Investigating Seed Dormancy in Pinus bungeana Zucc. ex Endl.: Understanding the Contributions of Enclosing Tissues and Temperature on Germination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Material
2.2. In Vitro Embryo Culture
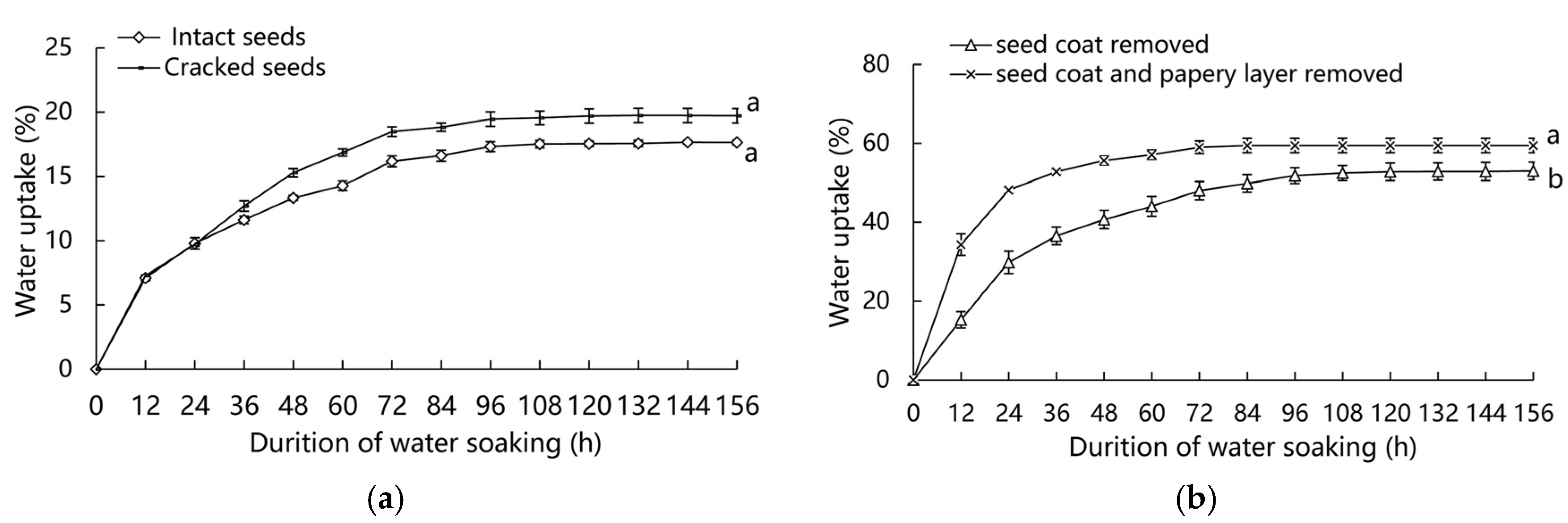
2.3. Water Uptake Test
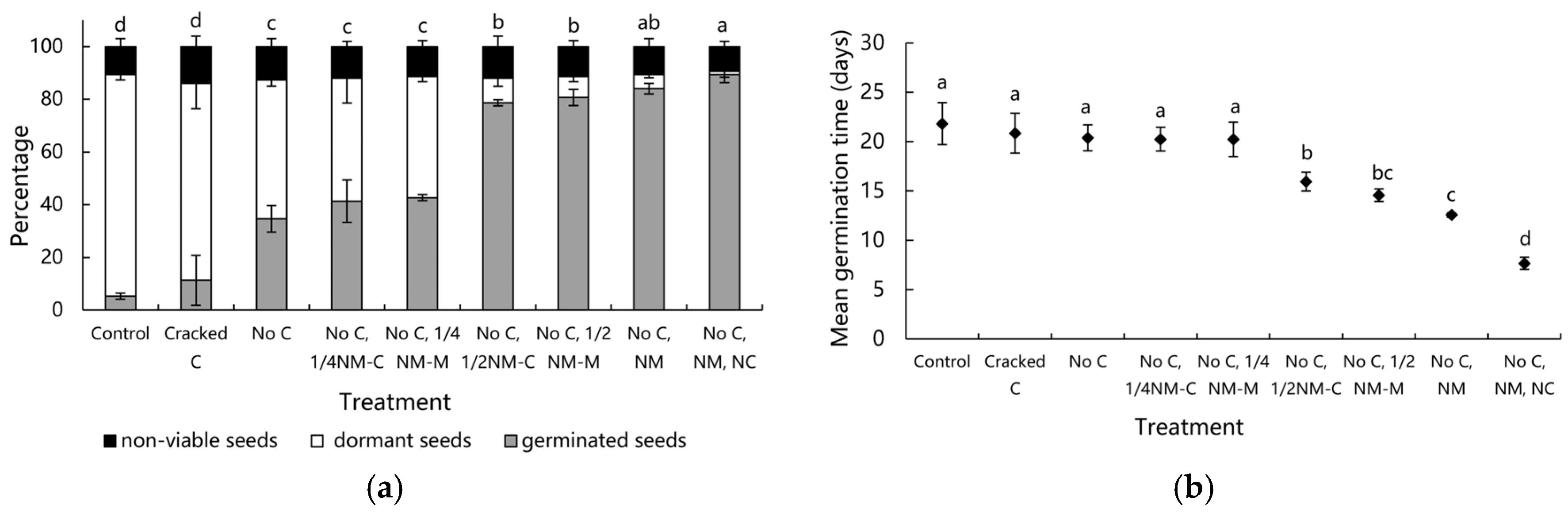
2.4. Determining Seed Tissues That Inhibit Germination
2.5. Effect of Temperature on Germination
2.6. Statistical Analyses
3. Results
3.1. In Vitro Embryo Culture
3.2. Water Uptake Test
3.3. Determining Seed Tissues That Inhibit Germination
3.4. Effect of Temperature on Germination
4. Discussion
4.1. Germination of Isolated Embryos
4.2. Water Imbibition of Seeds
4.3. Identification of Seed Structures Involved in Restricting Germination
4.4. Responses of Germination to Temperature
4.5. Roles Seed Structures Played in Germination at Different Temperatures
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yan, Z.; Xuezhong, Z.; Xiaoan, W. A study on geographical distribution law of Pinus bungeana natural forests in china. Acta Bot. Boreal. Occident. Sin. 1995, 15, 161–166. [Google Scholar]
- Wang, X.; Liu, J.; X.; Wang, J.; Liu, C. Geographical variation of morphological characteristics of Pinus bungeana seeds and cones. J. Beijing For. Univ. 1998, 20, 25–31. [Google Scholar]
- Kleczewski, N.M.; Herms, D.A.; Bonello, P. Effects of soil type, fertilization and drought on carbon allocation to root growth and partitioning between secondary metabolism and ectomycorrhizae of Betula papyrifera. Tree Physiol. 2010, 30, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Bradford, K.; Hilhorst, H.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Plenum Press: New York, NY, USA, 2014. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-X.; Li, Q.; Kong, L. Induction, development and maturation of somatic embryos in Bunge’s pine (Pinus bungeana Zucc. ex Endl.). Plant Cell Tissue Org. Cult. 2007, 91, 273–280. [Google Scholar] [CrossRef]
- Xin, Z. Seed dormancy mechanism of six pine species. J. Southwest For. Coll. 2008, 28, 5–9. [Google Scholar]
- Wang, X.; Liu, J.; Wang, J. Variation of dormancy characteristic of different Pinus bungeana seed sources. Chin. J. Appl. Ecol. 2000, 11, 9–12. [Google Scholar]
- Shulan, Y. Seed dormancy and germination of lacebark pine (Pinus bungeana). Seed 1983, 1, 24–28. [Google Scholar]
- Dong, L.; Shao, C.; Zhang, Z. Characteristics of embryo dormancy and germination of Pinus bungeana. Sci. Silv. Sin. 2003, 39, 47–54. [Google Scholar]
- Lifen, D.; Bin, X.; CuiPing, C. Investigating seed dormancy in Pinus bungeana. Shaanxi For. Sci. Technol. 1987, 1, 15–17. [Google Scholar]
- Bell, D.T.; Plummer, J.A.; Taylor, S.K. Seed germination ecology in Southwestern Western Australia. Bot. Rev. 1993, 59, 24–73. [Google Scholar] [CrossRef]
- Bellairs, S.M.; Bell, D.T. Temperature effects on the seed germination of ten kwongan species from Eneabba, Western Australia. Aust. J. Bot. 1990, 38, 451–458. [Google Scholar] [CrossRef]
- Marcos Filho, J. Fisiologia De Sementes De Plantas Cultivadas, 2nd ed.; Abrates: Londrina, Brazil, 2015. [Google Scholar]
- Ghildiyal, S.K.; Sharma, C.M. Effect of seed size and temperature treatments on germination of various seed sources of Pinus wallichiana and Pinus roxburghii from Garhwal Mmalaya. Indian For. 2005, 131, 56–65. [Google Scholar]
- Leskovar, D.; Esensee, V.; Belefant-Miller, H. Pericarp, leachate, and carbohydrate involvement in thermoinhibition of germinating spinach seeds. J. Am. Soc. Hortic. Sci. 1999, 124, 301–306. [Google Scholar]
- Chitwood, J.; Shi, A.; Evans, M.; Rom, C.E.; Gbur, E.; Motes, D.; Chen, P.; Hensley, D. Effect of temperature on seed germination in spinach (Spinacia oleracea). HortScience 2016, 51, 1475–1478. [Google Scholar] [CrossRef]
- Lopes, R.R.; Souza, C.H.L.D.; Bertoncelli, P.; Franke, L.B. Overcoming dormancy and determining optimal temperature for Slender serradella seed germination. Rev. Bras. De Zootec. 2015, 44, 413–419. [Google Scholar] [CrossRef]
- Ribeiro, J.N.S.; Costa, C.S.B. The effect of temperature regulation on seed germination of the tropical tree Myrsine parvifolia A. DC near its southern limit. South Afr. J. Bot. 2015, 98, 128–133. [Google Scholar] [CrossRef]
- Thanos, C.A.; Skordilis, A. The effects of light, temperature and osmotic stress on the germination of Pinus halepensis and P. brutia seeds. Seed Sci. Technol. 1987, 15, 163–174. [Google Scholar]
- Boydak, M.; Dirik, H.; Tilki, F.; Calikoglu, M. Effects of water stress on germination in six provenances of Pinus brutia seeds from different bioclimatic zones in Turkey. Turk. J. Agric. For. 2003, 27, 91–97. [Google Scholar]
- Dangasuk, O.G.; Panetsos, K.P. Altitudinal and longitudinal variations in Pinus brutia (Ten.) of Crete Island, Greece: Some needle, cone and seed traits under natural habitats. New For. 2004, 27, 269–284. [Google Scholar] [CrossRef]
- ISTA. International Seed Testing Association; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2005. [Google Scholar]
- Downie, B.; Bewley, J. Dormancy in white spruce (Picea glauca [Moench.] Voss.) seeds is imposed by tissues surrounding the embryo. Seed Sci. Res. 1996, 6, 9–15. [Google Scholar] [CrossRef]
- Bianco, J.; Garello, G.; Le Page-Degivry, M.T. De novo ABA synthesis and expression of seed dormancy in a gymnosperm: Pseudotsuga menziesii. Plant Growth Regul. 1997, 21, 115–119. [Google Scholar] [CrossRef]
- Ren, C.; Kermode, A.R. Analyses to determine the role of the megagametophyte and other seed tissues in dormancy maintenance of yellow cedar (Chamaecyparis nootkatensis) seeds: Morphological, cellular and physiological changes following moist chilling and during germination. J. Exp. Bot. 1999, 50, 1403–1419. [Google Scholar]
- Tillman-Sutela, E.; Kauppi, A. The morphological background to imbibition in seeds of Pinus sylvestris L. of different provenances. Trees 1995, 9, 123–133. [Google Scholar] [CrossRef]
- Tillman-Sutela, E.; Kauppi, A. The significance of structure for imbibition in seeds of the Norway spruce, Picea abies (L.) Karst. Trees 1995, 9, 269–278. [Google Scholar] [CrossRef]
- Barnett, J. Seed coat influences dormancy of loblolly pine seeds. Can. J. For. Res. 1972, 2, 7–10. [Google Scholar] [CrossRef]
- Barnett, J.P. Delayed germination of southern pine seeds related to seed coat constraint. Can. J. For. Res. 1976, 6, 504–510. [Google Scholar] [CrossRef]
- Cooke, J.; Cooke, B.; Gifford, D. Loblolly pine seed dormancy: Constraints to germination. New For. 2002, 23, 239–256. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Hoff, R.J. Dormancy in Pinus monticola seed related to stratification time, seed coat, and genetics. Can. J. For. Res. 1987, 17, 294–298. [Google Scholar] [CrossRef]
- Feurtado, J.A.; Ren, C.; Ambrose, S.J.; Cutler, A.J.; Ross, A.R.S.; Abrams, S.R.; Kermode, A.R. The coat-enhanced dormancy mechanism of western white pine (Pinus monticola Dougl. ex D. Don) seeds is mediated by abscisic acid homeostasis and mechanical restraint. Seed Sci. Technol. 2008, 36, 283–300. [Google Scholar] [CrossRef]
- Pacheco Junior, F.; Silva, J.; Rondinelli da Silva Negreiros, J.; Rose Gomes da Silva, M.; Braga Farias, S. Germination and vigor of long-pepper seeds (Piper hispidinervum) as a function of temperature and light. Revis. Cienc. Agron. 2013, 44, 325–333. [Google Scholar] [CrossRef]
- Ali-Rachedi, S.; Bouinot, D.; Wagner, M.-H.; Bonnet, M.; Sotta, B.; Grappin, P.; Jullien, M. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: Studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Plant. 2004, 219, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Endo, T.; Satoh, S. Restoration of seed germination at supraoptimal temperatures by fluridone, an inhibitor of abscisic acid biosynthesis. Plant Cell Physiol. 1998, 39, 307–312. [Google Scholar] [CrossRef]
- Barthe, P.; Garello, G.; Bianco-Trinchant, J.; le Page-Degivry, M.T. Oxygen availability and ABA metabolism in Fagus Sylvatica Seeds. Plant Growth Regulation. 2000, 30, 185–191. [Google Scholar] [CrossRef]
- Neill, S.J.; Horgan, R. Abscisic acid and related compounds. In Principles and Practice of Plant Hormone Analysis; Rivier, L., Crozier, A., Eds.; Academic Press: London, UK, 1987; pp. 111–167. [Google Scholar]
- Corbineau, F.; Côme, D. Control of seed germination and dormancy by the gaseous environment. In Seed Development and Germination; Kigel, J., Galili, G., Eds.; Dekker: New York, NY, USA, 1995; pp. 397–424. [Google Scholar]
- Poljakoff-Mayber, A.; Corbineau, F.; Côme, D. A possible mechanism of high temperature dormancy regulation in seeds of Avena sativa L. (cv. Moyencourt). Plant Growth Regul. 1990, 9, 147–156. [Google Scholar] [CrossRef]
- Walker-Simmons, M.K. Enhancement of ABA responsiveness in wheat embryos by high temperature. Plant Cell Environ. 1988, 11, 769–775. [Google Scholar] [CrossRef]
- Corbineau, F.; Côme, D.; Viémont, J.D.; Crabbé, J. Dormancy of cereal seeds as related to embryo sensitivity to ABA and water potential. In Dormancy in Plants: From Whole Plants Behaviour to Cellular Control; Viémont, J.D., Crabbé, J., Eds.; CAB International: Bringford, UK, 2000; pp. 183–191. [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Shen, Y.; Shi, F. Investigating Seed Dormancy in Pinus bungeana Zucc. ex Endl.: Understanding the Contributions of Enclosing Tissues and Temperature on Germination. Forests 2018, 9, 401. https://doi.org/10.3390/f9070401
Guo C, Shen Y, Shi F. Investigating Seed Dormancy in Pinus bungeana Zucc. ex Endl.: Understanding the Contributions of Enclosing Tissues and Temperature on Germination. Forests. 2018; 9(7):401. https://doi.org/10.3390/f9070401
Chicago/Turabian StyleGuo, Congcong, Yongbao Shen, and Fenghou Shi. 2018. "Investigating Seed Dormancy in Pinus bungeana Zucc. ex Endl.: Understanding the Contributions of Enclosing Tissues and Temperature on Germination" Forests 9, no. 7: 401. https://doi.org/10.3390/f9070401




