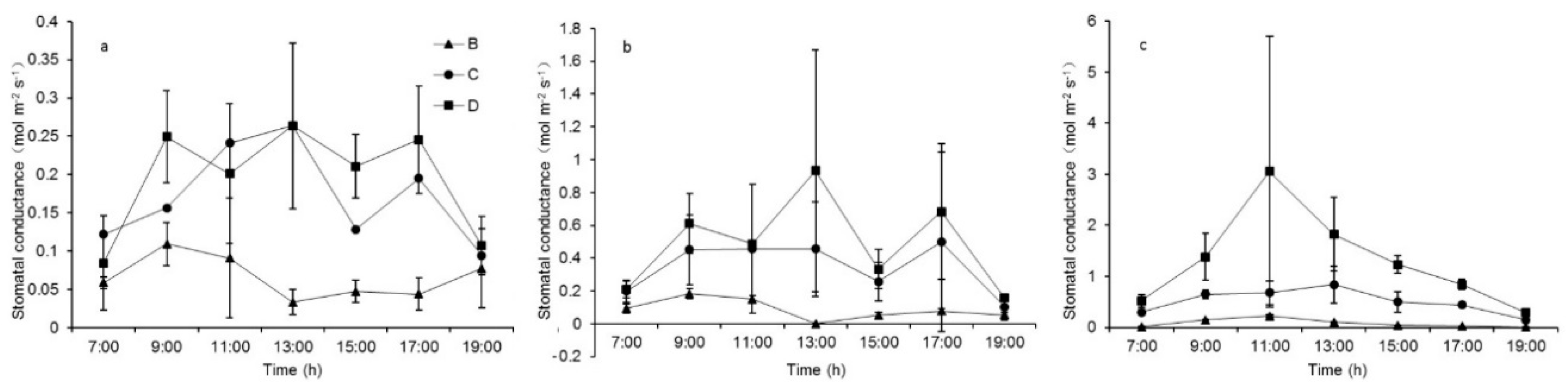
3.2. Fitting of Stomatal Conductance Model Parameters
3.3. Verification of the CWSI and IG Models
4. Discussion
4.1. Invalidity of Existing Stomatal Conductance Estimation Models in Psammophytic Plants
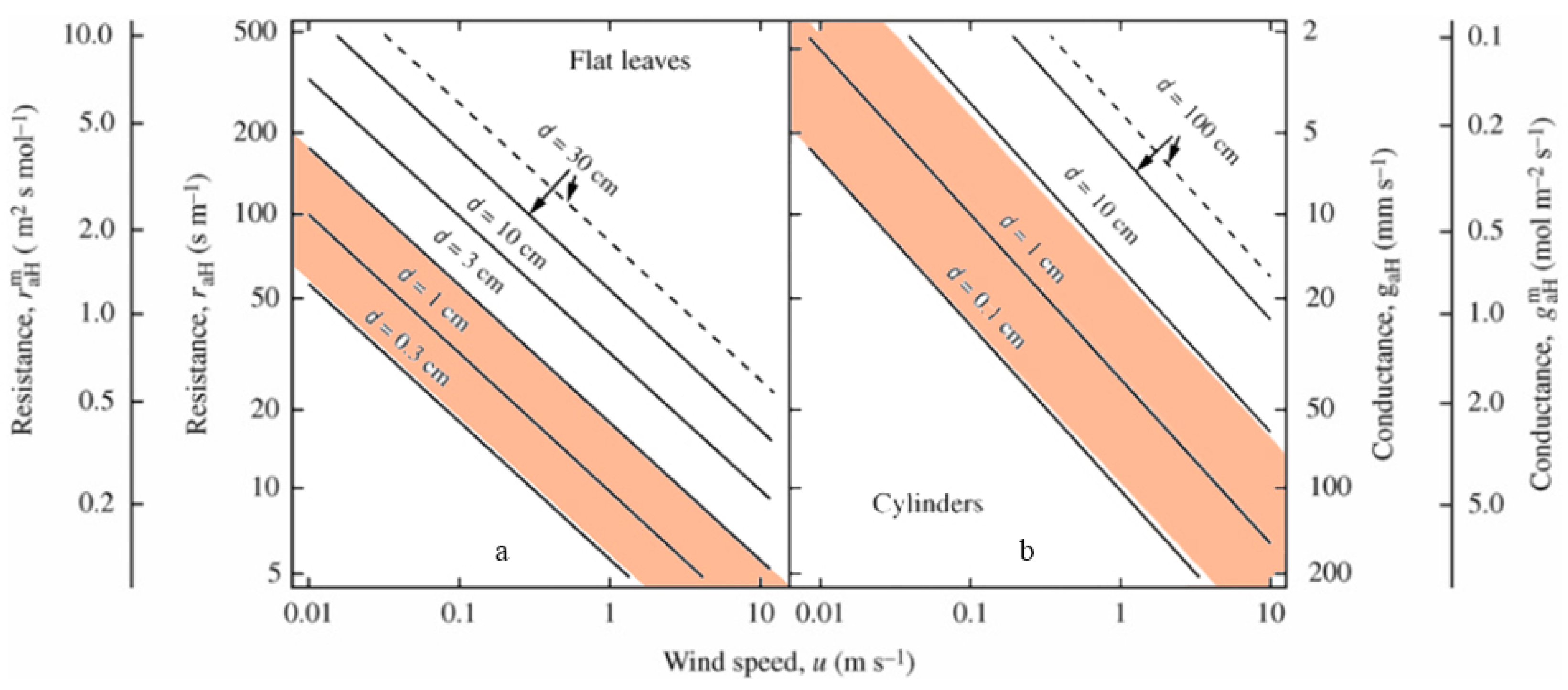
4.2. Factors and Mechanisms Affecting Leaf Temperature
- for flat leaves, raW = [6.62(μ/d)0.5]−1,
- for cylinders, raW = [4.03(μ0.6/d0.4)]−1, and
- for spheres, raW = [5.71(μ0.6/d0.4)]−1,
- for C. korshinskii, raW = [6.62(μ/d)0.5]−1 = 0.076μ−0.5,
- for S. psammophila, raW = [6.62(μ/d)0.5]−1 = 0.118μ−0.5, and
- for A. ordosica, raW = [4.03(μ0.6/d0.4)]−1 = 0.081μ−0.6.
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Cowan, I.R. Transport of water in the soil-plant-atmosphere system. J. Appl. Ecol. 1965, 2, 221–239. [Google Scholar] [CrossRef]
- Chaerle, L.; Straeten, D.V.D. Imaging techniques and the early detection of plant stress. Trends Plant Sci. 2000, 5, 495–501. [Google Scholar] [CrossRef]
- Meola, C.; Carlomagno, G.M. Recent advances in the use of infrared thermography. Meas. Sci. Technol. 2004, 15, 27–58. [Google Scholar] [CrossRef]
- Penfield, S. Temperature perception and signal transduction in plants. New Phytol. 2008, 79, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology, 3rd ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Franklin, K.A.; Knight, H. Unravelling plant temperature signaling networks. New Phytol. 2010, 185, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Georgiar, K.; Jackv, S.; Petera, A.; Mollya, W.; Stephend, T. A putative hybrid of Eucalyptus largiflorens growing on salt- and drought-affected floodplains has reduced specific leaf area and leaf nitrogen. Aust. J. Bot. 2012, 60, 358–367. [Google Scholar]
- Pallas, J.E.; Michel, B.E.; Harris, D.G. Photosynthesis, transpiration, leaf temperature, and stomatal activity of cotton plants under varying water potentials. Plant Physiol. 1967, 42, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.G.; Xia, S.J.; Chen, J.; Hu, N.; Yao, K.M. Plant temperature and its simulation model of thermo-sensitive genic male sterile rice. Rice Sci. 2008, 15, 223–231. [Google Scholar] [CrossRef]
- Idso, S.B.; Jackson, R.D.; Pinter, P.J.; Reginato, R.J.; Hatfield, J.L. Normalizing the stress-degree-day parameter for environmental variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Maes, W.H.; Steppe, K. Estimating evapotranspiration and drought stress with ground-based thermal remote sensing in agriculture: A review. J. Exp. Bot. 2012, 63, 4671–4712. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Shpiler, L.; Golan, G.; Mayer, J. Yield stability and canopy temperature of wheat genotypes under drought-stress. Field Crops Res. 1989, 22, 289–296. [Google Scholar] [CrossRef]
- Rashid, A.; Stark, J.C.; Tanveer, A.; Mustafa, T. Use of canopy temperature measurements as a screening tool for drought tolerance in spring wheat. J. Agron. Crop Sci. 1999, 182, 231–237. [Google Scholar] [CrossRef]
- Agam, N.; Cohen, Y.; Berni, J.A.J.; Alchanatis, V.; Kool, D.; Dag, A.; Yermiyahu, U.; Ben-Gala, A. An insight to the performance of crop water stress index for olive trees. Agric. Water Manag. 2013, 118, 79–86. [Google Scholar] [CrossRef]
- Jones, H.G. Use of infrared thermometry for estimation of stomatal conductance as a possible aid to irrigation scheduling. Agric. For. Meteorol. 1999, 95, 139–149. [Google Scholar] [CrossRef]
- Jones, H.G.; Stoll, M.; Santos, T.; Sousa, C.D.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Guilioni, L.; Jones, H.G.; Leinonen, I.; Lhomme, J.P. On the relationships between stomatal resistance and leaf temperatures in thermography. Agric. For. Meteorol. 2008, 148, 1908–1912. [Google Scholar] [CrossRef]
- Pou, A.; Diago, M.P.; Medrano, H.; Baluja, J.; Tardaguila, J. Validation of thermal indices for water status identification in grapevine. Agric. Water Manag. 2014, 134, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Mccuen, R.H. Evaluation of the Nash-Sutcliffe efficiency index. J. Hydrol. Eng. 2006, 11, 597–602. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Yu, M.H.; Ding, G.D.; Gao, G.L.; Zhao, Y.Y.; Yan, L.; Sai, K. Using plant temperature to evaluate the response of stomatal conductance to soil moisture deficit. Forests 2015, 6, 3748–3862. [Google Scholar] [CrossRef]
- Monteith, J.L.; Unsworth, M.H. Principles of Environmental Physics, 3rd ed.; Academic Press: Burlington, VT, USA, 2008. [Google Scholar]
- McCafferty, D.J.; Moncrieff, J.B.; Taylor, I.R. The effect of wind speed and wetting on thermal resistance of the barn owl. I: Total heat loss, boundary layer and total resistance. J. Therm. Biol. 1997, 22, 253–264. [Google Scholar] [CrossRef]
- Martin, T.A.; Hinckley, T.M.; Meinzer, F.C.; Sprugel, D.G. Boundary layer conductance, leaf temperature and transpiration of Abies amabilis branches. Tree Physiol. 1999, 19, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Leuning, R.; Cremer, K.W. Leaf temperatures during radiation frost Part I. Observations. Agric. For. Meteorol. 1988, 42, 121–133. [Google Scholar] [CrossRef]
- Bridge, L.J.; Franklin, K.A.; Homer, M.E. Impact of plant shoot architecture on leaf cooling: A coupled heat and mass transfer model. J. R. Soc. Interface 2013, 10, 20130326. [Google Scholar] [CrossRef] [PubMed]
- Ayeneh, A.; Ginkel, M.V.; Reynolds, M.P.; Ammar, K. Comparison of leaf, spike, peduncle and canopy temperature depression in wheat under heat stress. Field Crops Res. 2002, 79, 173–184. [Google Scholar] [CrossRef]


| Species | Within Subjects Effect | Mauchly’s W | Approx. Chi-Square | df | Sig. | Epsilona | ||
|---|---|---|---|---|---|---|---|---|
| Greenhouse-Geisser | Huynh-Feldt | Lower-Bound | ||||||
| C. korshinskii | Time | 0.000 | 0.000 | 20 | 0.000 | 0.171 | 0.219 | 0.167 |
| A. ordosica | Time | 0.000 | 0.000 | 20 | 0.000 | 0.177 | 0.229 | 0.167 |
| S. psammophila | Time | 0.000 | 0.000 | 20 | 0.000 | 0.172 | 0.220 | 0.167 |
| Species | Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|---|
| C. korshinskii | Time | 7777.755 | 1.028 | 7569.163 | 3132.020 | 0.000 |
| Time * stress gradient | 60.572 | 3.083 | 19.649 | 8.131 | 0.003 | |
| A. ordosica | Time | 7211.525 | 1.065 | 6773.545 | 3047.103 | 0.000 |
| Time * stress gradient | 204.490 | 3.194 | 64.024 | 28.801 | 0.000 | |
| S. psammophila | Time | 7773.256 | 1.030 | 7549.551 | 3105.961 | 0.000 |
| Time * stress gradient | 44.866 | 3.089 | 14.525 | 5.976 | 0.009 |
| Species | Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|---|
| C. korshinskii | Intercept | 72,860.635 | 1 | 72,860.635 | 3895.984 | 0.000 |
| Stress gradient | 177.694 | 3 | 59.231 | 3.167 | 0.046 | |
| Error | 224.418 | 12 | 18.701 | |||
| A. ordosica | Intercept | 73,069.456 | 1 | 73,069.456 | 3901.886 | 0.000 |
| Stress gradient | 113.097 | 3 | 37.699 | 2.013 | 0.044 | |
| Error | 224.720 | 12 | 18.727 | |||
| S. psammophila | Intercept | 75,321.794 | 1 | 75,321.794 | 3900.677 | 0.000 |
| Stress gradient | 70.266 | 3 | 23.422 | 1.213 | 0.034 | |
| Error | 231.719 | 12 | 19.310 |
| Plant Species | Upper Baseline | Lower Baseline |
|---|---|---|
| C. korshinskii | , R2 = 0.3861 | |
| A. ordosica | , R2 = 0.2864 | |
| S. psammophila | , R2 = 0.3599 |
| Model | Plant Species | Model Expression | R2 |
|---|---|---|---|
| CWSI | C. korshinskii | 0.5052 | |
| A. ordosica | 0.4575 | ||
| S. psammophila | 0.7885 | ||
| IG | C. korshinskii | 0.5214 | |
| A. ordosica | 0.7057 | ||
| S. psammophila | 0.5596 |
| Model | Plant Species | a | R2 | RMSE (mol m−2 s−1) | MAE (mol m−2 s−1) | MRE | Ef |
|---|---|---|---|---|---|---|---|
| CWSI | C. korshinskii | 0.86 | 0.09 | 0.23 | 0.19 | 0.50 | 0.43 |
| A. ordosica | 0.85 | 0.41 | 0.48 | 0.39 | 2.00 | 0.63 | |
| S. psammophila | 0.90 | 0.76 | 0.40 | 0.29 | 0.42 | 0.79 | |
| IG | C. korshinskii | 0.95 | 0.34 | 0.21 | 0.18 | 0.60 | 0.58 |
| A. ordosica | 0.94 | 0.51 | 0.45 | 0.37 | 1.09 | 0.59 | |
| S. psammophila | 0.87 | 0.46 | 0.32 | 0.27 | 0.33 | 0.56 |
| (a) Leaf Temperature | (b) Stomatal Conductance | ||||||
|---|---|---|---|---|---|---|---|
| Stress Gradient | Mean Square | F | Sig. | Stress Gradient | Mean Square | F | Sig. |
| B | 2.763 | 1.513 | 0.294 | B | 0.006 | 2.417 | 0.195 |
| C | 15.640 | 7.363 | 0.024 | C | 0.592 | 4.019 | 0.078 |
| D | 27.586 | 20.290 | 0.002 | D | 6.715 | 20.803 | 0.002 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Ding, G.; Gao, G.; Zhao, Y.; Sai, K. Leaf Temperature Fluctuations of Typical Psammophytic Plants and Their Application to Stomatal Conductance Estimation. Forests 2018, 9, 313. https://doi.org/10.3390/f9060313
Yu M, Ding G, Gao G, Zhao Y, Sai K. Leaf Temperature Fluctuations of Typical Psammophytic Plants and Their Application to Stomatal Conductance Estimation. Forests. 2018; 9(6):313. https://doi.org/10.3390/f9060313
Chicago/Turabian StyleYu, Minghan, Guodong Ding, Guanglei Gao, Yuanyuan Zhao, and Ke Sai. 2018. "Leaf Temperature Fluctuations of Typical Psammophytic Plants and Their Application to Stomatal Conductance Estimation" Forests 9, no. 6: 313. https://doi.org/10.3390/f9060313





