Abstract
Mediterranean plantations are the most suitable areas to assess vegetation dynamics and competitive interactions between native and exotic woody species. Our research was carried out in a coastal pine plantation (Sicily) where renaturalization by native species (Pistacia lentiscus L. and Olea europaea var. sylvestris) and invasion by Acacia saligna (Labill.) H.L.Wendl. simultaneously occur. The regeneration pattern of woody species in the pine understory was evaluated in six experimental plots along a stand density gradient, from 200 to approximately 700 pines per hectare. Both pine stand density and regeneration by native species had a significant negative relationship with Acacia natural regeneration. Olea regeneration was positively correlated with stand density, while Pistacia showed a non-significant relationship. Saplings of both native species were mostly less than 1 m high, whereas approximately 70% of Acacia individuals were higher than 1 m. We found that 400 pines per hectare should be considered a minimum stand density to keep Acacia under control, while favouring the establishment of native species in the understory. The successful control of Acacia requires an integrated management strategy, including different forest interventions according to stand density: thinning, control measures against Acacia, and renaturalization actions.
1. Introduction
The massive afforestation activities carried out on a large scale in the Mediterranean basin during the 20th century made extensive use of alien tree species [1,2]. The need to ensure rapid soil cover and the highly degraded conditions of substrates and slopes over large areas determined a clear preference for trees belonging to the genera Acacia, Eucalyptus, and, predominantly, Pinus [3], due to their fast-growing, pioneer traits, ecological plasticity, and easy propagation [4]. Another important reason was the supposed role of preparatory species: Introduced species were considered ideal for improving site conditions to allow recovery of native woody species. However, in many cases, afforestation/reforestation projects have slowed down, hindered, or completely stopped the natural evolutionary dynamics and the maturation of more complex, diversified, and stable Mediterranean forest ecosystems [5,6]. The role played by forest plantations has been a matter of intense debate in the Mediterranean [7,8], due to the marked heterogeneity of the ecological conditions and management practices. Furthermore, owing to prevailing purposes of soil protection, autoecological aspects have not been adequately considered in the choice of plant species and propagation material, thus determining the unsuitability of many afforested sites. Hence, in most cases, forest interventions are necessary to allow the gradual replacement of alien planted species by native ones. However, such necessary interventions (e.g., thinning) have not been performed or they have been performed without the guidance of clear forest planning and management objectives. In some cases, this has led to the complete failure of plantations, which have neither adequately protected the soil, enhanced local succession dynamics, nor provided adequate biodiversity conservation. As a result, the current main management choice for Mediterranean plantations is renaturalization, i.e., the gradual conversion towards more stable, complex, and diversified ecosystems, dominated by native broadleaved and evergreen trees; the manifestation of potential climax vegetation [9,10]. This objective is an absolute priority for protected coastal habitats, which have undergone rarefaction and marked alteration because of intense human exploitation of the territory.
Massive reforestation activity has provided a huge, experimental forest laboratory where we can observe and evaluate the evolution and dynamics of woody vegetation in the Mediterranean, as influenced by competition and/or facilitation interactions between native and exotic woody species [8,9]. New combinations between different tree species with varied characteristics, such as life-history strategies, origin, reproductive, and physiological traits, have resulted in artificial ecosystems, characterized by novel competitive interactions whose outcomes are not easily predictable nor can they be considered to have reached steady state [11]. They are strongly influenced by both local environmental and management conditions, as well as by the type of species that come into contact. In some cases, introduced species have shown a remarkable ability to adapt to the new ecosystem, achieving performance levels greater than that of the native co-existing species, with abundant regeneration abilities and quick soil coverage, thus, displaying invasive behaviour [12]. Such evidence further complicates the management of afforested areas in which interventions carried out to promote renaturalization may, at the same time, trigger the invasion process. This so-called paradox of ecological restoration occurs when the disturbance required to restore a forest ecosystem, which has been highly simplified by human intervention, may, on the contrary, favour invasive spread, bringing about significant negative ecological impacts [13]. Generally, invasive alien species are particularly able to exploit increases in available resources (light, nutrients) found in gaps or areas with reduced forest cover, such as those resulting from forest utilizations or restoration treatments, or even through wildfires and grazing [13,14]. Hence, selective cuttings are performed to counteract alien tree species of the overstory, while favouring the progressive replacement by native woody species, starting from the underlying layers [3].
Thinning within plantations is strictly necessary. On the one hand, such periodic interventions allow the enhancement of available resources for remaining trees, including light, soil water, and nutrients. Individual trees may grow better and increase in diameter size, and they may be healthier and less susceptible to pathogens, disease, and abiotic factors, thus rendering the whole forest stand more resistant and resilient. On the other hand, thinning has been proved to ameliorate understory microclimatic conditions, especially in terms of light available for native seedling establishment, growth, and development in coastal pine forests [15]. However, invasive tree species may also benefit from interventions, greatly hindering the natural dynamics and recovery of the functionality of forest ecosystems. Therefore, for the aforementioned reasons, active forest management, which includes appropriate silvicultural interventions, is mandatory.
Due to their high invasive potential many Acacia species are considered as ecosystem engineers [12], causing serious and long-lasting ecological impacts on entire ecosystems [16], including below-ground soil microbial communities [17] and native pollinators [18], as well as on soil and vegetation characteristics [16]. Some general traits seem to have favoured the worldwide distribution of Acacias as invasive species: Nitrogen symbiotic fixation, rapid initial growth rates, large seed production, and reproductive potential. The introduction of a new functional trait or of a new biochemical process into a native ecosystem is bound, generally, to bring about greater and longer-lasting ecological consequences on ecosystems [19]. The more diverse an invasive plant species is, compared to native communities, the greater the likelihood of it having larger negative impacts. Even after eradication, the altered conditions caused by an invasive species may remain for a longer period of time [20]. Emblematic cases are the invasion of Mediterranean coastal ecosystems by Carpobrotus spp. [21] and by Acacias [17,22].
Acacia saligna (Labill.) H.L.Wendl. (hereinafter, A. saligna) is one of the most widely used tree species in coastal-areas afforestation, covering approximately 600,000 hectares worldwide of which almost 10% is in South Africa alone [23]. It has been preferentially used in Mediterranean-climate areas to bind dunes and stabilize soils, along road systems, and to recover bare and degraded lands, in addition to use as a windbreak [24]. Whilst functioning reasonably well in soil protection, a number of peculiar traits make A. saligna one of the invasive species most suited to Mediterranean coastal ecosystems, even with limited nutrient availability. Traits include high growth rates, early achievement of sexual maturity, striking resprouting ability following cutting or other physical disturbance factors, and drought and abiotic stress tolerance, as well as its ability to establish symbiosis for nitrogen fixation [25,26]. A. saligna may have a profound effect on the structure and functioning of recipient ecosystems and seriously hamper vegetation dynamics of woody native species, significantly reducing their richness and cover [27]. A. saligna may cause a significant shift in native community assemblages, reducing the occurrence of guiding or focal species, whilst favouring the spread of opportunistic and ruderal species, including other invasive species [28,29]. In Mediterranean pine plantations, negative effects caused by A. saligna have been reported on small mammal communities and were attributed to the structural simplification and homogenization of the forest stand [30].
Only recently, in Italy, A. saligna has been listed amongst alien species posing a threat to native species and to almost all habitats of community interest linked to sandy shores and dune systems, including wooded dunes with Pinus pinea L. and/or Pinus pinaster Aiton (Habitat 2270) [29,31]. A. saligna was introduced to Sicily in the late nineteenth century [32], whilst the first cases of naturalization date back to the early years of the last century [33]. A. saligna has increasingly spread in the last decades, mainly invading the coastal dune habitats of SW Sicily and seriously threatening the vegetation communities dominated by species of considerable scientific and biogeographic interest, such as Retama raetam subsp. gussonei (Webb) Greuter [34] and Juniperus oxycedrus subsp. macrocarpa (Sm.) Ball [35]. The same plant communities are the most exposed to invasion by A. saligna in other Italian regions [31], as well as in other Mediterranean-type ecosystems [36]. Hence, urgent interventions of control and eradication have been invoked [37,38].
We assessed the regeneration of A. saligna and of co-occurring native woody species in the understory of a Mediterranean coastal plantation along a gradient of pine stand density, ranging from 200 to 700 stems per hectare. In our study site, two natural processes of opposing significance occur simultaneously: On the one hand, active processes of renaturalization of native woody species, particularly Olea europaea L. var. sylvestris (Mill.) Lehr. and Pistacia lentiscus L. (hereinafter, Olea and Pistacia) and, on the other hand, invasion by an alien tree species (A. saligna). Whilst the most favorable pine density values for the establishment and recruitment of Mediterranean native woody species are known, at least in part [39], only a limited body of knowledge exists on density values that allow the renaturalization process to occur without triggering plant invasion. Yet, this is crucial information for ecosystems where native and alien species coexist in the understory and compete. We hypothesized that there could be a threshold density value which favours the renaturalization process, whilst preventing invasive spread. Overall, we provide the most recommended management options for forest pine plantations, including specific forest interventions addressing the pine canopy (thinning), A. saligna (eradication), and native woody species (renaturalization).
2. Material and Methods
2.1. Study Area and Vegetation
Field surveys were carried out within the nature reserve “Foce del fiume Platani”, a 3.5 km long coastal belt consisting of 207 hectares, localized in the Agrigento Province (SW Sicily, Figure 1). Average annual precipitation is 496.7 mm, while the average annual temperature is 18.7 °C (Osservatorio delle Acque, 2016). Hence, the study area falls within the Thermomediterranean upper dry bioclimatic belt [40]. The dominant soil types in the study area are Typic Xeropsamments entisoils, with a sandy texture in the first meter of soil depth [41]. The reserve includes the alluvial plain originated by the delta of the Platani River. This coastal strip has not been affected by marked anthropic alterations and still hosts residual aspects of coastal dune and Mediterranean maquis vegetation, with high potential biodiversity, linked to the presence of the sea, coastal dunes, and backdune habitats. The woody vegetation is characterized by the coexistence of Mediterranean native species, such as Ephedra fragilis Desf., Pistacia lentiscus L., Chamaerops humilis L., and Olea europaea var. sylvestris, with planted alien tree species, especially Pinus spp., Acacia saligna and Eucalyptus camaldulensis Dehnh., and alien shrub species like Myoporum tenuifolium G.Forster [41]. The study area also harbours plant species of particular conservation value, such as Retama raetam subsp. gussonei and Juniperus turbinata Guss., characteristic of the association Ephedro-Juniperetum macrocarpae Bartolo, Brullo & Marcenò 1982 [34].
Figure 1.
The geographic location of the “Foce del Fiume Platani” Nature Reserve on the southwestern coast of Sicily.
2.2. Forest Management and Disturbance History
The forest plantation in the study area was established in 1952 [41], with the main aim of stabilizing sand dunes and backdunes, and as a windbreak to protect agricultural areas inland [24]. The plantation was mostly made with exotic tree species: Pines (Pinus pinea, Pinus halepensis Mill. and Pinus canariensis C.Sm.), eucalypts (Eucalyptus camaldulensis and Eucalyptus occidentalis Endl.), and Acacias, such as Acacia cyclops G.Don and Vachellia karroo (Hayne) Banfi & Galasso. A. saligna was also planted, although, interestingly, its felling began just 15 years later to favour the establishment of the pine forest [42]. However, until 1975, forest interventions were mainly limited to the elimination of dead trees. Subsequently, thinning was periodically carried out with the elimination of one tree row every three rows. The actual nature reserve was then established in 1984, with the decree 216 of the Regional Department of Territory and Environment, and, in 1988, it was assigned to the Regional Department of Rural and Territorial Development (DRSRT). The main goals were, “to ensure the conservation of bird communities, to promote the re-establishment of Mediterranean maquis vegetation, of halophilic plant communities and of dune fauna” (free authors’ translation from the original decree). Over the last two decades, periodic understory clearing and thinning of the exotic species were carried out to promote the gradual conversion towards native-species-dominated forest ecosystems. However, the increasing spread of A. saligna in the understory is making the achievement of this desirable goal extremely difficult. Continuous selective thinning interventions were always performed without the guidance of a forest management plan, whilst the control of A. saligna was never clearly addressed. No wildfires or grazing have occurred in the protected site over the last 50 years.
2.3. Experimental Plots and Sampling Design
To assess the natural regeneration performed by A. saligna, field surveys were carried out in 2017 over six experimental plots along a density gradient of dominant pine (Pinus pinea and Pinus halepensis), ranging from 200 to about 700 stems per hectare. Such values fall among the most commonly observed in thinned Mediterranean pine afforestations [43,44,45]. Pinus spp. are the only tree species composing the upper layer. Each study plot consisted of a 21 × 21 m square (441 m2) and was established after an in-depth investigation of all the study area to ensure homogeneity of main environmental factors (e.g., soil characteristics, slope, stoniness, herbaceous cover, distance from the sea) and silvicultural history. Specifically, the altitudinal difference was <5 m, the distance between plots was <500 m, all plots were located in a range of 250–350 m from the sea, and they were established with a minimum distance of 20 m from unpaved roads to minimize potential edge effects. All target tree species were present in all plots and in their surroundings. Hence, we considered the pine stand density as the most relevant driving factor of tree regeneration in the understory.
Within each plot, besides identification of the Pinus species, the following dendrometric parameters were surveyed for each individual tree: Diameter at breast height (DBH = D1.30 m), total tree height, and basal area. To assess natural regeneration by native woody species and by A. saligna, three 5 × 5 m subplots (25 m2) were established within each plot. Natural regeneration was evaluated in terms of species identity, density (N seedlings or saplings m−2), origin (seed-borne or vegetative sprout), and development (diameter at root collar, height of the largest saplings). We considered only regenerating individuals higher than 5 cm and the following four height (H) classes:
- ➢
- Class 1: H ≤ 50 cm;
- ➢
- Class 2: 50 cm < H ≤ 100 cm;
- ➢
- Class 3: 100 cm < H ≤ 200 cm;
- ➢
- Class 4: 200 cm < H ≤ 400 cm.
Within the upper height classes (3 and 4), we measured the diameter at the root collar in 10 randomly chosen individuals from each class. To determinate the age and the growth rate of A. saligna, we collected three discs from the stem of mature individuals above ground level (diameters = 3.0, 8.5 and 9.5 cm). The surface of the stem discs was sanded with progressively finer grades of sandpaper (up to 1000 grits) to produce a flat, polished surface on which tree ring boundaries were clearly visible under magnification. Growth rings were counted under the microscope (Leica Microsystems, Heerbrugg, Switzerland, Leica DFC 420C©) along three radii per disc, with close attention paid to the occurrence of wedging, missing, and/or false rings.
2.4. Statistical Analysis
Before performing statistical analysis, the assumption of data normality was assessed through the Anderson-Darling test. Both A. saligna (p > 0.15) and the native species (p > 0.15) regeneration density, as well as that of the pine stand (p > 0.15), followed a normal distribution. Linear regression analysis (p < 0.05) was used to assess the correlation between the density of A. saligna and the pine stand, and between the density of A. saligna and the native woody species. We also assessed differences between the height classes, regardless of woody species, and within each woody species between the height classes. As A. saligna data achieved normality after log transformation (Anderson-Darling Test; p = 0.114), we performed a one-way ANOVA followed by a Tukey’s HSD (Honestly Significant Difference) test. As Olea data achieved normality after log transformation (Anderson-Darling Test; p = 0.127), but variance was unequal, we performed a one-way ANOVA followed by a Tamhane’s T2 Test. As Pistacia data did not achieve normality, we performed a Kruskal-Wallis test (non-parametric) followed by a Conover-Inman Test. As data within each height class were not normally distributed, we again performed a Kruskal-Wallis test (non-parametric) followed by a Conover-Inman Test. Statistical analysis was performed using Systat Software, Inc., San Jose, California, CA, USA, 2009 (Version No.13.00.05).
3. Results
3.1. Dominant Pine Cover
Density and dendrometric parameters (DBH, height, and basal area) of the pine canopy cover showed a clear pattern (Table 1). First, a strong positive (r2 = 0.99) correlation between tree density and tree size, in terms of basal area, was found.

Table 1.
Main dendrometric parameters of the pine canopy layer of the study plots. Means are followed by standard deviations.
3.2. Regeneration Layer
A non-significant positive correlation was found between the overall regeneration density (native species plus A. saligna) and the pine stand density (F = 2.80, p = 0.169, r2 = 0.412). When analysed separately (Figure 2), A. saligna regeneration was negatively correlated (F = 11.15, p = 0.029, r2 = 0.736), whereas the native woody species regeneration was positively correlated to the pine density (F = 9.79, p = 0.035, r2 = 0.710). In effect, only Olea regeneration was positively correlated (F = 9.82, p = 0.035, r2 = 0.711) to the pine density, showing Pistacia a non-significant relationship (F = 0.31, p = 0.607, r2 = 0.072).
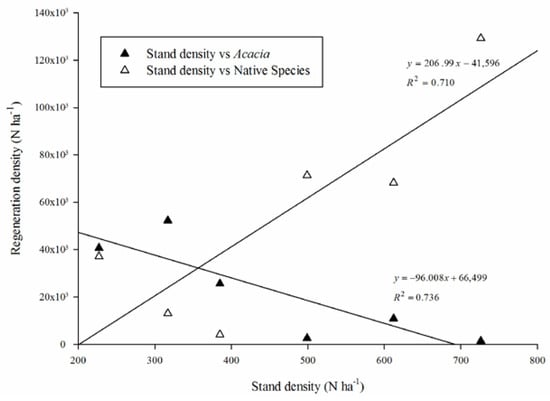
Figure 2.
Linear regression analysis between the pine stand density and the native woody species and Acacia saligna regeneration.
The intersection between the two trend lines allowed us to find a reference value, corresponding to a pine stand density of 356.75 ha−1.
A strong negative exponential relationship (r2 = 0.770) was found between the regeneration density of the native woody species and A. saligna (Figure 3).
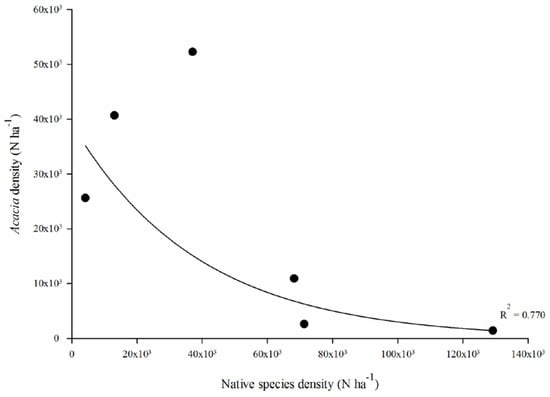
Figure 3.
Relationship between the density of the native woody species and Acacia saligna regeneration.
Overall, regeneration was found to be mainly concentrated in the smallest height classes (1 and 2), together accounting for more than 85% of all the individuals (Table 2). Height classes 3 and 4 accounted for a little over 12% of all the individuals, which was almost exclusively represented by A. saligna. Olea reached the highest regeneration density (mean value: 4.7 individuals m−2), followed by A. saligna (mean value: 2.2 individuals m−2), and Pistacia (mean value: 0.6 individuals m−2). However, large differences, in terms of height class, were found between species . Olea regeneration was clearly dominant in the smallest height classes (1 and 2), whilst A. saligna was by far the most prevalent in height classes 3 and 4. In terms of abundance, height classes 1 and 2 were not different from each other, but both were significantly higher than height classes 3 and 4, which, in turn, were equal with each other (Kruskal-Wallis 23.815, p < 0.001). Regarding A. saligna regeneration, we found very little variation in the diameter at the root collar in relation to plant height, with values ranging from 1 to 1.5 cm in height class 3 and from 2 to 3 cm in height class 4.

Table 2.
Distribution in height class (%) of the woody species regeneration. The relative contribution (%) of each species to each height class is reported within round brackets. Means are followed by the standard error.
No significant differences between the height classes were found in A. saligna Labill. regenerating individuals (F = 0.975, p = 0.414) (Figure 4). In Olea, height classes 1 and 2 were not different from each other and both were significantly higher than height classes 3 and 4 which, in turn, were equal with each other (F = 16.498, p < 0.001) (Figure 4). In Pistacia, height class 1 was significantly higher than the other height classes. Height class 2 was significantly higher than height classes 3 and 4 which, in turn, were equal with each other (Kruskal-Wallis 22.971, p < 0.001).
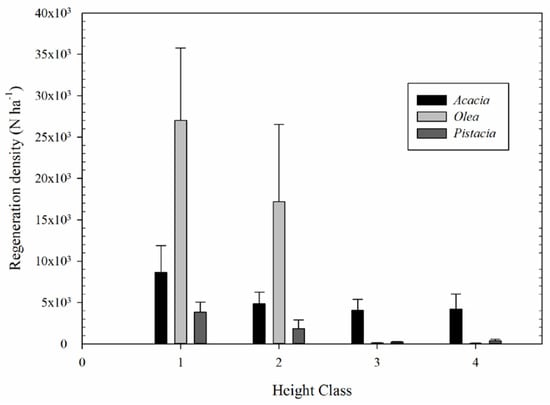
Figure 4.
Height class distribution of the woody species regeneration in the understory. Vertical bars show the standard error of the mean (±SE).
Regarding wood samples, it must be underlined that there are divergent results from different authors about the presence of growth rings in A. saligna. According to InsideWood database [46] and El-Sahhar et al. [47], the growth rings in this species are indistinct, whereas Crivellaro [48] showed that the growth rings boundaries are distinct. In our wood samples, the growth rings of A. saligna were visible in the wood anatomy as bands of marginal parenchyma that run around the entire disc. Counts of parenchyma bands produced disc ages of 6–12 years. We found that the average growth rate was 0.7 cm per year; similar results were found in Kenya by Jama [49].
4. Discussion
Acacia saligna is one of the most widespread invasive species in the Mediterranean basin, where it has been widely used for afforestation purposes and it now threatens native biodiversity and alters ecosystem structure and functioning of large areas. On the other hand, in many areas, Mediterranean afforestations are significantly affected by renaturalization processes by native woody species. Both processes are increasingly common in the Mediterranean basin and they require appropriate management practices. In the understory of a Mediterranean pine plantation, the regeneration pattern of woody native species and the invasive A. saligna were found to be significantly affected by pine stand density and, also, by forest management. The overall management of pine afforestation within the Platani Reserve would require varying types of forest interventions: Thinning of the dominant pine canopy, control measures against A. saligna, and renaturalization actions to favour native woody species.
4.1. Pine Management and Thinning
Stand density and dominant tree size both contribute to determining the amount of available resources in the understory, thus, affecting the competitive dynamics between woody species and altering the complex balance of facilitation and inhibition interactions [5,7,8,50]. Light, as well as soil water and nutrient content are known to play a crucial role in pine understory dynamics [5,43,51]. This is particularly true in Mediterranean coastal pine forests where water supply during summer is considered the main limiting factor of plant growth [9]. Very high stand densities (approximately 1150 pines per hectare) have strong inhibitory effects for shrub species, resulting from a reduction in light, soil water, and nutrient availability [6]. There is a large consensus and much field evidence regarding the negative role played by an excessive pine cover in the renaturalization process of Mediterranean pine plantations, as well as in the richness of the understory [8,13,30]. For this reason, thinning is, generally, performed with the aim of accelerating secondary succession and increasing the overall biodiversity, enhancing the heterogeneity and structural diversity of the forest stand and improving the status of the remaining trees [7,13,52]. Different thinning intensities are usually required for different forest types and species [52,53].
Within Mediterranean pine afforestations, moderate thinning is considered the most suitable option to enable the establishment of most native woody species, including Olea and Pistacia, with a recommended density ranging from approximately 330 to 550 pines per hectare [39,54,55]. Densities greater than 500 pines per hectare are generally required for thermophilous oaks, such as Quercus ilex L., which are shade-demanding at the seedling stage [8,56,57]. However, previous research was carried out in the absence of light-demanding invasive species, such as A. saligna. The variation in pine stand density in our study site depends on periodic thinning that felled, mainly, the largest tree individuals, while the remaining trees have not yet had enough time to grow. Indeed, larger pines were found in areas with higher stand density. Conversely, the number of individuals per unit area and average tree size are inversely correlated in natural forest stands [58]. Our research suggests the need to adopt different forest management practices according to the pine stand density. With densities of up to 400 pines per hectare, no thinning should be performed as a further decrease in stand density would cause the undisputed spread of A. saligna. A density greater than 400 is the minimum threshold to contain the invasive potential of A. saligna, while favouring the recruitment and establishment of native species. Densities higher than 500 pines per hectare were related to an increase in the regeneration density of approximately 6.5-fold for Olea and 2-fold for Pistacia, together with a concomitant decrease in A. saligna of approximately 8-fold. Low-intensity, localized, and gradual thinning is strongly recommended for densities exceeding 600 pines per hectare in which higher shading has kept A. saligna relatively under control. Mediterranean woody species, such as Quercus spp. and Olea, which may survive under a closed canopy during the early stages of life, need more light in subsequent sapling and juvenile phases [57]. Such necessary interventions should be preceded by the clearing of any adult A. saligna plants to avoid the risk of a new invasion.
4.2. Regeneration Pattern in the Understory
In our study site, we assessed the current regeneration pattern of Acacia saligna and native woody species in the understory of a Mediterranean coastal pine plantation. We found evidence of a species-specific response, with A. saligna being negatively correlated to the stand density, Olea being positively correlated, and Pistacia being not significantly correlated. At high pine densities (>500 pines per hectare), Olea regeneration is by far the most prevalent, while A. saligna regeneration is prevalent under low pine densities (<400 pines per hectare). At intermediate conditions (400–500 pines per hectare), Pistacia tends to prevail slightly, while both A. saligna and Olea are represented by just over 21% of regenerating individuals. As only current regeneration has been assessed, it should be considered that long-term fluctuations could arise and cannot be excluded.
Olea and Pistacia showed quite different behaviour based on the stand density. Olea attained an impressive regeneration density that linearly increased with stand density, with the maximum value exceeding 120,000 seedlings per hectare (mean: 47,110 N ha−1). Previous regeneration data obtained from field investigations carried out in the same protected area suggest that Olea has experienced a marked increase in the last 10–15 years [41]. Such a sizable change may be due to an increase in the effective seed dispersal by birds and to possible shifts in their natural population [59]. Conversely, Pistacia showed a much lower regeneration density (mean: 5,700 N ha−1), with non-significant variations based on the stand density. Such a pattern was not completely unexpected as Pistacia was found to dominate the understory in high pine cover conditions [30] and its considerable ecological plasticity to solar radiation is recognized [60]. However, saplings of both native species were much less developed than A. saligna, being mostly less than one meter high in Olea (>99%) and Pistacia (>91%). Conversely, approximately 70% of A. saligna saplings were taller than one meter, suggesting a clearly higher reproductive potential.
4.3. Acacia Management
Our field surveys and widespread evidence collected worldwide suggest that the control and eradication of A. saligna is a difficult and time-consuming task. After heavy thinning or a natural disturbance event, this species seems to be better equipped than native species to occupy free space and exploit available resources, thus proving more competitive. Under such circumstances, A. saligna may exert strong negative effects on the growth and establishment of native woody species, seriously hindering the renaturalization process and any chance of an autonomous evolution towards more complex and diversified forest stands. A. saligna has proven to be particularly favoured by high light availability, such as can be found in natural gaps or recently cut areas [29]. However, its regeneration has also been found in high pine densities, underlining its remarkable adaptability to a wide spectrum of light conditions [50]. Furthermore, pine cover may have played a key role in the initial stages of invasion, offering suitable microclimatic conditions for regeneration in the backdunes and providing protection from direct sun exposure, marine salt-spray, and frequent winds, as well as, presumably, higher soil water and nutrient availability [45,51].
The temporal dynamics of A. saligna invasion should be considered carefully as time has significant consequences for the efficacy of control actions. The ecological impacts A. saligna exerts on the recipient ecosystem [16] and the average time needed for ecosystem recovery may vary significantly with the duration of the invasion. The alterations in soil chemical characteristics, such as increased pH and soil organic matter and nutrients (N and P), are time-dependent effects that are generally associated with A. saligna [27,61,62]. For fast-growing trees, like Acacias, a time span of 20-25 years is already considered a long-term invasion [61,62]. A factor explaining the massive regeneration of A. saligna in recent decades could be the improvement in edaphic conditions due to Acacia litter accumulation, as was found for Acacia cyclops on the Island of Lampedusa [63]. Time has also allowed A. saligna to express its maximum reproductive potential. Annually, A. saligna mother plants may release approximately 5,400 seeds per square meter to the soil [64]. While less than 10% of seeds germinate in the first year, the remaining seeds help to build up a consistent and long-lasting soil seed bank [65,66]. In South African invaded stands, more than 44,000 seeds per square meter may be found in the seed bank, including the soil and litter layers [67,68]. This huge amount depends on the long viability of the seed in the soil, resisting up to 50 years in the absence of mechanical scarification [65] and even 10 years after clearing [62,66,68]. Resistance to fire events is also highly influential for Mediterranean ecosystems [65]. A. saligna could have reached its maximum potential soil seed bank in the Platani nature reserve as this is related to the stem diameter reaching 6 cm [68], a value commonly found in our field surveys.
The successful management of A. saligna can be achieved only if its reproductive potential is destroyed or at least severely limited. The interventions required should aim to reduce the population of adult individuals and, therefore, to curb the current seed production and to deplete seed banks by destroying seeds and/or triggering mass germination [65]. Regarding clearing treatments, excluding the use of herbicides, a possible option could be felling and burning for the combined effect on A. saligna living trees and regeneration, as well as on the soil seed bank [27,64,66]. However, in our protected site, the use of prescribed burning is not recommended due to difficulties in controlling the spread of fire outside well-confined areas and, also, due to negative ecological effects on the overall biodiversity. Simply stopping seed fall may lead to reductions in the soil seed bank of 80% in four to six years [67]. Hence, it is crucial to intervene with systematic cuttings of A. saligna mature plants before dissemination, i.e., every year before summer. This is not simple as A. saligna reaches reproductive maturity extremely quickly: We observed individuals at just one meter tall already fruiting in the study area. It is also essential to eliminate seedlings that may emerge after a disturbance event [68]. The absence of continuous forest interventions may lead to the comeback of the seed bank at pre-intervention levels in only seven years [67]. After stand clearing, the altered soil conditions caused by the invasion of A. saligna can last up to 10 years [62] and may, especially, threaten the presence of native species which have a narrow ecological niche and are accustomed to ecosystems with limited nutrient availability [69]. In our study site, it is highly likely that no fewer than 10 years of continuous and capillary interventions will be necessary for the effective eradication of A. saligna. Considering this, an effective possibility could be the production of woodchips of A. saligna and other wood residuals (i.e., from pruning, thinning, ecc.).
Another factor that further complicates the management of pine afforestation is the concomitant occurrence of other non-native tree species, such as Acacia cyclops A.Cunn. ex G.Don and Pinus canariensis [70], Eucalyptus occidentalis Endl. [71], Vachellia karroo (Hayne) Banfi & Galasso, and Myoporum tenuifolium G.Forst. All these species experienced full naturalization in the protected site and could spread further in the near future, completely altering local vegetation dynamics, with unpredictable consequences. Such potentially invasive species may benefit from the increased nutrient availability provided by A. saligna and the clearing treatments against it. The threat also posed by potential secondary invasion has to be carefully evaluated during the necessary periodic monitoring of the study area [72].
4.4. Renaturalization
After eradication, especially in areas with sufficient cover of native woody species, specific interventions addressing renaturalization of the forest plantation could be carried out. It is necessary to assess whether local species can autonomously recover their role in the ecosystem, starting from the understory. This opportunity depends on various ecological, management, and landscape characteristics. Three main aspects must be considered: Soil seed bank dynamics, the dispersal ability of native species, and the occurrence and abundance of suitable seed dispersers. Firstly, it should be noted that A. saligna is able to reduce the soil seed bank of native species over time [61]. Forest ecosystems in the surrounding areas of the Platani reserve are very rare and fragmented; mature woody individuals, therefore, are too few and/or too far away, thus compromising the chance of passive restoration processes via bird communities [73]. Active restoration through direct sowing or planting of native woody species is required [8,27,62]. The effective renaturalization is, however, quite difficult to achieve as many biotic and abiotic factors limiting seed and seedling establishment and affecting mortality rates need to be considered. For instance, Li et al. [74] found that the successful strategy to reintroduce native woody species in the understory of conifer plantations is using seedlings older than six months or fencing the area.
Then, as native woody species in the Platani reserve all bear fleshy fruits or acorns, their dissemination strongly depends upon the occurrence of seed dispersers, especially frugivorous birds [73,75,76]. Gómez-Aparicio et al. [8] found that 4 km is the maximum seed dispersal distance by Garrulus glandarius L. to enable the effective establishment of Holm oak in Mediterranean contexts. Some scattered regenerating individuals of Quercus ilex and Quercus pubescens Willd. s.l. have been found in the study area, probably due to dissemination by the jay [76]. The recovery of woody vegetation could be relatively fast as many bird seed dispersers, such as the Eurasian jay (Garrulus glandarius Linnaeus), the song thrush (Turdus philomelos Brehm), the common starling (Sturnus vulgaris Linnaeus), and the blackbird (Turdus merula Linnaeus) are already present in the protected site or in its surrounding area (T. La Mantia and R. Bueno, pers. obs.).
5. Conclusions
In Mediterranean pine plantations threatened by A. saligna, forest interventions should differ according to pine stand density. The present case study may offer many useful pointers for the management of similar Mediterranean afforestations where thinning, aimed at favouring renaturalization by native woody species, could, otherwise, trigger the spread of invasive alien species, thus counterbalancing the positive effects of necessary interventions. In the absence of any active management in the Platani reserve, an inexorable increase in the invasion process by A. saligna is likely. Adaptive forest management capable of maintaining sufficient pine cover in the areas most at risk of invasion, while increasing the light available in areas with excessive stand density, appears to be the best trade-off to foster natural succession processes, whilst preventing invasion by A. saligna. The complex understory dynamics and competitive interactions between native species and A. saligna highlight the need for periodic interventions, as well as for follow-up treatments and regular and constant monitoring.
Author Contributions
E.B., R.d.S.B., D.S.L.M.V., S.P. and T.L.M. conceived and designed the study, carried out field work, analyzed data and prepared the manuscript; O.C. conceived the study and provided information about historical aspects; M.G. carried out field work and analyzed data; G.S. carried out field work, analyzed data, in particular dendrochronological aspects, and contributed to prepare the manuscript.
Funding
The authors are indebted to the Sicilian Department of Rural and Territorial Development for the logistic support during field surveys. This work was financially supported by the PRIN2012 “IDEM” (Development of innovative methods for forest ecosystems monitoring based on remote sensing; Grant 2012EWEY2S, national coordinator: G. Chirici), and the PRIN2010-11 “CARBOTREES” (Climate change mitigation strategies in tree crops and forestry in Italy, national coordinator: R. Valentini), both funded by the Italian Ministry of Education, University and Research
Acknowledgments
We thank Dott. Ruvolo and Andrea La Mantia for their kind support during field activities. We thank Giovanni Mughini for its support in bibliographic research. We are grateful to L.B. Hornsby for the revision of the English version of the manuscript. Finally, we are grateful to the reviewers who significantly improved the quality of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sheffer, E. A review of the development of Mediterranean pine-oak ecosystems after land abandonment and afforestation: Are they novel ecosystems? Ann. For. Sci. 2012, 69, 429–443. [Google Scholar] [CrossRef]
- Pausas, J.G.; Bladé, C.; Valdecantos, A.; Seva, J.P.; Fuentes, D.; Alloza, J.; Vilagrosa, A.; Bautista, S.; Cortina, J.; Vallejo, R. Pines and oaks in the restoration of Mediterranean landscapes of Spain: New perspectives for an old practice—A review. Plant Ecol. 2004, 171, 209–220. [Google Scholar] [CrossRef]
- Van der Meulen, F.; Salman, A.H.P.M. Management of Mediterranean coastal dunes. Ocean Coast. Manag. 1996, 30, 177–195. [Google Scholar] [CrossRef]
- Vallejo, R. Restoring Mediterranean Forests. In Forest Restoration in Landscapes; Mansourian, S., Vallauri, D., Dudley, N., Eds.; Springer-Verlag: New York, NY, USA, 2005; pp. 313–319. [Google Scholar]
- Bellot, J.; Maestre, F.T.; Chirino, E.; Hernández, N.; De Urbina, J.O. Afforestation with Pinus halepensis reduces native shrub performance in a Mediterranean semiarid area. Acta Oecol. 2004, 25, 7–15. [Google Scholar] [CrossRef]
- Moreno-Gutiérrez, C.; Battipaglia, G.; Cherubini, P.; Delgado Huertas, A.; Querejeta, J.I. Pine afforestation decreases the long-term performance of understorey shrubs in a semi-arid Mediterranean ecosystem: A stable isotope approach. Funct. Ecol. 2015, 29, 15–25. [Google Scholar] [CrossRef]
- Pasta, S.; La Mantia, T.; Rühl, J. The impact of Pinus halepensis afforestation on Mediterranean spontaneous vegetation: Do soil treatment and canopy cover matter? J. For. Res. 2012, 23, 517–528. [Google Scholar] [CrossRef][Green Version]
- Gómez-Aparicio, L.; Zavala, M.A.; Bonet, F.J.; Zamora, R. Are pine plantations valid tools for restoring Mediterranean forests? An assessment along gradients of climatic conditions, stand density and distance to seed sources. Ecol. Appl. 2009, 19, 2124–2141. [Google Scholar] [CrossRef] [PubMed]
- Osem, Y.; Zangy, E.; Bney-Moshe, E.; Moshe, Y.; Karni, N.; Nisan, Y. The potential of transforming simple structured pine plantations into mixed Mediterranean forests through natural regeneration along a rainfall gradient. For. Ecol. Manag. 2009, 259, 14–23. [Google Scholar] [CrossRef]
- Badalamenti, E.; La Mantia, T.; La Mantia, G.; Cairone, A.; La Mela Veca, D.S. Living and dead aboveground biomass in Mediterranean forests: Evidence of old-growth traits in a Quercus pubescens Willd. s.l. stand. Forests 2017, 8, 187. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Arico, S.; Aronson, J.; Baron, J.S.; Bridgewater, P.; Cramer, V.A.; Epstein, P.R.; Ewel, J.J.; Klink, C.A.; Lugo, A.E.; et al. Novel ecosystems: Theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 2006, 15, 1–7. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pysek, P.; Rejmánek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- McGlone, C.M.; Springer, J.D.; Laughlin, D.C. Can pine forest restoration promote a diverse and abundant understory and simultaneously resist nonnative invasion? For. Ecol. Manag. 2009, 258, 2638–2646. [Google Scholar] [CrossRef]
- Radtke, A.; Ambraß, S.; Zerbe, S.; Tonon, G.; Fontana, V.; Ammer, C. Traditional coppice forest management drives the invasion of Ailanthus altissima and Robinia pseudoacacia into deciduous forests. For. Ecol. Manag. 2013, 291, 308–317. [Google Scholar] [CrossRef]
- Zhu, J.J.; Matsuzaki, T.; Lee, F.Q.; Gonda, Y. Effect of gap size created by thinning on seedling emergency, survival and establishment in a coastal pine forest. For. Ecol. Manag. 2003, 182, 339–354. [Google Scholar] [CrossRef]
- Le Maitre, D.C.; Gaertner, M.; Marchante, E.; Ens, E.-J.; Holmes, P.M.; Pauchard, A.; O’Farrell, P.J.; Rogers, A.M.; Blanchard, R.; Blignaut, J.; et al. Impacts of invasive Australian acacias: Implications for management and restoration. Divers. Distrib. 2011, 17, 1015–1029. [Google Scholar] [CrossRef]
- Crisóstomo, J.A.; Rodríguez-Echeverría, S.; Freitas, H. Co-introduction of exotic rhizobia to the rhizosphere of the invasive legume Acacia saligna, an intercontinental study. Appl. Soil Ecol. 2013, 64, 118–126. [Google Scholar] [CrossRef]
- Montesinos, D.; Castro, S.; Rodríguez-Echeverría, S. Two invasive acacia species secure generalist pollinators in invaded communities. Acta Oecol. 2016, 74, 46–55. [Google Scholar] [CrossRef]
- Corbin, J.D.; D’Antonio, C.M. Effects of exotic species on soil nitrogen cycling: Implications for restoration. Weed Technol. 2004, 18, 1464–1467. [Google Scholar] [CrossRef]
- Grove, S.; Parker, I.M.; Haubensak, K.A. Persistence of a soil legacy following removal of a nitrogen-fixing invader. Biol. Invasions 2015, 17, 2621–2631. [Google Scholar] [CrossRef]
- Badalamenti, E.; Gristina, L.; Laudicina, V.A.; Novara, A.; Pasta, S.; La Mantia, T. The impact of Carpobrotus cfr. acinaciformis (L.) L. Bolus on soil nutrients, microbial communities structure and native plant communities in Mediterranean ecosystems. Plant Soil 2016, 409, 19–34. [Google Scholar] [CrossRef]
- Badalamenti, E.; Gristina, L.; La Mantia, T.; Novara, A.; Pasta, S.; Lauteri, M.; Fernandes, P.; Correia, O.; Máguas, C. Relationship between recruitment and mother plant vitality in the alien species Acacia cyclops A. Cunn. ex G. Don. For. Ecol. Manag. 2014, 331, 237–244. [Google Scholar] [CrossRef]
- Griffin, A.R.; Midgley, S.J.; Bush, D.; Cunningham, P.J.; Rinaudo, A.T. Global uses of Australian acacias-recent trends and future prospects. Divers. Distrib. 2011, 17, 837–847. [Google Scholar] [CrossRef]
- La Mantia, T. I rimboschimenti delle dune. In I Cambiamenti Nell’ecosistema della Riserva Naturale di Vendicari e Gli Effetti Sull’avifauna; Ientile, R., Rühl, J., La Mantia, T., Massa, B., Eds.; Danaus: Palermo, Italy, 2011; pp. 97–109. ISBN 978-88-904929-3-8. [Google Scholar]
- Souza-Alonso, P.; Rodríguez, J.; González, L.; Lorenzo, P. Here to stay. Recent advances and perspectives about Acacia invasion in Mediterranean areas. Ann. For. Sci. 2017, 74, 1–20. [Google Scholar] [CrossRef]
- Quatrini, P.; Scaglione, G.; Incannella, G.; Badalucco, L.; Puglia, A.M.; La Mantia, T. Microbial inoculants on woody legumes to recover a municipal landfill site. Water Air Soil Pollut. Focus 2003, 3, 189–199. [Google Scholar] [CrossRef]
- Mostert, E.; Gaertner, M.; Holmes, P.M.; Rebelo, A.G.; Richardson, D.M. Impacts of invasive alien trees on threatened lowland vegetation types in the Cape Floristic Region, South Africa. South Afr. J. Bot. 2017, 108, 209–222. [Google Scholar] [CrossRef]
- Holmes, P.M.; Cowling, R.M. The effects of invasion by Acacia saligna on the guild structure and regeneration capabilities of South African fynbos shrublands. J. Appl. Ecol. 1997, 34, 317. [Google Scholar] [CrossRef]
- Del Vecchio, S.; Acosta, A.; Stanisci, A. The impact of Acacia saligna invasion on Italian coastal dune EC habitats. Comptes Rendus Biol. 2013, 336, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Manor, R.; Cohen, O.; Saltz, D. Community homogenization and the invasiveness of commensal species in Mediterranean afforested landscapes. Biol. Invasions 2008, 10, 507–515. [Google Scholar] [CrossRef]
- Biondi, E.; Blasi, C.; Burrascano, S.; Casavecchia, S.; Copiz, R.; Del Vico, E.; Galdenzi, D.; Gigante, D.; Lasen, C.; Spampinato, G.; et al. Italian Interpretation Manual of the 92/43/EEC Directive Habitats. Ministero dell’Ambiente e della Tutela del Territorio e del Mare. 2009. Available online: http://vnr. unipg.it/habitat/ (accessed on 10 April 2018).
- Terracciano, A. Osservazioni fenologiche. Boll. R. Orto Bot. Palermo 1898, 2, 66–88. [Google Scholar]
- Bazan, G.; Speciale, M. Processi di spontaneizzazione in Sicilia di Acacia saligna (Mimosaceae, Magnoliophyta). Quad. Bot. Ambient. Appl. 2002, 12, 99–100. [Google Scholar]
- Troìa, A.; Spallino, R.E. Conferma della presenza nella sicilia occidentale di Retama raetam (Forssk.) Webb subsp. gussonei (Webb) W. Greuter (Fabaceae Cytiseae), specie a rischio della flora italiana. Nat. Sicil. 2009, 33, 305–314. [Google Scholar]
- Sciandrello, S.; Tomaselli, G.; Minissale, P. The role of natural vegetation in the analysis of the spatio-temporal changes of coastal dune system: A case study in Sicily. J. Coast. Conserv. 2015, 19, 199–212. [Google Scholar] [CrossRef]
- Gutierres, F.; Gil, A.; Reis, E.; Lob, A.; Neto, C.; Calado, H.; Costa, J.C. Acacia saligna (Labill.) H. Wendl in the Sesimbra County: Invaded habitats and potential distribution modeling. J. Coast. Res. 2011, 403–407. [Google Scholar]
- Guarino, R.; Guglielmo, A.; Ronsisvalle, F.; Sciandrello, S. Il progetto ECONET-COHAST: Strategie per la conservazione degli habitat costieri di Torre Manfria (Sicilia merid.). Fitosociologia 2008, 44, 333–337. [Google Scholar]
- Pasta, S.; La Mantia, T. Le specie vegetali aliene in alcuni SIC siciliani: Analisi del grado di invasività e misure di controllo. Mem. Soc. It. Sci. nat. Museo civ. Stor. nat. Milano 2008, 36, 79. [Google Scholar]
- Del Favero, R. I Boschi delle Regioni meridionali E Insulari D’Italia; Cleup: Padova, Italy, 2008; ISBN 978-88-6129-176-8. [Google Scholar]
- Rivas-Martínez, S. Global Bioclimatics (Clasificación Bioclimática de la Tierra); Universidad Complutense: Madrid, Spain, 1994. [Google Scholar]
- Terrasi, R. Analisi dei processi evolutivi della vegetazione nella Riserva Naturale Orientata “Foce Del Fiume Platani”; Università degli Studi di Palermo: Palermo, Italy, 2002. [Google Scholar]
- Capuano, D.; Sammartano, G.; Palmeri, A.; Oieni, S.; Lopez, T.; Butera, S.; Giuliani, N.; Caputo, G.; Morello, S.; Cascio, A.; Leone, P.; Marguglio, T. Boschi di Sicilia; La Cartogr: Palermo, Italy, 1967. [Google Scholar]
- Maestre, F.T.; Cortina, J.; Bautista, S. Mechanisms underlying the interaction between Pinus halepensis and the native late-successional shrub Pistacia lentiscus in a semi-arid plantation. Ecography 2004, 27, 776–786. [Google Scholar] [CrossRef]
- Tartarino, P.; Galante, W.; Greco, R. Using the hart-becking spacing index in a study of the naturalisation of Pinus halepensis Miller plantation stands in the South-Eastern Salento peninsula. Option Méditerranéennes. Série A Semin. Méditerranéennes 2007, 75, 175–188. [Google Scholar]
- Bouachir, B.B.; Khorchani, A.; Guibal, F.; El Aouni, M.H.; Khaldi, A. Dendroecological study of Pinus halepensis and Pinus pinea in Northeast coastal dunes in Tunisia according to distance from the shoreline and dieback intensity. Dendrochronologia 2017, 45, 62–72. [Google Scholar] [CrossRef]
- InsideWood Database. Available online: http://insidewood.lib.ncsu.edu/search (accessed on 30 March 2018).
- El-Sahhar, K.F.; Nassar, D.M.; Amer, W.M.; Qasem, L.A. Morphological and anatomical studies of Acacia saligna the dominant plant species in Al-Ahrash protectorate-Rafah-North Sinai Egypt. Bull. Fac. Agric. 2009, 60, 43–60. [Google Scholar]
- Crivellaro, A.; Schweingruber, F.H. Atlas of Wood, Bark and Pith Anatomy of Eastern Mediterranean Trees and Shrubs; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-37234-6. [Google Scholar]
- Jama, B.; Nair, P.K.R.; Kurira, P.W. Comparative growth performance of some multipurpose trees and shrubs grown at Machakos, Kenya. Agrofor. Syst. 1989, 9, 17–27. [Google Scholar] [CrossRef]
- Bonari, G.; Acosta, A.T.R.; Angiolini, C. Mediterranean coastal pine forest stands: Understorey distinctiveness or not? For. Ecol. Manag. 2017, 391, 19–28. [Google Scholar] [CrossRef]
- Rascher, K.G.; Große-Stoltenberg, A.; Máguas, C.; Werner, C. Understory invasion by Acacia longifolia alters the water balance and carbon gain of a Mediterranean pine forest. Ecosystems 2011, 14, 904–919. [Google Scholar] [CrossRef]
- Otto, R.; García-del-Rey, E.; Méndez, J.; Fernández-Palacios, J.M. Effects of thinning on seed rain, regeneration and understory vegetation in a Pinus canariensis plantation (Tenerife, Canary Islands). For. Ecol. Manag. 2012, 280, 71–81. [Google Scholar] [CrossRef]
- Pérez-De-Lis, G.; García-González, I.; Rozas, V.; Arévalo, J.R. Effects of thinning intensity on radial growth patterns and temperature sensitivity in Pinus canariensis afforestations on Tenerife Island, Spain. Ann. For. Sci. 2011, 68, 1093–1104. [Google Scholar] [CrossRef]
- Cullotta, S.; Pizzurro, G.; Garfì, G.; La Mantia, T. Analisi dei processi di rinaturalizzazione nelle pinete mediterranee artificiali dei Monti di Palermo (Sicilia Nord-Occidentale). In III Congresso Nazionale S.I.S.E.F.—Atti 3—Alberi E Foreste Per Il Nuovo Millennio; SISEF—Società Italiana Selvicoltura Ecologia Forestale: Bologna, Italy, 2001; pp. 457–465. [Google Scholar]
- Pastorella, F. Analisi del microclima luminoso, in pinete artificiali a pino d’Aleppo, idoneo alla rinnovazione delle principali specie forestali dell’area del Mediterraneo. Dendronatura 2011, 2, 53–73. [Google Scholar]
- Lookingbill, T.R.; Zavala, M.A. Spatial pattern of Quercus ilex and Quercus pubescens recruitment in Pinus halepensis dominated woodlands. J. Veg. Sci. 2000, 11, 607–612. [Google Scholar] [CrossRef]
- Navarro-González, I.; Pérez-Luque, A.J.; Bonet, F.J.; Zamora, R. The weight of the past: Land-use legacies and recolonization of pine plantations by oak trees. Ecol. Appl. 2013, 23, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Zeide, B. A relationship between size of trees and their number. For. Ecol. Manag. 1995, 72, 265–272. [Google Scholar] [CrossRef]
- Aslan, C.E. Implications of newly-formed seed-dispersal mutualisms between birds and introduced plants in northern California, USA. Biol. Invasions 2011, 13, 2829–2845. [Google Scholar] [CrossRef]
- Nahum, S.; Inbar, M.; Ne’eman, G.; Ben-Shlomo, R. Phenotypic plasticity and gene diversity in Pistacia lentiscus L. along environmental gradients in Israel. Tree Genet. Genomes 2008, 4, 777–785. [Google Scholar] [CrossRef]
- Holmes, P.; Cowling, R. Diversity, composition and guild structure relationships between soil stored seed banks and mature vegetation in alien plant invaded South African shrublands. Plant Ecol. 1997, 133, 107–122. [Google Scholar] [CrossRef]
- Nsikani, M.M.; Novoa, A.; van Wilgen, B.W.; Keet, J.H.; Gaertner, M. Acacia saligna’s soil legacy effects persist up to 10 years after clearing: Implications for ecological restoration. Austral Ecol. 2017, 42, 880–889. [Google Scholar] [CrossRef]
- Pasta, S.; Badalamenti, E.; La Mantia, T. Acacia cyclops A. Cunn. ex G. Don (Leguminosae) in Italy: First cases of naturalization. Anal. Jard. Bot. Madrid 2012, 69, 193–200. [Google Scholar] [CrossRef][Green Version]
- Richardson, D.M.; Kluge, R.L. Seed banks of invasive Australian Acacia species in South Africa: Role in invasiveness and options for management. Perspect. Plant Ecol. Evol. Syst. 2008, 10, 161–177. [Google Scholar] [CrossRef]
- Milton, S.J.; Hall, A.V. Reproductive biology of Australian acacias in the Outh-Western Cape Province, South Africa. Trans. R. Soc. South Afr. 1981, 44, 465–487. [Google Scholar] [CrossRef]
- Holmes, P.M. Implications of alien Acacia seed bank viability and germination for clearing. South Afr. J. Bot. 1988, 54, 281–284. [Google Scholar] [CrossRef]
- Holmes, P.M.; Macdonald, I.A.W.; Juritz, J. Effects of clearing treatment on seed banks of the alien invasive shrubs Acacia saligna and Acacia cyclops in the Southern and South-Western Cape, South Africa. J. Appl. Ecol. 1987, 24, 1045–1051. [Google Scholar] [CrossRef]
- Strydom, M.; Veldtman, R.; Ngwenya, M.Z.; Esler, K.J. Invasive Australian Acacia seed banks: Size and relationship with stem diameter in the presence of gall-forming biological control agents. PLoS ONE 2017, 12, e0181763. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, E.T.F. Growth and competition between seedlings of Protea repens (L.) L. and the alien invasive, Acacia saligna (Labill.) Wendl. in relation to nutrient availability. Funct. Ecol. 1991, 5, 101–110. [Google Scholar] [CrossRef]
- Badalamenti, E.; La Mantia, T.; Pasta, S. Primo caso di naturalizzazione di Pinus canariensis C. Sm. (Pinaceae) per la Sicilia e prima stazione di Acacia cyclops G. Don (Fabaceae) sull’isola maggiore. Nat. Sicil. 2013, 37, 497–503. [Google Scholar]
- Badalamenti, E.; Cusimano, D.; La Mantia, T.; Pasta, S.; Romano, S.; Troìa, A.; Ilardi, V. The ongoing naturalisation of Eucalyptus spp. in the Mediterranean Basin: New threats to native species and habitats. Austral. For. 2018, in press. [Google Scholar]
- Kuebbing, S.E.; Nuñez, M.A.; Simberloff, D. Current mismatch between research and conservation efforts: The need to study co-occurring invasive plant species. Biol. Conserv. 2013, 160, 121–129. [Google Scholar] [CrossRef]
- Zamora, R.; Hódar, J.A.; Matías, L.; Mendoza, I. Positive adjacency effects mediated by seed disperser birds in pine plantations. Ecol. Appl. 2010, 20, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-T.; Lo, Y.-H.; Lin, Y.-C.; Guan, B.; Blanco, J.; You, C.-H. Bringing the natives back: Identifying and alleviating establishment limitations of native hardwood species in a conifer plantation. Forests 2018, 9, 3. [Google Scholar] [CrossRef]
- Gómez, J.M. Spatial patterns in long-distance dispersal of Quercus ilex acorns by jays in a heterogeneous landscape. Ecography 2003, 26, 573–584. [Google Scholar] [CrossRef]
- La Mantia, T.; da Silveira Bueno, R. Colonization of Eurasian jay Garrulus glandarius and holm oaks Quercus ilex: The establishment of ecological interactions in urban areas. Avocetta 2016, 40, 85–87. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).