Native Forests Have a Higher Diversity of Macrofungi Than Comparable Plantation Forests in the Greater Mekong Subregion
Abstract
1. Introduction
- (1)
- How does macrofungal diversity differ between native and plantation forests?
- (2)
- How does macrofungal community composition differ between forests in temperate and tropical climate zones?
- (3)
- How does macrofungal diversity and community composition change with environmental factors (elevation, latitude, tree species richness)?
2. Materials and Methods
2.1. Study Area and Study Plots
2.2. Fungal Sampling and Identification
2.3. Data Analysis
3. Results
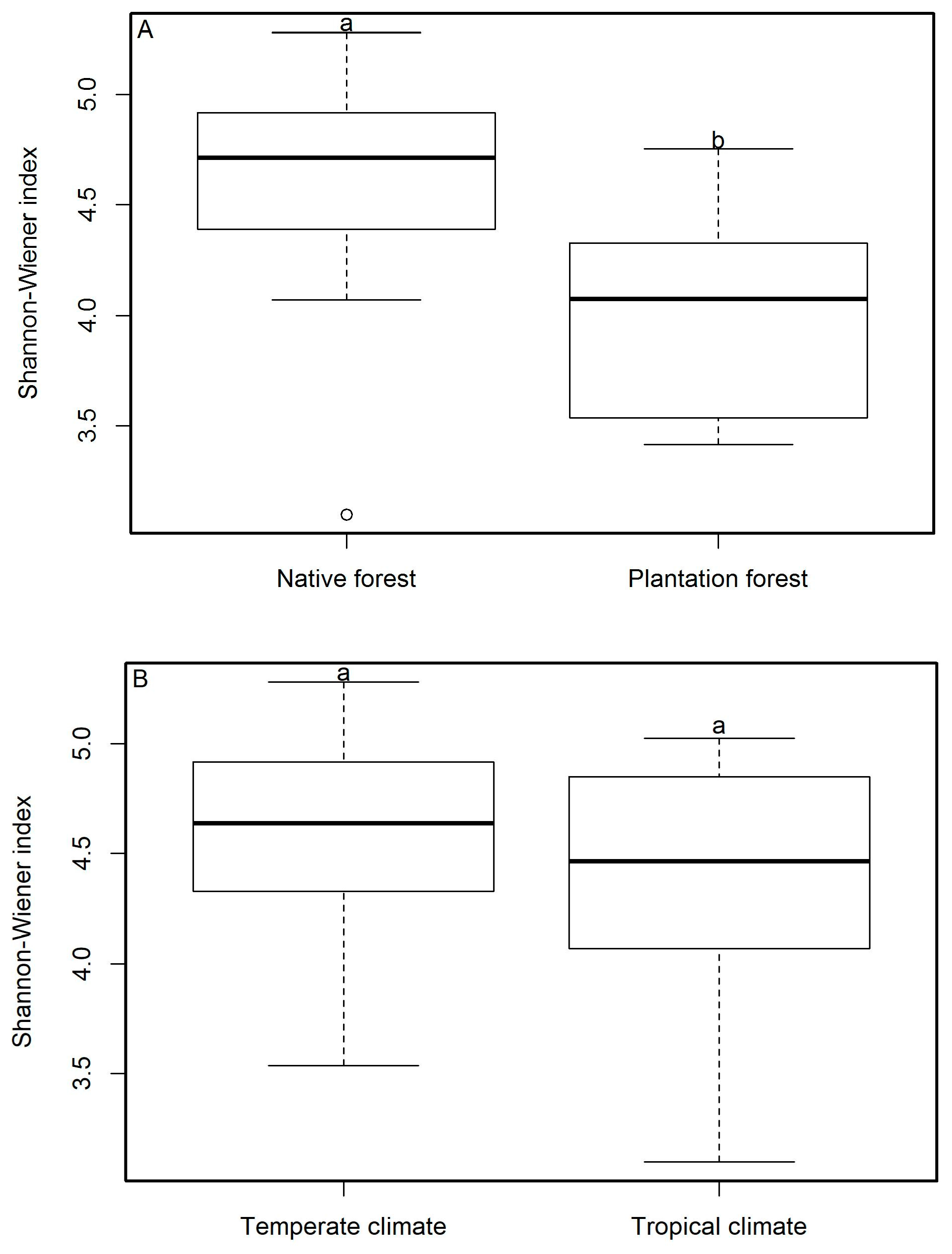
3.1. Macrofungal Diversity
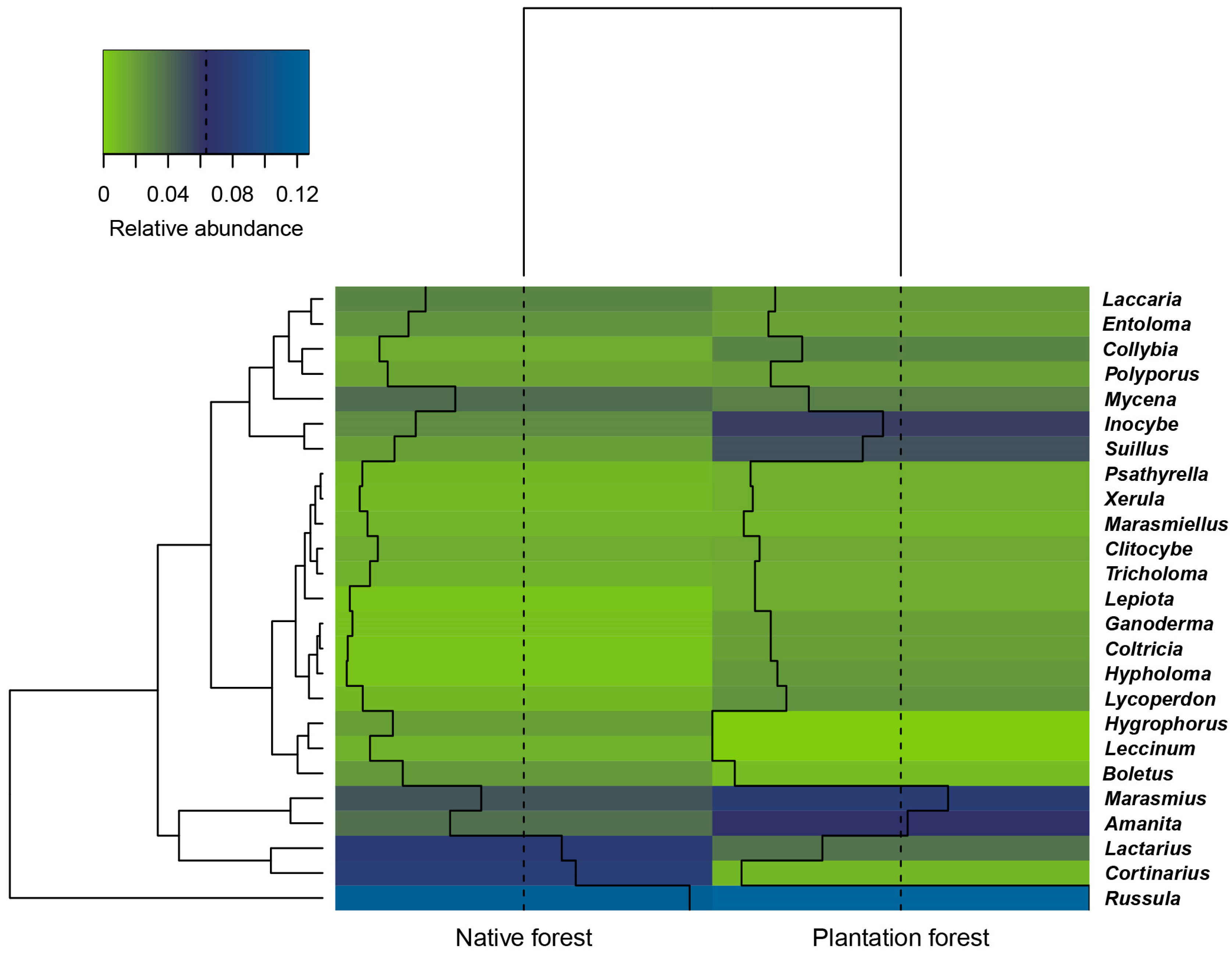
3.2. Macrofungal Composition
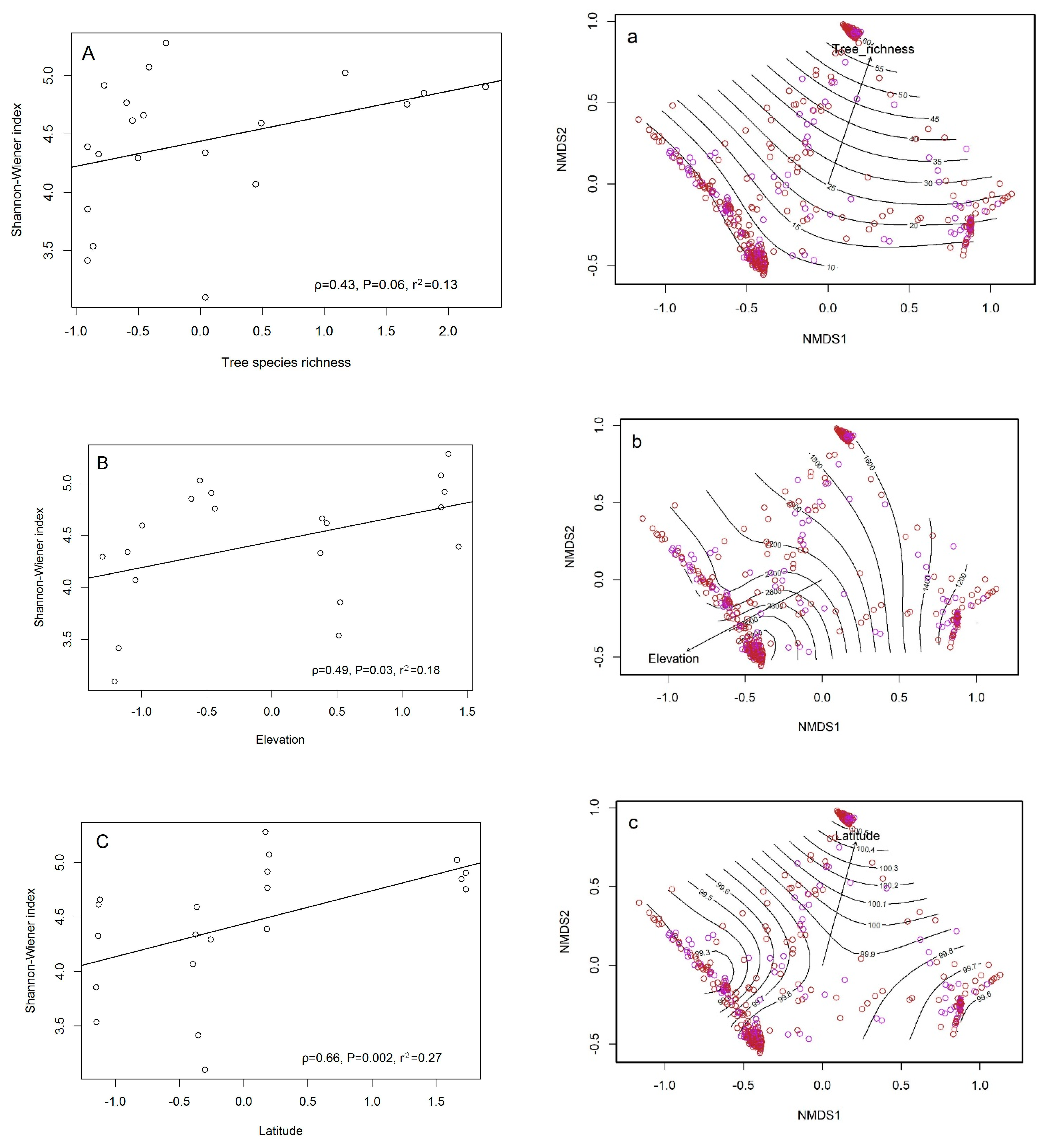
3.3. Correlation between Ecological Diversity and Environmental Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reid, W.V. Biodiversity hotspots. Trends Ecol. Evol. 1998, 13, 275–280. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lu, P.; Liu, D.; Jin, P. Pinpointing source of mekong and measuring its length through analysis of satellite imagery and field investigations. Geo-Spat. Inf. Sci. 2007, 10, 51–56. [Google Scholar] [CrossRef]
- Tang, L.; Yang, Z. Recent studies on fungal species diversity in the Lancang-Mekong River Watershed. Resour. Sci. 2014, 36, 282–295. [Google Scholar]
- Moore, D.; Nauta, M.M.; Evans, S.E.; Rotheroe, M. Fungal Conservation: Issues and Solutions; Cambridge University Press: Cambridge, UK, 2009; pp. 1–256. [Google Scholar] [CrossRef]
- McLellan, T.; Brown, M. Mushrooms and cash crops can coexist in mountain livelihoods: Wild mushrooms as economic and recreational resources in the Greater Mekong. Mt. Res. Dev. 2017, 37, 108–120. [Google Scholar] [CrossRef]
- Gong, M.; Wang, F.; Chen, Y.; Chen, Y.; Cao, J.; Su, L. Protecting the eco-environment of Tricholoma matsutake and improving its sustainable development. For. Res. 2000, 13, 562–567. [Google Scholar]
- Mortimer, P.E.; Karunarathna, S.C.; Li, Q.; Gui, H.; Yang, X.; Yang, X.; He, J.; Ye, L.; Guo, J.; Li, H. Prized edible Asian mushrooms: Ecology, conservation and sustainability. Fungal Divers. 2012, 56, 31–47. [Google Scholar] [CrossRef]
- Yu, F.; Wang, X.; Liu, P. Prospects of exploitation and utilization on edible fungi resource in Yunnan. Chin. Wild Plant Resour. 2002, 21, 21–25. [Google Scholar]
- Hodge, S.; Peterken, G. Deadwood in british forests: Priorities and a strategy. For. Int. J. For. Res. 1998, 71, 99–112. [Google Scholar] [CrossRef]
- Kangas, J.; Kuusipalo, J. Integrating biodiversity into forest management planning and decision-making. For. Ecol. Manag. 1993, 61, 1–15. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Sawyer, J.W. Standards for biodiversity: A proposal based on biodiversity standards for forest plantations. Biodivers. Conserv. 1996, 5, 447–459. [Google Scholar] [CrossRef]
- Catovsky, S.; Bradford, M.A.; Hector, A. Biodiversity and ecosystem productivity: Implications for carbon storage. Oikos 2002, 97, 443–448. [Google Scholar] [CrossRef]
- Smith, J.; Molina, R.; Huso, M.M.; Luoma, D.; McKay, D.; Castellano, M.; Lebel, T.; Valachovic, Y. Species richness, abundance, and composition of hypogeous and epigeous ectomycorrhizal fungal sporocarps in young, rotation-age, and old-growth stands of Douglas-fir (Pseudotsuga menziesii) in the cascade range of oregon, USA. Can. J. Bot. 2002, 80, 186–204. [Google Scholar] [CrossRef]
- O’Dell, T.E.; Ammirati, J.F.; Schreiner, E.G. Species richness and abundance of ectomycorrhizal basidiomycete sporocarps on a moisture gradient in the Tsuga heterophylla zone. Can. J. Bot. 2000, 77, 1699–1711. [Google Scholar] [CrossRef]
- Luoma, D.L.; Frenkel, R.E.; Trappe, J.M. Fruiting of hypogeous fungi in oregon Douglas-fir forests: Seasonal and habitat variation. Mycologia 1991, 335–353. [Google Scholar] [CrossRef]
- Bonet, J.; Fischer, C.; Colinas, C. The relationship between forest age and aspect on the production of sporocarps of ectomycorrhizal fungi in Pinus sylvestris forests of the central Pyrenees. For. Ecol. Manag. 2004, 203, 157–175. [Google Scholar] [CrossRef]
- Tedersoo, L.; Nara, K. General latitudinal gradient of biodiversity is reversed in ectomycorrhizal fungi. New Phytol. 2010, 185, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Sadam, A.; Zambrano, M.; Valencia, R.; Bahram, M. Low diversity and high host preference of ectomycorrhizal fungi in western Amazonia, a neotropical biodiversity hotspot. ISME J. 2010, 4, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Gabel, A.; Gabel, M. Comparison of diversity of macrofungi and vascular plants at seven sites in the black hills of south Dakota. Am. Midl. Nat. 2007, 157, 258–296. [Google Scholar] [CrossRef]
- Bills, G.; Holtzman, G.; Miller, O., Jr. Comparison of ectomycorrhizal–basidiomycete communities in red spruce versus northern hardwood forests of West Virginia. Can. J. Bot. 1986, 64, 760–768. [Google Scholar] [CrossRef]
- Ferrer, A.; Gilbert, G.S. Effect of tree host species on fungal community composition in a tropical rain forest in Panama. Divers. Distrib. 2003, 9, 455–468. [Google Scholar] [CrossRef]
- Villeneuve, N.; Grandtner, M.M.; Fortin, J.A. Frequency and diversity of ectomycorrhizal and saprobic macrofungi in the Laurentide mountains of Quebec. Can. J. Bot. 1989, 67, 2616–2629. [Google Scholar] [CrossRef]
- Bahram, M.; Polme, S.; Koljalg, U.; Zarre, S.; Tedersoo, L. Regional and local patterns of ectomycorrhizal fungal diversity and community structure along an altitudinal gradient in the hyrcanian forests of Northern Iran. New Phytol. 2012, 193, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Karunarathna, S.; Luo, Y.; Xu, K.; Xu, J.; Chamyuang, S.; Mortimer, P. Drivers of macrofungal composition and distribution in Yulong Snow mountain, Southwest China. Mycosphere 2016, 7, 727–740. [Google Scholar] [CrossRef]
- Brown, N.; Bhagwat, S.; Watkinson, S. Macrofungal diversity in fragmented and disturbed forests of the Western Ghats of India. J. Appl. Ecol. 2006, 43, 11–17. [Google Scholar] [CrossRef]
- Liang, Z. Flora Fungorum Sinicorum. Vol. 32. Cordyceps; Science Press: Beijing, China, 2007. [Google Scholar]
- Peng, Y.; Liu, B.; Fan, L. Flora Fungorum Sinicorum. Vol. 2. Tremellales Et Dacrymycetales; Science Press: Beijing, China, 1992. [Google Scholar]
- Wu, X.; Yang, Z.; Li, T. Fungi of Tropical China; Science Press: Beijing, China, 2010. [Google Scholar]
- Yang, Z. Flora Fungorum Sinicorum. Vol. 27. Amanitaceae; Science Press: Beijing, China, 2005. [Google Scholar]
- Zhao, J.; Zhang, X. Flora Fungorum Sinicorum. Vol. 18. Ganodermataceae; Science Press: Beijing, China, 2000. [Google Scholar]
- Zhou, T.; Chen, Y.; Zhao, L.; Fu, H.; Yang, B. Flora Fungorum Sinicorum. Vol. 36. Geastraceae and Nidulariaceae; Science Press: Beijing, China, 2006. [Google Scholar]
- Index Fungorum. Available online: http://www.indexfungorum.org/names (accessed on 1 April 2018).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Kruskal, J.B. Nonmetric multidimensional scaling: A numerical method. Psychometrika 1964, 29, 115–129. [Google Scholar] [CrossRef]
- O’Hanlon, R.; Harrington, T.J.; Berch, S.M.; Outerbridge, R.A. Comparisons of macrofungi in plantations of sitka spruce (Picea sitchensis) in its native range (British Columbia, Canada) versus non-native range (Ireland and Britain) show similar richness but different species composition. Can. J. For. Res. 2013, 43, 450–458. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Humphrey, J.; Newton, A.; Peace, A.; Holden, E. The importance of conifer plantations in Northern Britain as a habitat for native fungi. Biol. Conserv. 2000, 96, 241–252. [Google Scholar] [CrossRef]
- Shi, L.L.; Mortimer, P.E.; Slik, J.F.; Zou, X.M.; Xu, J.; Feng, W.T.; Qiao, L. Variation in forest soil fungal diversity along a latitudinal gradient. Fungal Divers. 2014, 64, 305–315. [Google Scholar] [CrossRef]
- Durall, D.M.; Gamiet, S.; Simard, S.W.; Kudrna, L.; Sakakibara, S.M. Effects of clearcut logging and tree species composition on the diversity and community composition of epigeous fruit bodies formed by ectomycorrhizal fungi. Botany 2006, 84, 966–980. [Google Scholar] [CrossRef]
- Gomez-Hernandez, M.; Williams-Linera, G. Diversity of macromycetes determined by tree species, vegetation structure, and microenvironment in tropical cloud forests in Veracruz, Mexico. Botany 2011, 89, 203–216. [Google Scholar] [CrossRef]
- Gilbert, G.S. The Dimensions of Plant Disease in Tropical Forests; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Gilbert, G.S.; Ferrer, A.; Carranza, J. Polypore fungal diversity and host density in a moist tropical forest. Biodivers. Conserv. 2002, 11, 947–957. [Google Scholar] [CrossRef]
- Osono, T. Diversity, resource utilization, and phenology of fruiting bodies of litter-decomposing macrofungi in subtropical, temperate, and subalpine forests. J. For. Res. 2015, 20, 60–68. [Google Scholar] [CrossRef]
- Lodge, D.J. Factors related to diversity of decomposer fungi in tropical forests. Biodivers. Conserv. 1997, 6, 681–688. [Google Scholar] [CrossRef]
- Geml, J.; Pastor, N.; Fernandez, L.; Pacheco, S.; Semenova, T.A.; Becerra, A.G.; Wicaksono, C.Y.; Nouhra, E.R. Large-scale fungal diversity assessment in the andean Yungas forests reveals strong community turnover among forest types along an altitudinal gradient. Mol. Ecol. 2014, 23, 2455–2472. [Google Scholar] [CrossRef] [PubMed]


| Study site | Zhongdian | Baoshan | Xishuangbanna | Doi Mae Salong |
|---|---|---|---|---|
| Location | Yunnan Province, China | Yunnan Province, China | Yunnan Province, China | Chiang Rai Province, Thailand |
| Number of plots | 5 | 5 | 4 | 6 |
| Plot size | 100 m × 100 m | 100 m × 100 m | 100 m × 100 m | 100 m × 100 m |
| Mean elevation (m) | 3265 | 2490 | 1661 | 1126 |
| Climate type | Temperate | Temperate | Tropical | Tropical |
| Mean monthly temperature (°C) | 13.3 | 20.3 | 23.5 | 25.7 |
| Mean monthly precipitation (mm) | 82.4 | 127.2 | 164.4 | 212 |
| Mean monthly relative humidity (%) | 70.3 | 72.2 | 74.5 | 80.3 |
| Management type | Five native forests | Three pine plantations, Two native forests | One tea plantation, Three native forests | One mixed rubber and coffee plantation, One pine plantation, four native forests |
| Dominant trees | Pinus densata Mast., Picea likiangensis (Franchet) E. Pritzel, Rhododendron rubiginosum Franchet, Lyonia ovalifolia var. hebecarpa (Franchet ex Forbes & Hemsley) Chun, Rhododendron tapetiforme Balf. f. & Kingdon-Ward, Betula platyphylla Sukaczev | Pine plantations: Pinus armandii Franchet, Pinus yunnanensis Franchet Native forests: Pinus yunnanensis, Castanopsis orthacantha Franchet, Quercus rehderiana Handel-Mazzetti | Tea plantation: Camellia sinensis var. assamica (J. W. Masters) Kitamura; Native forests: Syzygium brachythyrsum Merrill & L. M. Perry, Xanthophyllum flavescens Roxburgh, Macaranga henryi (Pax & K. Hoffmann) Rehder, Cryptocarya hainanensis Merrill, Myrsine seguinii H. Leveille, Anneslea fragrans Wallich, Schima wallichii (Candolle) Korthals, Castanopsis mekongensis A. Camus, Litsea cubeba (Loureiro) Persoon, Castanopsis calathiformis (Skan) Rehder & E. H. Wilson | Mixed rubber and coffee plantation: Hevea brasiliensis (Willdenow ex A. Jussieu) Müller Argoviensis, Coffea arabica Linnaeus; Pine planation: Pinus kesiya Royle ex Gordon; Native forests: Schima wallichii, Pittosporopsis kerrii Craib, Parashorea chinensis H. Wang, Baccaurea ramiflora Loureiro, Knema furfuracea (Hook.f. & Thomson) Warb., Castanopsis tribuloides (Smith) A., Castanopsis diversifolia King ex Hook. f., Castanopsis calathiformis, Lithocarpus polystachyus (Wall. ex A. DC.) Rehder, Erythrina stricta Roxb., Heliciopsis terminalis (Kurz) Sleumer |
| Management Type | Total Fungi | Ectomycorrhizal Fungi | Non-Ectomycorrhizal Fungi |
|---|---|---|---|
| Native forests | 163.4 ± 19.8 A/a | 87.7 ± 15.6 A/b | 75.7 ± 13.7 A/b |
| Plantation forests | 90.3 ± 21.5 B/a | 35.5 ± 6.9 A/a | 55 ± 16.6 A/a |
| Climate type | |||
| Temperate | 158.8 ± 25.5 A/a | 103.7 ± 19.8 A/a | 55.1 ± 7.2 A/b |
| Tropical | 124.2 ± 21.8 A/a | 40.4 ± 5.6 B/b | 83.9 ± 19.9 A/ab |
| Native mixed forest type | |||
| CTCF | 214.4 ± 32.1 A/a | 151.8 ± 19.2 A/a | 62.6 ± 13.5 B/b |
| WTCF | 139.5 ± 9.5 A/a | 89 ± 6 B/ab | 50.5 ± 3.5 B/b |
| MEBF | 200 ± 6.3 A/a | 39 ± 3.6 B/b | 161.3 ± 5.5 A/b |
| TEBF | 84 ± 24 B/a | 43.5 ± 13.5 B/a | 40.5 ± 10.7 B/a |
| Jaccard Index | Baoshan | Xishuangbanna | Doi Mae Salong | Zhongdian |
|---|---|---|---|---|
| Baoshan | --- | |||
| Xishuangbanna | 0.7661322 | --- | ||
| Doi Mae Salong | 0.7287382 | 0.7479675 | --- | |
| Zhongdian | 0.6964778 | 0.8319783 | 0.8407662 | --- |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Guo, J.; Karunarathna, S.C.; Ye, L.; Xu, J.; Hyde, K.D.; Mortimer, P.E. Native Forests Have a Higher Diversity of Macrofungi Than Comparable Plantation Forests in the Greater Mekong Subregion. Forests 2018, 9, 402. https://doi.org/10.3390/f9070402
Li H, Guo J, Karunarathna SC, Ye L, Xu J, Hyde KD, Mortimer PE. Native Forests Have a Higher Diversity of Macrofungi Than Comparable Plantation Forests in the Greater Mekong Subregion. Forests. 2018; 9(7):402. https://doi.org/10.3390/f9070402
Chicago/Turabian StyleLi, Huili, Jiayu Guo, Samantha C. Karunarathna, Lei Ye, Jianchu Xu, Kevin D. Hyde, and Peter E. Mortimer. 2018. "Native Forests Have a Higher Diversity of Macrofungi Than Comparable Plantation Forests in the Greater Mekong Subregion" Forests 9, no. 7: 402. https://doi.org/10.3390/f9070402
APA StyleLi, H., Guo, J., Karunarathna, S. C., Ye, L., Xu, J., Hyde, K. D., & Mortimer, P. E. (2018). Native Forests Have a Higher Diversity of Macrofungi Than Comparable Plantation Forests in the Greater Mekong Subregion. Forests, 9(7), 402. https://doi.org/10.3390/f9070402






