Joint Control of Net Primary Productivity by Climate and Soil Nitrogen in the Forests of Eastern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Datasets
2.2.1. Estimation of NPP
2.2.2. Measurements of Soil N
2.2.3. Climate Data
2.3. Statistical Analysis
3. Results
3.1. Latitudinal Pattern and Statistics of NPP
3.2. The Impact of Climatic Factors on the Spatial Pattern of NPP
3.3. The Impact of Soil N on the Spatial Pattern of NPP
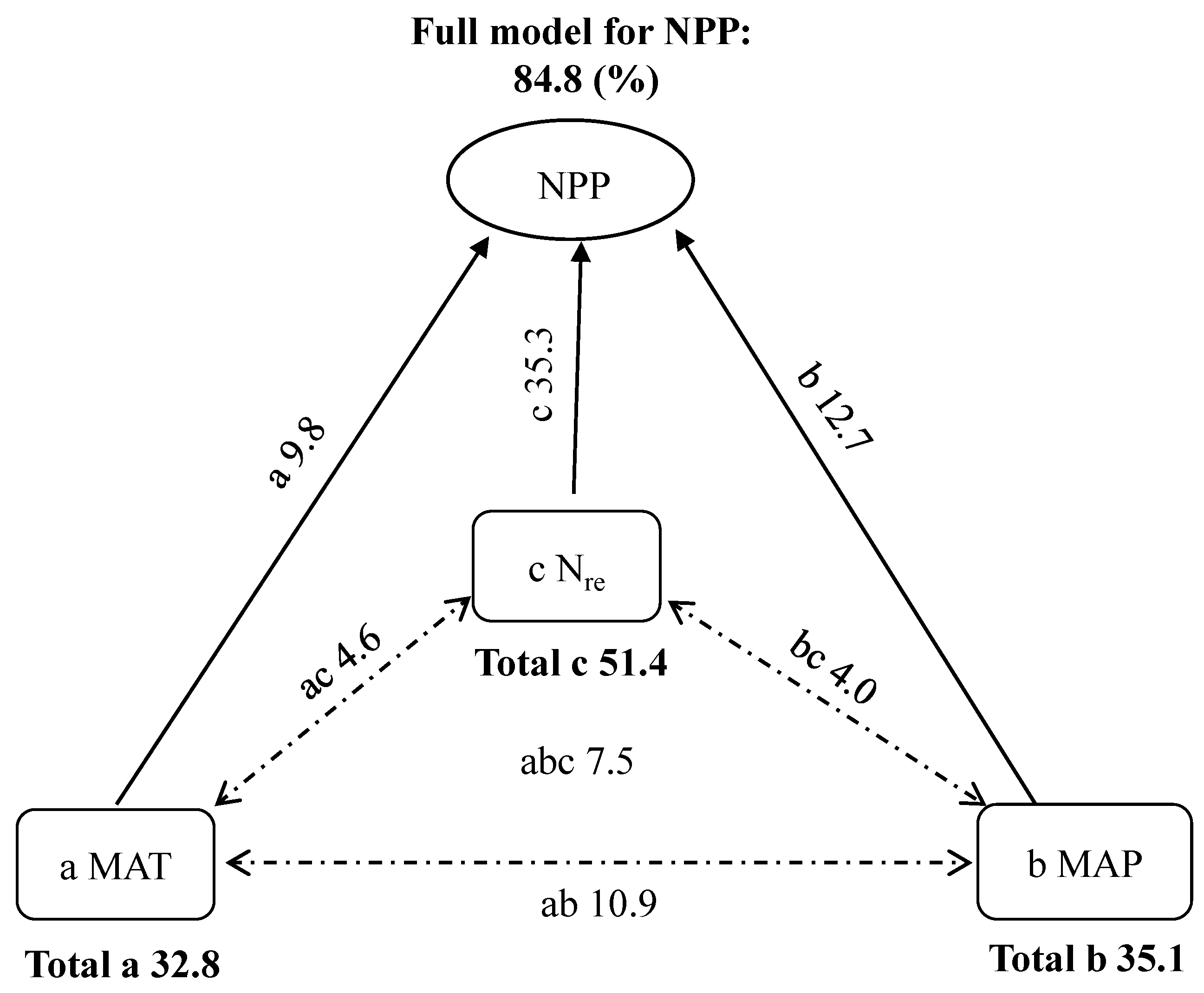
3.4. The Combined Impact of Climate and Soil on the Spatial Pattern of NPP
4. Discussion
4.1. Carbon Budget
4.2. Explanations for the Distribution Pattern of NPP
4.2.1. Climate Control
4.2.2. N Control
4.2.3. Joint Control
4.3. Uncertainty Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dixon, R.K.; Brown, S.; Houghton, R.A.; Solomon, A.M.; Trexler, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystmes. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Niemeijer, D. Developing indicators for environmental policy: Data-driven and theory-driven approaches examined by example. Environ. Sci. Policy 2002, 5, 91–103. [Google Scholar] [CrossRef]
- Kitayama, K.; Aiba, S.I. Ecosystem structure and productivity of tropical rain forests along altitudinal gradients with contrasting soil phosphorus pools on Mount Kinabalu, Borneo. J. Ecol. 2002, 90, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Raich, J.W.; Russell, A.E.; Kitayama, K.; Parton, W.J.; Vitousek, P.M. Temperature influences carbon accumulation in moist tropical forests. Ecology 2006, 87, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Keeling, H.C.; Phillips, O.L. The global relationship between forest productivity and biomass. Glob. Ecol. Biogeogr. 2007, 16, 618–631. [Google Scholar] [CrossRef]
- Matsushita, B.; Xu, M.; Chen, J.; Kameyama, S.; Tamura, M. Estimation of regional net primary productivity (NPP) using a process-based ecosystem model: How important is the accuracy of climate data? Ecol. Model. 2004, 178, 371–388. [Google Scholar] [CrossRef]
- Chapin, F.S.; McFarland, J.; McGuire, A.D.; Euskirchen, E.S.; Ruess, R.W.; Kielland, K. The changing global carbon cycle: Linking plant-soil carbon dynamics to global consequences. J. Ecol. 2009, 97, 840–850. [Google Scholar]
- Lieth, H. Primary productivity of the biosphere. In Modeling the Primary Productivity of the World; Lieth, H., Whittaker, R.H., Eds.; Springer: New York, NY, USA, 1975; pp. 237–264. [Google Scholar]
- Knapp, A.K.; Smith, M.D. Variation among biomes in temporal dynamics of aboveground primary production. Science 2001, 291, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.X.; Pan, Y.D.; Ouyang, H.; Shi, P.L.; Luo, J.; Yu, Z.L.; Lu, Q. Leaf area index and net primary productivity along subtropical to alpine gradients in the Tibetan Plateau. Glob. Ecol. Biogeogr. 2004, 13, 345–358. [Google Scholar] [CrossRef] [Green Version]
- Del Grosso, S.; Parton, W.; Stohlgren, T.; Zheng, D.L.; Bachelet, D.; Prince, S.; Hibbard, K.; Richard Olson, A. Global potential net primary production predicted from vegetation class, precipitation, and temperature. Ecology 2008, 89, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Sala, O.E.; Parton, W.J.; Joyce, L.A.; Lauenroth, W.K. Primary production of the central grassland region of the United States. Ecology 1988, 69, 40–45. [Google Scholar] [CrossRef]
- Clark, D.A.; Brown, S.; Kicklighter, D.W.; Chambers, J.Q.; Thomlinson, J.R.; Ni, J.; Holland, E.A. Net primary production in tropical forests: An evaluation and synthesis of existing field data. Ecol. Appl. 2001, 11, 371–384. [Google Scholar] [CrossRef]
- Raich, J.W.; Russell, A.E.; Vitousek, P.M. Primary productivity and ecosystem development along an elevational gradient on Mauna Loa, Hawaii. Ecology 1997, 78, 707–721. [Google Scholar]
- Schuur, E.A.G.; Matson, P.A. Net primary productivity and nutrient cycling across a mesic to wet precipitation gradient in Hawaiian montane forest. Oecologia 2001, 128, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; La Pierre, K.J.; Collins, S.L.; Knapp, A.K.; Gross, K.L.; Barrett, J.E.; Frey, S.D.; Gough, L.; Miller, R.J.; Morris, J.T.; et al. Global environmental change and the nature of aboveground net primary productivity responses: Insights from long-term experiments. Oecologia 2015, 177, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Scurlock, J.M.O.; Johnson, K.; Olson, R.J. Estimating net primary productivity from grassland biomass dynamics measurements. Glob. Chang. Biol. 2002, 8, 736–753. [Google Scholar] [CrossRef] [Green Version]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Chen, A.P.; Peng, C.H.; Zhao, S.Q.; Ci, L. Changes in forest biomass carbon storage in China between 1949 and 1998. Science 2001, 292, 2320–2322. [Google Scholar] [CrossRef] [PubMed]
- Ni, J. Net primary productivity in forests of China: Scaling-up of national inventory data and comparison with model predictions. For. Ecol. Manag. 2003, 176, 485–495. [Google Scholar] [CrossRef]
- Fang, J.Y.; Guo, Z.D.; Piao, S.L.; Chen, A.P. Terrestrial vegetation carbon sinks in China, 1981–2000. Sci. China Ser. D 2007, 50, 1341–1350. [Google Scholar] [CrossRef]
- Peng, S.L.; Zhao, P.; Ren, H.; Zheng, F.Y. The possible heat-driven pattern variation of zonal vegetation and agricultural ecosystem, along the north-south of China under the global change. Earth. Sci. Front. 2002, 9, 218–226. [Google Scholar]
- Hobbie, S.E. Plant species effects on nutrient cycling: Revisiting litter feed backs. Trends Ecol. Evol. 2015, 30, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Woodward, F.I.; Lomas, M.R.; Kelly, C.K. Global climate and the distribution of plant biomes. Philos. Trans. Soc. B 2004, 359, 1465–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, T.X. Patterns of Biological Production and Its Mathematical Models for Main Forest Types of China; Committee of Synthesis Investigation of Natural Resources, Chinese Academy of Sciences: Beijing, China, 1996. [Google Scholar]
- Pan, Y.D.; Luo, T.X.; Birdsey, R.; Hom, J.; Melillo, J. New estimates of carbon storage and sequestration in China’s forests: Effects of age-class and method on inventory-based carbon estimation. Clim. Chang. 2004, 67, 211–236. [Google Scholar] [CrossRef]
- Keith, H.; Mackey, B.G.; Lindenmayer, D.B. Re-evaluation of forest biomass carbon stocks and lessons from the world’s most carbon-dense forests. Proc. Natl. Acad. Sci. USA 2009, 106, 11635–11640. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Cao, M.K.; Li, K.R.; Gu, F.X.; Ji, J.J.; Huang, M.; Zhang, L.M. Spatial patterns of terrestrial net ecosystem productivity in China during 1981–2000. Sci. China Ser. D 2007, 50, 745–753. [Google Scholar] [CrossRef]
- Heikkinen, R.K.; Luoto, M.; Kuussaari, M.; Poyry, J. New insights into butterfly-environment relationships using partitioning methods. Proc. Biol. Sci. 2005, 272, 2203–2210. [Google Scholar] [CrossRef] [PubMed]
- Gower, S.T.; Krankina, O.; Olson, R.J.; Apps, M.; Linder, S.; Wang, C. Net primary production and carbon allocation patterns of boreal forest ecosystems. Ecol. Appl. 2001, 11, 1395–1411. [Google Scholar] [CrossRef]
- Scarascia Mugnozza, G.A.B.; Persoon, H.G.; Matteucci, A. Carbon and Nitrogen Cycling in European Forest Ecosystems. In Tree Biomass, Growth and Nutrient Pools; Schulze, E.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 49–62. [Google Scholar]
- Raupach, M.R. Carbon cycle pinning down the land carbon sink. Nat. Clim. Chang. 2011, 1, 148–149. [Google Scholar] [CrossRef]
- Hirata, R.; Saigusa, N.; Yamamoto, S.; Ohtani, Y.; Ide, R.; Asanuma, J.; Gamo, M.; Hirano, T.; Kondo, H.; Kosugi, Y.; et al. Spatial distribution of carbon balance in forest ecosystems across East Asia. Agric. For. Meteorol. 2008, 148, 761–775. [Google Scholar] [CrossRef]
- Saigusa, N.; Yamamoto, S.; Hirata, R.; Ohtani, Y.; Ide, R.; Asanuma, J.; Gamo, M.; Hirano, T.; Kondo, H.; Kosugi, Y.; et al. Temporal and spatial variations in the seasonal patterns of CO2 flux in boreal, temperate, and tropical forests in East Asia. Agric. For. Meteorol. 2008, 148, 700–713. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Matson, P.A.; Mooney, H.A. Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2002. [Google Scholar]
- Luyssaert, S.; Inglima, I.; Jung, M.; Richardson, A.D.; Reichstein, M.; Papale, D.; Piao, S.L.; Schulze, E.D.; Wingate, L.; Matteucci, G.; et al. CO2 balance of boreal, temperate, and tropical forests derived from a global database. Glob. Chang. Biol. 2007, 13, 2509–2537. [Google Scholar] [CrossRef]
- Kato, T.; Tang, Y.H. Spatial variability and major controlling factors of CO2 sink strength in Asian terrestrial ecosystems: Evidence from eddy covariance data. Glob. Chang. Biol. 2008, 14, 2333–2348. [Google Scholar] [CrossRef]
- Ni, J.; Zhang, X.S.; Scurlock, J.M.O. Synthesis and analysis of biomass and net primary productivity in Chinese forests. Ann. For. Sci. 2001, 58, 351–384. [Google Scholar] [CrossRef] [Green Version]
- Matson, P.; Lohse, K.A.; Hall, S.J. The globalization of nitrogen deposition: Consequences for terrestrial ecosystems. Ambio 2002, 31, 113–119. [Google Scholar] [CrossRef] [PubMed]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Finzi, A.C.; Norby, R.J.; Calfapietra, C.; Gallet-Budynek, A.; Gielen, B.; Holmes, W.E.; Hoosbeek, M.R.; Iversen, C.M.; Jackson, R.B.; Kubiske, M.E.; et al. Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc. Natl. Acad. Sci. USA 2007, 104, 14014–14019. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.C.; Townsend, A.R.; Taylor, P.; Alvarez-Clare, S.; Bustamante, M.M.C.; Chuyong, G.; Dobrowski, S.Z.; Grierson, P.; Harms, K.E.; Houlton, B.Z.; et al. Relationships among net primary productivity, nutrients and climate in tropical rain forest: A pan-tropical analysis. Ecol. Lett. 2011, 14, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Li, L.H.; Han, X.G.; Chen, S.P.; Wang, Z.W.; Chen, Q.S.; Wang, Z.W.; Chen, Q.S.; Bai, Y.F. Nitrogen response efficiency increased monotonically with decreasing soil resource availability: A case study from a semiarid grassland in northern China. Oecologia 2006, 148, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Litton, C.M.; Ryan, M.G.; Knight, D.H. Effects of tree density and stand age on carbon allocation patterns in postfire lodgepole pine. Ecol. Appl. 2004, 14, 460–475. [Google Scholar] [CrossRef]
- Wang, S.Q.; Zhou, L.; Chen, J.M.; Ju, W.M.; Feng, X.F.; Wu, W.X. Relationships between net primary productivity and stand age for several forest types and their influence on China’s carbon balance. J. Environ. Manag. 2011, 92, 1651–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, C.J.; Lind, E.M.; Hautier, Y.; Harpole, W.S.; Borer, E.T.; Hobbie, S.E.; Seabloom, E.W.; Ladwig, L.M.; Bakker, J.D.; Chu, C.; et al. Anthropogenic nitrogen deposition predicts local grassland primary production worldwide. Ecology 2015, 96, 1459–1465. [Google Scholar] [CrossRef] [Green Version]




| Climate Zone | N | NPP (t ha−1 a−1) | ||||
|---|---|---|---|---|---|---|
| Mean | Max | Min | SE | CV (%) | ||
| Boreal | 4 | 12.1 ab | 24.6 | 1.9 | 4.7 | 78.4 |
| Temperate | 28 | 5.5 c | 14.1 | 0.8 | 0.8 | 73.2 |
| Subtropical | 42 | 10.1 b | 29.6 | 1.2 | 0.9 | 54.7 |
| Tropical | 13 | 15.3 a | 24.2 | 8.6 | 1.3 | 31.6 |
| Overall | 87 | 9.5 | 29.6 | 0.8 | 0.7 | 64.0 |
| Variables | Model Type | N | R2 | F | RMSE | p |
|---|---|---|---|---|---|---|
| MAT | linear | 87 | 0.166 | 16.9 | 5.6 | <0.001 |
| exponential | 87 | 0.202 | 22.0 | 5.6 | <0.001 | |
| MAP | linear | 87 | 0.218 | 21.5 | 5.4 | <0.001 |
| exponential | 87 | 0.237 | 23.2 | 5.3 | <0.001 | |
| MAT+MAP | linear | 87 | 0.243 | 23.8 | 4.2 | <0.001 |
| Nsoil | linear | 42 | 0.106 | 4.7 | 7.0 | <0.05 |
| exponential | 42 | 0.148 | 4.5 | 6.7 | <0.05 | |
| Nre | linear | 86 | 0.284 | 30.1 | 3.4 | <0.001 |
| Nsoil + Nre | linear | 42 | 0.382 | 32.7 | 2.2 | <0.001 |
| MAT + MAP + Nsoil + Nre | linear | 42 | 0.543 | 10.6 | 1.6 | <0.001 |
| Variables | Data Range | MAT | MAP | Nsoil |
|---|---|---|---|---|
| MAT (°C) | 0–23.5 | 1 | ||
| MAP (mm) | 500.0–2000.0 | 0.670 ** | ||
| Nsoil (kg ha−1) | 115.6–163.5 | 0.213 | 0.365 | |
| Nre (kg ha−1 a−1) | 1.9–172.2 | 0.288 ** | 0.242 ** | 0.161 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiaoyun, Z.; Minghang, G.; Tibin, Z. Joint Control of Net Primary Productivity by Climate and Soil Nitrogen in the Forests of Eastern China. Forests 2018, 9, 322. https://doi.org/10.3390/f9060322
Xiaoyun Z, Minghang G, Tibin Z. Joint Control of Net Primary Productivity by Climate and Soil Nitrogen in the Forests of Eastern China. Forests. 2018; 9(6):322. https://doi.org/10.3390/f9060322
Chicago/Turabian StyleXiaoyun, Zhan, Guo Minghang, and Zhang Tibin. 2018. "Joint Control of Net Primary Productivity by Climate and Soil Nitrogen in the Forests of Eastern China" Forests 9, no. 6: 322. https://doi.org/10.3390/f9060322




