Coarse Woody Debris Variability Due to Human Accessibility to Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Accessibility CWD Classes and CWD Amount
3.2. Components of CWD
3.3. Decay Class of CWD
3.4. Species of CWD
3.5. Diameter of CWD
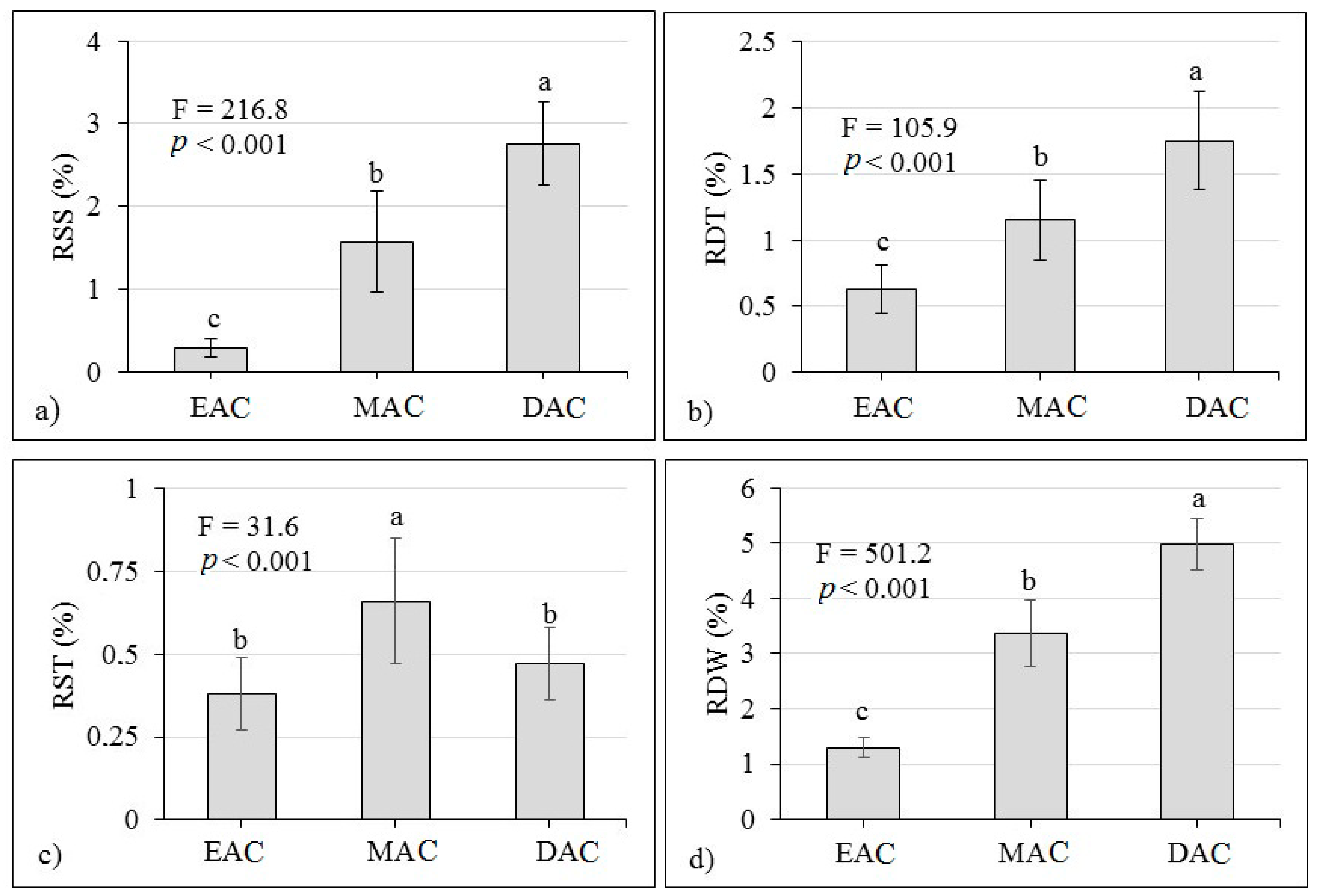
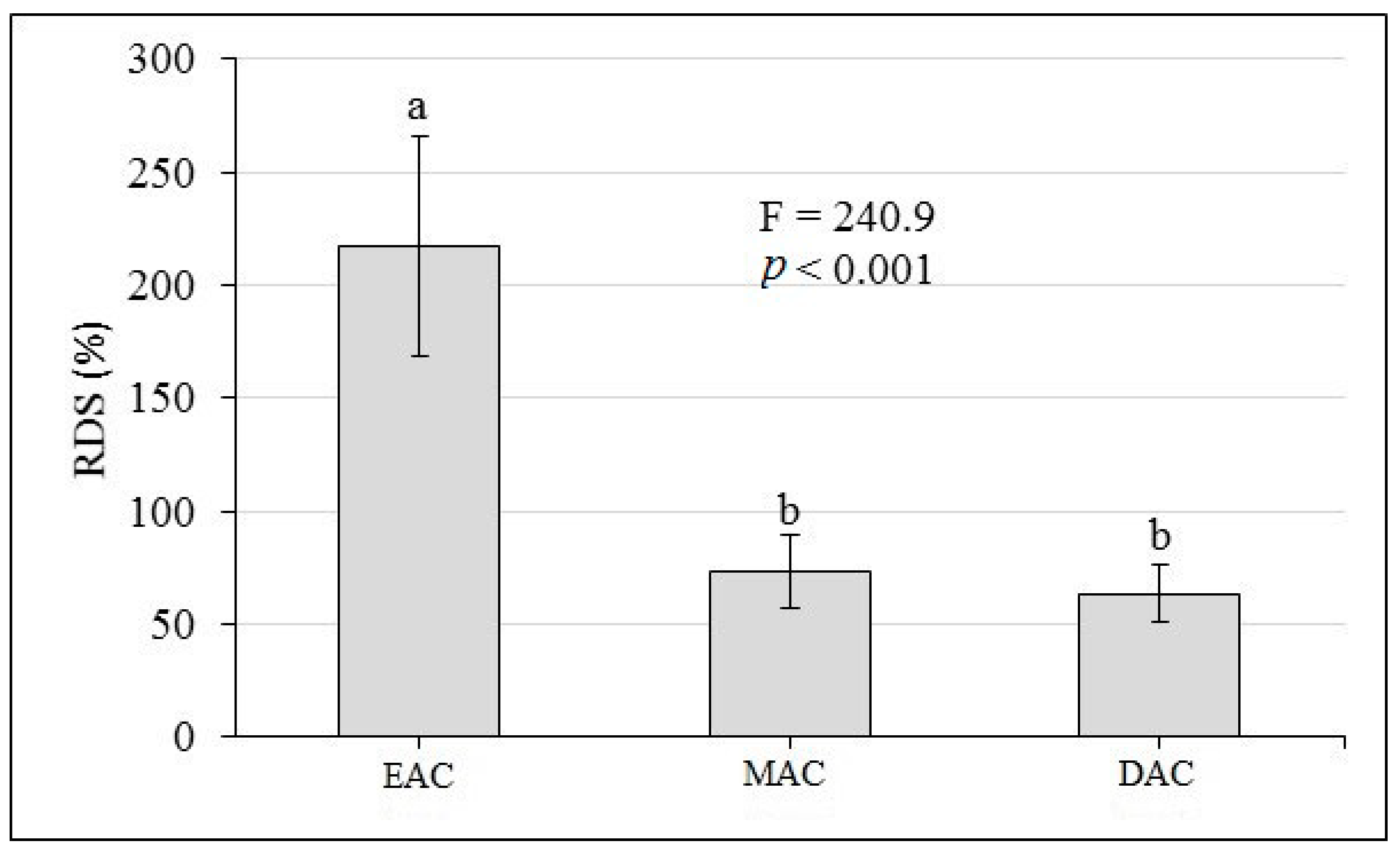
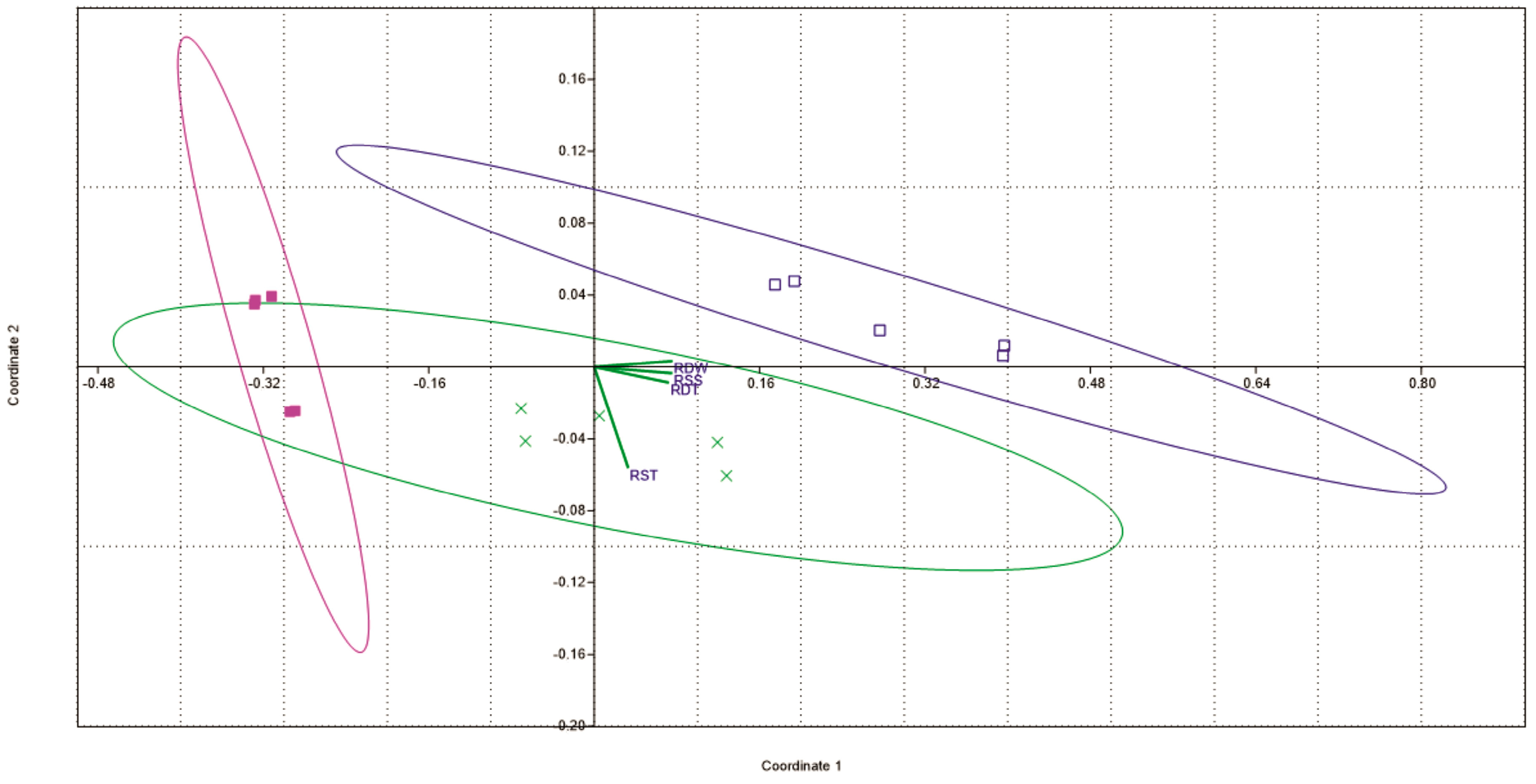
3.6. RSS, RDT, RST, RDW and RDS
4. Discussion
4.1. Human Accessibility Classes and CWD Amount
4.2. Components of CWD
4.3. Decay Class of CWD
4.4. Species of CWD
4.5. Diameter of CWD
4.6. RSS, RDT, RST, RDW and RDS
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghadiri Khanaposhtani, M.; Kaboli, M.; Karami, M.; Etemad, V.; Baniasadi, S. Effects of logged and unlogged forest patches on avifaunal diversity. Environ. Manag. 2013, 51, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Sefidi, K.; Marvie Mohadjer, M.R.; Mosand, R.; Copenheaver, C. Coarse and fine woody debris in mature Oriental Beech. Nat. Area J. 2013, 3, 248–255. [Google Scholar] [CrossRef]
- Moghimian, N.; Jalali, S.G.; Kooch, Y.; Rey, A. Downed logs improve soil properties in old-growth temperate forests of Northern Iran. Pedosphere 2017. [Google Scholar] [CrossRef]
- Sefidi, K.; Etemad, V. Dead wood characteristics influencing macrofungi species abundance and diversity in Caspian natural beech (Fagus orientalis Lipsky) forests. For. Syst. 2015, 24, eSC03. [Google Scholar] [CrossRef]
- Tavankar, F.; Nikoy, M.; Picchio, R.; Venanzi, R.; Lo Monaco, A. Long-term effects of single-tree selection cutting management on coarse woody debris in natural mixed beech stands in the Caspian forest (Iran). iForest 2017, 10, 652–658. [Google Scholar] [CrossRef] [Green Version]
- Nourzad Moghaddam, M.; Shamekhi, T.; Etemad, V.; Hemmat, M.A. Firewood consumption by rural households in upland of the Caspian forests in the northern Iran and related factors (Case study: Upland villages in southern parts of Kheyrud Forest). Iran J. For. 2014, 6, 113–125. [Google Scholar]
- Prasad, A.E. Tree community change in a tropical dry forest: the role of roads and exotic plant invasion. Environ. Conserv. 2009, 36, 201–207. [Google Scholar] [CrossRef]
- Coffin, A.W. From roadkill to road ecology: A review of the ecological effects of roads. J. Trans. Geogr. 2002, 15, 396–406. [Google Scholar] [CrossRef]
- Ozturk, T.; Inan, M.; Mustafa Akgul, M. Environmental damages of forest road construction by bulldozer on steep terrain. Afr. J. Biotechnol. 2009, 8, 4547–4552. [Google Scholar]
- Tavankar, F.; Nkkooy, M.; Venanzi, R.; Lo Monaco, A.; Picchio, R. Study of forest road effect on tree community and stand structure in three Italian and Iranian temperate forests. Croat. J. For. Eng. 2018, 39, 57–70. [Google Scholar]
- Caliskan, E. Environmental impacts of forest road construction on mountainous terrain. Iran. J. Environ. Health Sci. Eng. 2013, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gumus, S.; Acar, H.H.; Toksoy, D. Functional forest road network planning by consideration of environmental impact assessment for wood harvesting. Environ. Monit. Assess. 2008, 142, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tunay, M. The assessment of environmentally sensitive forest road construction in Calabrian pine forest areas of Turkey. J. Environ. Biol. 2006, 27, 529–535. [Google Scholar] [PubMed]
- Tavankar, F.; Bonyad, A.E.; Nikoy, M.; Picchio, R.; Venanzi, R.; Calienno, L. Damages to soil and tree species by cable-skidding in Caspian forests of Iran. For. Syst. 2017, 26, 1–9. [Google Scholar] [CrossRef]
- Bate, L.J.; Wisdom, M.J.; Wales, B.C. Snag densities in relation to human access and associated management factors in forests of northeastern Oregon, USA. Landsc. Urban Plan. 2007, 80, 278–291. [Google Scholar] [CrossRef]
- Wisdom, M.J.; Bate, L.J. Snag density varies with intensity of timber harvest and human access. For. Ecol. Manag. 2008, 255, 2085–2093. [Google Scholar] [CrossRef]
- Bertolotto, P.; Calienno, L.; Conforti, M.; D’Andrea, E.; Lo Monaco, A.; Magnani, E.; Marinšek, A.; Micali, M.; Picchio, R.; Sicuriello, F.; et al. Assessing indicators of forest ecosystem health. Ann. Silv. Resear. 2016, 40, 64–69. [Google Scholar]
- Picchio, R.; Spina, R.; Calienno, L.; Venanzi, R.; Lo Monaco, A. Forest operations for implementing silvicultural treatments for multiple purposes. Ital. J. Agron. 2016, 11, 156–161. [Google Scholar]
- Tavankar, F.; Picchio, R.; Nikooy, M.; Lo Monaco, A.; Venanzi, R.; Iranparast Bodaghi, A. Healing rate of logging wounds on broadleaf trees in Hyrcanian forest with some technological implications. Drewno 2017, 60, 16. [Google Scholar] [CrossRef]
- Tavankar, F.; Picchio, R.; Lo Monaco, A.; Bonyad, A.E. Forest management and snag characteristics in Northern Iran lowland forests. J. For. Sci. 2014, 60, 431–441. [Google Scholar] [CrossRef]
- Britzke, E.R.; Harvey, M.J.; Loeb, S.C. Indiana bat, Myotis sodalis, maternity roosts in the southern United States. Southwest Nat. 2003, 2, 235–242. [Google Scholar] [CrossRef]
- Bursell, J. Winter abundance of hole-nesting birds in natural and managed woods of Zealand (Denmark). Acta Ornithol. 2002, 37, 67–74. [Google Scholar] [CrossRef]
- Humphrey, J.W.; Davey, S.; Peace, A.J.; Ferris, R.; Harding, K. Lichens and bryophite communities of planted and semi-natural forests in Britain: the influence of site type, stand structure and deadwood. Biol. Conserv. 2002, 107, 165–180. [Google Scholar] [CrossRef]
- Hanberry, B.B.; Hanberry, P.; Demarais, S.; Jones, J.C. Importance of residual trees to birds in regenerating pine plantations. iForest 2012, 5, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Lonsdale, D.; Pautasso, M.; Holdenriede, O. Wood-decaying fungi in the forest: Conservation needs and management options. Eur. J. For. Res. 2008, 127, 1–22. [Google Scholar] [CrossRef]
- Allard, J.; Park, A. Woody debris volumes and carbon accumulation differ across a chronosequence of boreal red pine and jack pine stands. Can. J. For. Res. 2013, 43, 768–775. [Google Scholar] [CrossRef]
- Matsuzaki, E.; Sanborn, P.; Fredeen, A.L.; Shaw, C.H.; Hawkins, C. Carbon stocks in managed and unmanaged old-growth western red cedar and western hemlock stands of Canada’s inland temperate rainforests. For. Ecol. Manag. 2013, 297, 108–119. [Google Scholar] [CrossRef]
- Kim, R.; Son, Y.; Lim, J.H.; Lee, I.K.; Seo, K.W.; Koo, J.W.; Noh, N.J.; Ryu, S.; Hong, S.K.; Ihm, B.S. Coarse woody debris mass and nutrients in forest ecosystems of Korea. Ecol. Res. 2006, 21, 819–827. [Google Scholar] [CrossRef]
- Laiho, R.; Prescott, C.E. The contribution of coarse woody debris to carbon, nitrogen and phosphorous cycles in three Rocky Mountain coniferous forests. Can. J. For. Res. 1999, 29, 1592–1603. [Google Scholar] [CrossRef]
- Strukelj, M.; Brais, S.; Quideaua, S.A.; Angers, V.A.; Kebli, H.; Drapeau, P.; Oh, S. Chemical transformations in downed logs and snags of mixed boreal species during decomposition. Can. J. For. Res. 2013, 43, 785–798. [Google Scholar] [CrossRef]
- Böhl, J.; Brändli, U.B. Deadwood volume assessment in the third Swiss national forest inventory: Methods and first results. Eur. J. For. Res. 2007, 126, 449–457. [Google Scholar]
- Kunttu, P.; Junninen, K.; Kouki, J. Dead wood as an indicator of forest naturalness: A comparison of methods. For. Ecol. Manag. 2015, 353, 30–40. [Google Scholar] [CrossRef]
- Di Cosmo, L.; Gasparini, P.; Paletto, A.; Nocetti, M. Deadwood basic density values for national-level carbon stock estimates in Italy. For. Ecol. Manag. 2013, 295, 51–58. [Google Scholar] [CrossRef]
- Rondeux, J.; Sanchez, C. Review of indicators and field methods for monitoring biodiversity within national forest inventories. Core variable: Deadwood. Environ. Monit. Assess. 2010, 164, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Teissier du Cros, R.; Lopez, S. Preliminary study on the assessment of deadwood volume by the French national forest inventory. Ann. For. Sci. 2009, 66, 1–10. [Google Scholar] [CrossRef]
- Woodall, C.W.; Rondeux, J.; Verkerk, P.J.; Ståhl, G. Estimating dead wood during national forest inventories: A review of inventory methodologies and suggestions for harmonization. Environ. Manag. 2009, 44, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Forest Europe—State of Europe’s Forests. Ministerial Conference on the Protection of Forests in Europe. Forest Europe Liaison Unit Madrid, 2015. Available online: https://www.foresteurope.org/docs/fullsoef2015.pdf (accessed on 4 May 2018).
- Larrieu, L.; Cabannettes, A. Species, live status and diameter are important tree features for diversity and abundance of tree microhabitats in subnatural montane beech-fir forests. Can. J. For. Res. 2012, 42, 1433–1445. [Google Scholar] [CrossRef]
- Perry, R.W.; Thill, R.E. Comparison of snag densities among regeneration treatments in mixed pine-hardwood forests. Can. J. For. Res. 2013, 43, 619–626. [Google Scholar] [CrossRef]
- De Long, S.C.; Sutherland, G.D.; Daniels, L.D.; Heemskerk, B.H.; Storaunet, K.O. Temporal dynamics of snags and development of snag habitats in wet spruce-fir stands in east-central British Columbia. For. Ecol. Manag. 2008, 255, 3613–3620. [Google Scholar] [CrossRef]
- Hurlbert, S.H. Pseudorepliction and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Forestry of Guilan. Tariff of Hyrcanian Beech Forests; Hyrcanian Forest Management Office: Guilan, Iran, 2010. [Google Scholar]
- Corace, R.G.; Seefelt, N.E.; Goebel, P.C.; Shaw, H.L. Snag longevity and decay class development in a recent Jack Pine clearcut in Michigan. North J. App. For. 2010, 27, 125–131. [Google Scholar]
- Palett, A.; De Meo, I.; Cantiani, P.; Ferretti, F. Effects of forest management on the amount of deadwood in Mediterranean oak ecosystems. Ann. For. Sci. 2014, 71, 791–800. [Google Scholar] [CrossRef] [Green Version]
- Garbarino, M.; Marzano, R.; Shaw, J.D.; Long, J.N. Environmental drivers of deadwood dynamics in woodlands and forests. Ecosphere 2015, 6, 30. [Google Scholar] [CrossRef]
- Siipola, A.L.; Siitonen, J.; Kallio, R. Amount and quality of coarse woody debris in natural and managed coniferous forests near the timberline in Finnish Lapland Scand. J. For. Res. 1998, 13, 204–214. [Google Scholar]
- Siitonen, J.; Martikainen, P.; Punttila, P.; Rauh, J. Coarse woody debris and stand characteristics in mature managed and old-growth boreal mesic forests in southern Finland. For. Ecol. Manag. 2000, 128, 211–225. [Google Scholar] [CrossRef]
- Christensen, M.; Hahn, K.; Mountford, E.P.; Odor, P.; Standovar, T.; Rozenbergar, D.; Diaci, J.; Wijdeven, S.; Meyer, P.; Winter, S.; et al. Deadwood in European beech (Fagus sylvatica) forest Reserves. For. Ecol. Manag. 2005, 210, 267–282. [Google Scholar] [CrossRef]
- Norden, B.; Gotmark, F.; Tonnberg, M.; Ryberg, M. Dead wood in semi-natural temperate broadleaved woodland: Contribution of coarse and fine dead wood, attached dead wood and stumps. For. Ecol. Manag. 2004, 194, 235–248. [Google Scholar] [CrossRef]
- Commarmot, B.; Bachofen, H.; Bundziak, Y.; Burgi, A.; Ramp, B.; Shparyk, Y.; Sukhariuk, D.; Viter, R.; Zingg, A. Structure of virgin and managed beech forests in Uholka (Ukraine) and Sihlwald (Switzerland): A comparative study. For. Snow. Landsc. Res. 2005, 79, 45–56. [Google Scholar]
- Rouvinen, S.; Rautiainen, A.; Kouki, J. A relation between historical forest use and current dead woody material in a boreal protected old-growth forest in Finland. Silva Fenn 2005, 39, 21–36. [Google Scholar] [CrossRef]
- Amanzadeh, B.; Sagheb-Talebi, K.; Foumani, B.S.; Fadaie, F.; Camarerond, J.J.; Linares, J.C. Spatial Distribution and Volume of Dead Wood in Unmanaged Caspian Beech (Fagus orientalis) Forests from Northern Iran. Forests 2013, 4, 751–765. [Google Scholar] [CrossRef]
- Behjou, F.K.; Mollabashi, O.G.; Amirahmadi, N. Effects of management on the amount and characteristics of woody debris in mixed stands of Caspian forests. BioResources 2014, 9, 4108–4116. [Google Scholar]
- Sefidi, K.; Marvie Mohadjer, M.R. Characteristics of coarse woody debris in successional stages of natural beech (Fagus orientalis) forests of Northern Iran. J. For. Sci. 2010, 56, 7–17. [Google Scholar] [CrossRef]
- Kahl, T.; Bauhus, J. An index of forest management intensity based on assessment of harvested tree volume, tree species composition and dead wood origin. J. Nat. Conserv. 2014, 7, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Herrero, C.; Monleon, V.J.; Gómez, N.; Bravo, F. Distribution of dead wood volume and mass in mediterranean Fagus sylvatica L. forests in Northern Iberian Peninsula. Implications for field sampling inventory. For. Syst. 2016, 25, e069. [Google Scholar] [CrossRef]
- Marquez-Reynoso, M.I.; Ramírez-Marcial, N.; Cortina-Villar, S.; Ochoa-Gaona, S. Purpose, preferences and fuel value index of trees used for firewood in El Ocote Biosphere Reserve, Chiapas, Mexico. Biomass Bioenergy 2017, 100, 1–9. [Google Scholar] [CrossRef]
- Barreca, L.; Cutini, A.; Mercurio, R. Dead wood characterisation in Quercus frainetto stands in Calabria (Southern Italy). Forest@ 2008, 5, 187–194. [Google Scholar] [CrossRef]
- Caviedes, J.; Ibarra, J.T. Influence of anthropogenic disturbances on stand structural complexity in andean temperate forests: Implications for managing key habitat for biodiversity. PLoS ONE 2017, 12, e0169450. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Hjältén, J.; Dynesius, M. Wood-Inhabiting Beetles in Low Stumps, High Stumps and Logs on Boreal Clear-Cuts: Implications for Dead Wood Management. PLoS ONE 2015, 10, e0118896. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Johansson, V.; Dahlberg, A.; Frisch, A.; Thor, G.; Ranius, T. The relative importance of stand and dead wood types for wood-dependent lichens in managed boreal forests. Fungal Ecol. 2016, 20, 166–174. [Google Scholar] [CrossRef]
- Jonsson, B.G.; Ekström, M.; Esseen, P.; Grafström, A.; Ståhl, G.; Westerlund, B. Dead wood availability in managed Swedish forests —Policy outcomes and implications for biodiversity. For. Ecol. Manag. 2016, 376, 174–182. [Google Scholar] [CrossRef]
- Pedlar, J.H.; Pearce, J.L.; Venier, L.A.; McKenney, D.W. Coarse woody debris in relation to disturbance and forest type in boreal Canada. For. Ecol. Manag. 2002, 158, 189–194. [Google Scholar] [CrossRef]
- Castagneri, D.; Garbarino, M.; Berretti, R.; Motta, R. Site and stand effects on coarse woody debris in montane mixed forests of Eastern Italian Alps. For. Ecol. Manag. 2010, 260, 1592–1598. [Google Scholar] [CrossRef] [Green Version]
- Garbarino, M.; Weisberg, P.J.; Motta, R. Interacting effects of physical environment and anthropogenic disturbances on the structure of European larch (Larix decidua Mill.) forests. For. Ecol. Manag. 2009, 257, 1794–1802. [Google Scholar] [CrossRef]
- D’Amato, A.W.; Orwig, D.A.; Foster, D.R. The influence of successional processes and disturbance on the structure of Tsuga canadensis forests. Ecol. Appl. 2008, 18, 1182–1199. [Google Scholar] [CrossRef] [PubMed]
- De Groot, M.; Zapponi, L.; Badano, D.; Corezzola, S.; Mason, F. Influenza delle pratiche di gestione forestale sulla conservazione dei vertebrati. Ital. J. Agron. 2016, 11, 32–37. [Google Scholar]
- Müller, J.; Wende, B.; Strobl, C.; Eugster, M.; Gallenberger, I.; Floren, A.; Steffan-Dewenter, I.; Linsenmair, K.E.; Weisser, W.W.; Gossner, M.M. Forest management and regional tree composition drive the host preference of saproxylic beetle communities. J. Appl. Ecol. 2015, 52, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Van Der Wal, A.; Ottosson, E.; De Boer, W. Neglected role of fungal community composition in explaining variation in wood decay rates. Ecology 2015, 96, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Boddy, L. Fungal Community Ecology and Wood Decomposition Processes in Angiosperms: From Standing Tree to Complete Decay of Coarse Woody Debris. Ecol. Bull. 2001, 49, 43–56. [Google Scholar]
- Dunn, C.J.; Bailey, J.D. Temporal dynamics and decay of coarse wood in early seral habitats of dry-mixed conifer forests in Oregon’s Eastern Cascades. For. Ecol. Manag. 2012, 276, 71–81. [Google Scholar] [CrossRef]
- Yatskov, M.; Harmon, M.E.; Krankina, O.N. A chronosequence of wood decomposition in the boreal forests of Russia. Can. J. For. Res. 2003, 33, 1211–1226. [Google Scholar] [CrossRef]
- Sanchez, E.; Gallery, R.; Dalling, J. Importance of nurse logs as a substrate for the regeneration of pioneer tree species on Barro Colorado Island, Panama. J. Trop. Ecol. 2009, 25, 429–437. [Google Scholar] [CrossRef]
- Szewczyk, J.; Szwagrzyk, J. Tree regeneration on rotten wood and on soil in old-growth stand. Vegetatio 1996, 122, 37–46. [Google Scholar] [CrossRef]
- Brunner, A.; Kimmins, J.P. Nitrogen fixation in coarse woody debris of Thuja plicata and Tsuga heterophylla forests on northern Vancouver Island. Can. J. For. Res. 2003, 33, 1670–1682. [Google Scholar] [CrossRef]
- Creed, I.F.; Morrison, D.L.; Nicholas, N.S. Is coarse woody debris a net sink or source of nitrogen in the red spruce–Fraser fir forest of the southern Appalachians, U.S.A.? Can. J. For. Res. 2004, 34, 716–727. [Google Scholar] [CrossRef]
- Nascimbene, J.; Thor, G.; Nimis, P.L. Effects of forest management on epiphytic lichens in temperate deciduous forests of Europe—a review. For. Ecol. Manag. 2013, 298, 27–38. [Google Scholar] [CrossRef]
- Spies, T.A.; Franklin, J.F.; Thomas, T.B. Coarse woody debris in Douglas-fir forests of western Oregon and Washington. Ecology 1988, 69, 689–702. [Google Scholar] [CrossRef]
- Mason, F.; Zapponi, L. The forest biodiversity artery: towards forest management for saproxylic conservation. iForest 2015, 9, 205–216. [Google Scholar] [CrossRef]
- Ahtikoski, A.; Hänninen, R.; Siipilehto, J.; Hynynen, J.; Siitonen, J.; Koskela, T.; Kojola, S. Cost-Efficiency of Alternative Forest Conservation Targets, a Case Study from Finland. J. Biodivers. Manag. For. 2017, 6, 4. [Google Scholar] [CrossRef]
- Ando, A.; Chen, X. Optimal contract lengths for voluntary ecosystem service provision with varied dynamic benefit functions. Conserv. Lett. 2011, 4, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Banaś, J.; Bujoczek, L.; Zięba, S.; Drozd, M. The effects of different types of management, functions and characteristics of stands in Polish forests on the amount of coarse woody debris. Eur. J. For. Res. 2014, 133, 1095–1107. [Google Scholar] [CrossRef]
- Ranius, T.; Ekvall, H.; Jonsson, M.; Bostedt, G. Cost-efficiency of measures to increase the amount of coarse woody debris in managed Norway spruce forests. For. Ecol. Manag. 2005, 206, 119–133. [Google Scholar] [CrossRef]
- Sassen, M.; Sheil, D.; Giller, K.E. Fuelwood collection and its impacts on a protected tropical mountain forest in Uganda. For. Ecol. Manag. 2015, 354, 56–67. [Google Scholar] [CrossRef]
- Regnery, B.; Paillet, Y.; Couvet, D.; Kerbiriou, C. Which factors influence the occurrence and density of tree microhabitats in Mediterranean oak forests? For. Ecol. Manag. 2013, 295, 118–125. [Google Scholar] [CrossRef]
- Ylläsjärvi, I.; Berglund, H.; Kuuluvainen, T. Relationships between wood-inhabiting fungal species richness and habitat variables in old-growth forest stands in the Pallas-Yllästunturi National Park, northern boreal Finland. Silva Fenn 2011, 45, 995–1013. [Google Scholar] [CrossRef]
- Ghadiri Khanaposhtani, M.; Kaboli, M.; Karami, M.; Etemad, V. Effect of habitat complexity on richness, abundance and distributional pattern of forest birds. Environ. Manag. 2012, 50, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Madžule, L.; Brūmelis, G.; Tjarve, D. Structures determining bryophyte species richness in a managed forest landscape in boreo-nemoral Europe. Biodivers. Conserv. 2012, 21, 437–450. [Google Scholar] [CrossRef]
- Thomas, J.W.; Anderson, R.G.; Maser, C.; Bull, E.L. Snags, Wildlife Habitats in Managed Forests: The Blue Mountains of Oregon and Washington; Agricultural Handbook No. 553; USDA Forest Service: Portland, OR, USA, 1979.
- Tillon, L.; Bouget, C.; Paillet, Y.; Aulagnier, S. How does deadwood structure temperate forest bat assemblages? Eur. J. For. Res. 2016, 135, 433–449. [Google Scholar] [CrossRef]
- Russell, M.B.; Kenefic, L.S.; Weiskitte, A.R.; Puhlick, J.J.; Brissette, J.C. Assessing and modeling standing deadwood attributes under alternative silvicultural regimes in the Acadian forest region of Maine, USA. Can. J. For. Res. 2012, 42, 1873–1883. [Google Scholar] [CrossRef]
- Angers, V.A.; Drapeau, P.; Bergeron, Y. Mineralization rates and factors influencing snag decay in four North American boreal tree species. Can. J. For. Res. 2012, 42, 157–166. [Google Scholar] [CrossRef]
- Annesi, T.; Calienno, L.; Picchio, R.; De Simone, D.; Lo Monaco, A. Degradation of some technological features in wood of ornamental species cause by Inonotus rickii (Pat.) Reid. Drewno 2015, 58, 5–18. [Google Scholar]
- Lõhmus, A.; Kraut, A.; Rosenvald, R. Dead wood in clearcuts of semi-natural forests in Estonia: Sitetype variation, degradation and the influences of tree retention and slash harvest. Eur. J. For. Res. 2013, 132, 335–349. [Google Scholar] [CrossRef]
- Kohm, K.; Franklin, J.F. Forestry in 21st Century; Island Press: Covelo, CA, USA, 1997. [Google Scholar]
- Rabe, M.J.; Morrell, T.E.; Green, H.; DeVos, J.C.; Miller, C.R. Characteristics of ponderosa pine snag roosts used by reproductive bats in northern Arizona. J. Wildl. Manag. 1998, 62, 612–621. [Google Scholar] [CrossRef]

| Slope (%) | Slope Direction | Road Type | No of Sample Plots in Total | No of Sample Plots Selected for Study | HAC |
|---|---|---|---|---|---|
| <20 | Flat | Adjacent Open | 30 | 30 | EAC |
| <20 | Flat | Adjacent Closed | 1 | 0 | - |
| <20 | Flat | Not Adjacent | 2 | 0 | - |
| 20–40 | Uphill | Adjacent Open | 2 | 0 | - |
| 20–40 | Uphill | Adjacent Closed | 3 | 0 | - |
| 20–40 | Uphill | Not Adjacent | 2 | 0 | - |
| 20–40 | Downhill | Adjacent Open | 3 | 0 | - |
| 20–40 | Downhill | Adjacent Closed | 45 | 30 | MAC |
| 20–40 | Downhill | Not Adjacent | 3 | 0 | - |
| >40 | Uphill | Adjacent Open | 3 | 0 | - |
| >40 | Uphill | Adjacent Closed | 4 | 0 | - |
| >40 | Uphill | Not Adjacent | 43 | 30 | DAC |
| >40 | Downhill | Adjacent Open | 3 | 0 | - |
| >40 | Downhill | Adjacent Closed | 3 | 0 | - |
| >40 | Downhill | Not Adjacent | 3 | 0 | - |
| HAC | Slope (%) | Slope Direction | Road Type |
|---|---|---|---|
| EAC | <20 | Flat | Adjacent, open |
| MAC | 20–40 | Downhill | Adjacent, closed |
| DAC | >40 | Uphill | Not adjacent |
| Types | Decay class | |||||
|---|---|---|---|---|---|---|
| Character | 1 | 2 | 3 | 4 | 5 | |
| Snags | Leaves | Present | Absent | Absent | Absent | As logs |
| Bark | Tight | Loose | Partly present | Absent | ||
| Crown, branches and twigs | All present | Only branches present | Only large branch stubs present | Absent | ||
| Bole | Recently dead | Standing, firm | Standing, decayed | Heavily decayed, Soft and block structure | ||
| Indirect measure | Cambium still fresh, died <1 year | Cambium decayed, knife blade penetrates a few millimetres | Knife blade penetrates <2 cm | Knife blade penetrates 2–5 cm | Knife blade penetrates all the way | |
| Logs | Structural integrity | Sound | Sapwood slightly rotting, heartwood sound | Sapwood missing, heartwood mostly sound | Heartwood decayed | Soft |
| Leaves | Present | Absent | Absent | Absent | Absent | |
| Branches | All twigs present | Larger twigs present | Larger branches present | Branch stubs present | Absent | |
| Bark | Present | Present | Often present | Often present | Absent | |
| Bole shape | Round | Round | Round | Round to oval | Oval to flat | |
| Wood consistency | Solid | Solid | Semisolid | Partly soft | Fragmented, powdery | |
| Colour of wood | Original colour | Original colour | Original colour to faded | Original colour to faded | Heavily faded | |
| Portion of log on ground | Elevated on support point | Elevated on support point | Near or on ground | Whole log on ground | Whole log on ground | |
| Indirect measure | Cambium still fresh, died | Cambium decayed, knife blade penetrates a few mm | Knife blade penetrates <2 cm | Knife blade penetrates 2–5 cm | Knife blade penetrates all the way | |
| Stumps | Indirect measure | Cambium still fresh, died <1 year | Cambium decayed, knife blade penetrates a few mm | Knife blade penetrates <2 cm | Knife blade penetrates 2–5 cm | Knife blade penetrates all the way |
| HAC | Area of HAC (ha) | Tree Density 1 (stem ha−1) | Stand Volume 1 (m3 ha−1) |
|---|---|---|---|
| EAC | 25.0 | 166.1 ± 23.2 | 309.2 ± 31.7 a |
| MAC | 74.2 | 169.0 ± 22.8 | 262.8 ± 25.3 b |
| DAC | 53.1 | 171.5 ± 19.8 | 298.6 ± 28.6 a |
| F-value | - | 0.386 N.S. | 21.463 ** |
| HAC | Snag | Downed Log | Stump | Total (CWD) |
|---|---|---|---|---|
| EAC | 0.90 ± 0.28 c | 1.96 ± 0.29 c | 1.17 ± 1.07 | 4.03 ± 1.17 c |
| (22.3%) | (48.6%) | (29.1%) | ||
| MAC | 4.11 ± 0.72 b | 3.01 ± 0.54 b | 1.72 ± 1.29 | 8.84 ± 1.54 b |
| (46.5%) | (34.0%) | (19.5%) | ||
| DAC | 8.24 ± 1.13 a | 5.23 ± 0.59 a | 1.40 ± 1.01 | 14.87 ± 1.31 a |
| (55.4%) | (35.2%) | (9.4%) | ||
| ANOVA | ||||
| F-value | 645.4 | 342.6 | 1.78 | 485.5 |
| p-value | <0.001 | <0.001 | 0.174 | <0.001 |
| HAC | DC1 | DC2 | DC3 | DC4 | DC5 |
|---|---|---|---|---|---|
| EAC | 0.35 ± 0.10 c | 0.36 ± 0.13 b | 0.72 ± 0.21 b | 1.08 ± 0.28 b | 1.52 ± 0.31 b |
| (8.7%) | (9.0%) | (17.9%) | (26.8%) | (37.7%) | |
| MAC | 0.78 ± 0.26 b | 1.02 ± 0.34 a | 1.11 ± 0.19 b | 2.59 ± 0.49 a | 3.34 ± 0.34 a |
| (8.8%) | (11.5%) | (12.6%) | (29.3%) | (37.8%) | |
| DAC | 2.52 ± 0.58 a | 1.15 ± 0.23 a | 4.23 ± 0.46 a | 2.87 ± 0.41 a | 4.10 ± 0.34 a |
| (16.9%) | (7.7%) | (28.4%) | (19.3% | (27.6%) | |
| ANOVA | |||||
| F-value | 266.7 | 87.9 | 1133.5 | 170.7 | 383.6 |
| p-value | <0.001 | <0.001 | 0<.001 | <0.001 | <0.001 |
| Tree Species | EAC | MAC | DAC | F-Value | p-Value |
|---|---|---|---|---|---|
| Beech | 3.10 ± 0.39 c | 6.13 ± 1.24 b | 9.40 ± 1.62 a | 218.8 | <0.001 |
| (76.0%) | (69.3%) | (63.2%) | |||
| Hornbeam | 0.62 ± 0.18 c | 1.22 ± 0.30 b | 3.13 ± 0.91 a | 173.2 | <0.001 |
| (15.2%) | (14.6%) | (21.0%) | |||
| Alder | 0.22 ± 0.05 c | 0.64 ± 0.20 b | 1.20 ± 0.55 a | 64.8 | <0.001 |
| (5.4%) | (7.7%) | (8.1%) | |||
| Maple | 0.09 ± 0.04 c | 0.50 ± 0.18 b | 0.72 ± 0.26 a | 95.4 | <0.001 |
| (2.2) | (6.0) | (4.8) | |||
| Other Sp. | 0.05 ± 0.02 b | 0.35 ± 0.15 a | 0.42 ± 0.11 a | 88.0 | <0.001 |
| (1.2%) | (4.2%) | (2.8%) |
| CWD Component | HAC | Diameter of CWD (cm) | ||||
|---|---|---|---|---|---|---|
| 7.5–25 | 26–50 | 51–75 | 76–100 | LINE SUM | ||
| Snag | EAC | 0.05 ± 0.03 b | 0.09 ± 0.04 b | 0.15 ± 0.07 c | 0.67 ± 0.09 c | 0.96 |
| MAC | 0.25 ± 0.09 b | 0.38 ± 0.13 b | 0.63 ± 0.11 b | 2.85 ± 0.39 b | 4.11 | |
| DAC | 0.87 ± 0.19 a | 1.05 ± 0.30 a | 1.66 ± 0.27 a | 4.66 ± 0.67 a | 8.24 | |
| ANOVA | F-value | 361.2 | 200.8 | 583.4 | 580.3 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Downed log | EAC | 0.09 ± 0.06 c | 0.19 ± 0.06 b,c | 0.25 ± 0.09 c | 1.43 ± 0.06 b | 1.96 |
| MAC | 0.25 ± 0.07 a,b | 0.47 ± 0.08 a,b | 0.77 ± 0.11 b | 1.52 ± 0.14 b | 3.01 | |
| DAC | 0.48 ± 0.14 a,b | 0.79 ± 0.18 a | 1.21 ± 0.14 a | 2.75 ± 0.44 a | 5.23 | |
| ANOVA | F-value | 122.5 | 173.0 | 484.9 | 546.2 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Stump | EAC | 0.09 ± 0.08 | 0.25 ± 0.08 b | 0.40 ± 0.08 b | 0.43 ± 0.07 c | 1.17 |
| MAC | 0.11 ± 0.07 | 0.39 ± 0.10 a | 0.55 ± 0.09 a | 0.67 ± 0.09 a | 1.72 | |
| DAC | 0.09 ± 0.05 | 0.30 ± 0.08 a | 0.46 ± 0.12 b | 0.55 ± 0.09 b | 1.40 | |
| ANOVA | F-value | 1.3 | 20.7 | 14.6 | 52.2 | |
| p-value | 0.270 | <0.001 | <0.001 | <0.001 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behjou, F.K.; Lo Monaco, A.; Tavankar, F.; Venanzi, R.; Nikooy, M.; Mederski, P.S.; Picchio, R. Coarse Woody Debris Variability Due to Human Accessibility to Forest. Forests 2018, 9, 509. https://doi.org/10.3390/f9090509
Behjou FK, Lo Monaco A, Tavankar F, Venanzi R, Nikooy M, Mederski PS, Picchio R. Coarse Woody Debris Variability Due to Human Accessibility to Forest. Forests. 2018; 9(9):509. https://doi.org/10.3390/f9090509
Chicago/Turabian StyleBehjou, Farshad Keivan, Angela Lo Monaco, Farzam Tavankar, Rachele Venanzi, Mehrdad Nikooy, Piotr S. Mederski, and Rodolfo Picchio. 2018. "Coarse Woody Debris Variability Due to Human Accessibility to Forest" Forests 9, no. 9: 509. https://doi.org/10.3390/f9090509







