EU Legislation on Forest Plant Health: An Overview with a Focus on Fusarium circinatum
Abstract
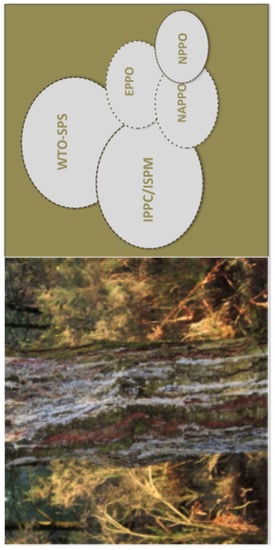
:1. Introduction
2. Current European Plant Health Legislation
- protect plant health in agriculture, forestry, and the uncultivated environment;
- develop an international strategy against the introduction and spread of pests (including invasive alien plants) that damage cultivated and wild plants, in agricultural and natural ecosystems and protect biodiversity;
- encourage harmonization of phytosanitary regulations and all other areas of official plant protection action;
- promote the use of modern, safe, and effective pest control methods;
- provide a documentation and information service on plant protection.
2.1. The Case of Fusarium circinatum
2.1.1. EU Legislation
- Annex III (A), prohibits the introduction of plants or plant parts of Pinus spp. and Pseudotsuga menziesii (host plants) other than fruit and seed from non-European countries in all member states. Annex III (A) also prohibits the introduction of soil and growing medium as such in all member states;
- Annex II (A) and IV(A) specify import requirements for growing media attached to plants and coniferous wood;
- Wood packaging material must comply with the requirements as specified in ISPM 15.
2.1.2. Emergency Measures and Contingency Plans
- (i)
- they have been grown throughout their life in countries where the specified organism is not known to occur, or
- (ii)
- they have been grown throughout their life in a pest-free area, established by the national plant protection organisation in the country of origin in accordance with relevant International Standards for Phytosanitary Measures. The name of the pest-free area shall be mentioned under the rubric “place of origin”, or
- (iii)
- they originate in a place of production where no signs of the specified organism have been observed during official inspections within a period of two years prior to export, and have been tested immediately prior to export.
- (i)
- they have been grown throughout their life or since their introduction into the Community in a place of production of a Member State where the organism is not known to occur, or
- (ii)
- they have been grown throughout their life or since their introduction into the Community, in a place of production in a pest-free area, established by the responsible official body in a Member State, in accordance with relevant International Standards for Phytosanitary Measures, or
- (iii)
- they originate in a place of production where no signs of the specified organism have been observed during official inspections within a period of two years prior to movement and have been tested immediately prior to movement.
- (i)
- an infected zone where the presence of the specified organism has been confirmed and which includes all specified plants showing symptoms caused by the specified organism, and
- (ii)
- a buffer zone with a boundary at least 1 km beyond the infected zone. In cases where several buffer zones overlap or are geographically close, a wider demarcated area shall be defined which includes the relevant demarcated areas and the areas between them.
- (i)
- appropriate measures aimed at eradicating the specified organism;
- (ii)
- intensive monitoring (surveillance) for the presence of the specified organism through appropriate inspections.
- (a)
- the roles and responsibilities of the bodies involved in the execution of the plan in the event of an outbreak;
- (b)
- access of competent authorities to premises of operators, laboratories, equipment, personnel, external expertise and resources necessary for the rapid and effective eradication or containment of the priority pest;
- (c)
- official publication and communication of findings and measures taken against the priority pest;
- (d)
- a pest risk assessment regarding the risk of the priority pest concerned for its territory and the risk management measures to be taken;
- (e)
- principles for the geographical demarcation of demarcated areas;
- (f)
- protocols describing the methods of visual examinations, sampling and laboratory testing, and principles concerning the training of personnel.
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Convention on Biological Diversity. What Are Invasive Alien Species? Available online: https://www.cbd.int/invasive/WhatareIAS.shtml (accessed on 18 June 2018).
- Santini, A.; Ghelardini, L.; De Pace, C.; Desprez-Loustau, M.L.; Capretti, P.; Chandelier, A.; Cech, T.; Chira, D.; Diamandis, S.; Gaitniekis, T.; et al. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 2013, 197, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Roques, A.; Rabitsch, W.; Rasplus, J.-Y.; Lopez-Vamonde, C.; Nentwig, W.; Kenis, M. Alien terrestrial invertebrates of Europe. In Handbook of Alien Species in Europe; Delivering Alien Invasive Species Inventories for Europe (DAISIE), Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 63–79. [Google Scholar]
- Desprez-Loustau, M.-L. The alien fungi of Europe. In Handbook of Alien Species in Europe; Delivering Alien Invasive Species Inventories for Europe (DAISIE), Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 15–28. [Google Scholar]
- Liebhold, A.; Brockerhoff, E.; Garrett, L.; Parke, J.; Britton, K. Live plant imports: The major pathway for the forest insect and pathogen invasions of the US. Front. Ecol. Environ. 2012, 10, 135–143. [Google Scholar] [CrossRef]
- Eschen, R.; Holmes, T.; Smith, D.; Roques, A.; Santini, A.; Kenis, M. Likelihood of establishment of tree pests and diseases based on their worldwide occurrence as determined by hierarchical cluster analysis. For. Ecol. Manag. 2014, 315, 103–111. [Google Scholar] [CrossRef]
- Brasier, C.M. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathol. 2008, 57, 792–808. [Google Scholar] [CrossRef] [Green Version]
- Gonthier, P.; Anselmi, N.; Capretti, P.; Bussotti, F.; Feducci, M.; Giordano, L.; Honorati, T.; Lione, G.; Luchi, N.; Michelozzi, M.; et al. An integrated approach to control the introduced forest pathogen Heterobasidion irregulare in Europe. Forestry 2014, 87, 471–481. [Google Scholar] [CrossRef]
- EPPO. Situation of Diabrotica virgifera in Serbia (YU). In Proceedings of the International Workshop “Western Corn Rootworm in Europe 95”, Gödöllö, Hungary, 8 November 1995. [Google Scholar]
- Aukema, J.E.; McCullough, D.G.; Von Holle, B.; Liebhold, A.M.; Britton, K.; Frankel, S.J. Historical accumulation of non indigenous forest pests in the Continental United States. Bioscience 2010, 60, 886–897. [Google Scholar] [CrossRef]
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic Impacts of Non-Native Forest Insects in the Continental United States. PLoS ONE 2011, 6, E24587. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, causal agent of pine wilt disease on Pinus pinaster in North-western Spain. EPPO Bull. 2011, 39, 344–353. [Google Scholar] [CrossRef]
- Fonseca, L.; Lopes, A.; Cardoso, J.; Pestana, M.; Abreu, F.; Nunes, N.; Mota, M.; Abrantes, I. The pinewood nematode, Bursaphelenchus xylophilus, in Madeira Island. Helminthologia 2012, 49, 96–103. [Google Scholar] [CrossRef]
- Robertson, L.; Arcos, C.S.; Escuer, M.; Merino, S.R.; Esparrago, G.; Abelleira, A.; Navas, A. Incidence of the pinewood nematode Bursaphelenchus xylophlius Steiner &Buhrer, 1934 (Nickle, 1970) in Spain. Nematology 2011, 13, 755–757. [Google Scholar] [CrossRef]
- Rodrigues, J. Eradication program for the pinewood nematode in Portugal. In Pine wilt Disease: A Worldwide Threat to Forest Ecosystems; Mota, M., Vieira, P., Eds.; Springer: Heidelberg, Germany, 2008; pp. 5–14. [Google Scholar]
- FVO—Food and Veterinary Office. Final Report of a Mission Carried Out in Portugal from 02 June to 06 June 2008 in Order to Assess the Implementation of Commission Decision 2006/133/Ec and the National Eradication Programme for Bursaphelenchus xylophilus (Pine Wood Nematode); DG SANCO/2008/7991–MR–Final, European Commission. 2008. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/ph_biosec_legis_em-measures_pwn-task-force_en.pdf (accessed on 18 June 2018).
- Soliman, T.; Mourits, M.C.M.; van der Werf, W.; Hengeveld, G.M.; Robinet, C.; Lansink, A.G.J.M.O. Framework for modelling economic impacts of invasive species, applied to pine wood nematode in Europe. PLoS ONE 2012, 7, E45505. [Google Scholar] [CrossRef] [PubMed]
- Økland, B.; Skarpaas, O.; Schroeder, M.; Magnusson, C.; Lindelow, Å.; Thunes, K. Is eradication of the Pinewood Nematode (Bursaphelenchus xylophilus) likely? An evaluation of current contingency plans. Risk Anal. 2010, 30, 1424–1439. [Google Scholar] [CrossRef] [PubMed]
- Mascheretti, S.; Croucher, P.J.P.; Vettraino, A.M.; Prospero, S.; Garbelotto, M. Reconstruction of the sudden oak death epidemic in California through microsatellite analysis of the pathogen Phytophtho raramorum. Mol. Ecol. 2008, 11, 2755–2768. [Google Scholar] [CrossRef] [PubMed]
- Ivors, K.; Garbelotto, M.; Vries, I.; Ruyter–Spira, C.; Hekkert, B.; Rosenzweig, N.; Bonants, P. Microsatellite markers identify three lineages of Phytophtho raramorum in US nurseries, yet single lineages in US forest and European nursery populations. Mol. Ecol. 2006, 15, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Kanaskie, A.; Hansen, E.M.; Goheen, E.M.; McWilliams, M.; Reeser, P.; Sutton, W. Phytophtho raramorum in Oregon forests: Six years of detection, eradication, and disease spread. In Proceedings of the Fourth Meeting of the International Union of Forest Research Organizations (IUFRO) Working Party S07.02.09: Phytophthoras in Forests and Natural Ecosystems. Gen. Tech. Rep. PSW-GTR-221; Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2009; pp. 170–172. [Google Scholar]
- Haack, R.A.; Herard, F.; Sun, J.; Turgeon, J.J. Managing invasive populations of Asian Longhorned Beetle and Citrus Longhorned Beetle: A worldwide perspective. Ann. Rev. Entomol. 2010, 55, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Swett, C.L.; Gordon, T.R. First report of grass species (Poaceae) as naturally occurring hosts of the pine pathogen Gibberella circinata. Plant Dis. 2012, 96, 908. [Google Scholar] [CrossRef]
- Hernandez-Escribano, L.; Iturritxa, E.; Elvira-Recuenco, M.; Berbegal, M.; Campos, J.A.; Renobales, G.; García, I.; Raposo, R. Herbaceous plants in the understory of a pitch canker-affected Monterey pine plantation are endophytically infected with Fusarium circinatum. Fungal Ecol. 2018, 32, 65–71. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Bailey, M.J.; Bainbridge, I.P.; Brereton, T.; Dick, J.T.A.; Drewitt, J.; Dulvy, N.K.; Dusic, N.R.; Freckleton, R.P.; Gaston, K.J.; et al. Future novel threats and opportunities facing UK biodiversity identified by horizon scanning. J. Appl. Ecol. 2008, 45, 821–833. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Baker, R.H.A.; Coakley, S.M.; Harrington, R.; Kriticos, D.J.; Scherm, H. Pests under global change—Meeting your future landlords. In Terrestrial Ecosystems in a Changing World; Canadell, J.G., Pataki, D.E., Pitelka, L.F., Eds.; Springer: Berlin, Germany, 2007; pp. 211–226. [Google Scholar]
- Ghini, R.; Hamada, E.; Bettiol, W. Climate change and plant diseases. Sci. Agric. 2008, 65, 98–107. [Google Scholar] [CrossRef] [Green Version]
- MacLeod, A.; Pautasso, M.; Jeger, M.J.; Haines-Young, R. Evolution of the international regulation of plant pests and challenges for future plant health. Food Secur. 2010, 2, 49–70. [Google Scholar] [CrossRef]
- WTO-SPS. Agreement on the Application of Sanitary and Phytosanitary Measures; World Trade Organization: Rome, Italy, 1995. [Google Scholar]
- Eschen, R.; Britton, K.; Brockerhoff, E.; Burgess, T.; Dalley, V.; Epanchin-Niell, R.S.; Gupta, K.; Hardy, G.; Huang, Y.; Kenis, M.; et al. International variation in phytosanitary legislation and regulations governing importation of plants for planting. Environ. Sci. Policy 2015, 51, 228–237. [Google Scholar] [CrossRef] [Green Version]
- International Plant Protection Convention (IPPC). Available online: https://www.ippc.int (accessed on 18 June 2018).
- International Plant Protection Convention (IPPC). ISPM No. 15. Guidelines for regulating wood packaging material in international trade. In International Standards for Phytosanitary Measures; FAO: Rome, Italy, 2006. [Google Scholar]
- International Plant Protection Convention (IPPC). ISPM No. 38 on the International Movement of Seed. In International Standards for Phytosanitary Measures; FAO: Rome, Italy, 2017. [Google Scholar]
- International Plant Protection Convention (IPPC). ISPM 39. In International movement of wood. In International Standards for Phytosanitary Measures; FAO: Rome, Italy, 2017. [Google Scholar]
- Ebbels, D.L.; King, J.E. Plant Health: The Scientific Basis for Control of Plant Diseases and Pests; Blackwell Scientific Pubs.: Oxford, UK, 1979; 322p. [Google Scholar]
- International Plant Protection Convention (IPPC). ISPM 36—Integrated Measures for Plants for Planting. ISPM no. 36. In International Standards for Phytosanitary Measures; FAO: Rome, Italy, 2012. [Google Scholar]
- NAPPO. RSPM 24—Integrated Pest Risk Management Measures for the Importation of Plants for Planting into NAPPO Member Countries. NAPPO: Ontario, Canada, 2013; Available online: http://www.nappo.org/english/products/regional-standards/regional-phytosanitary-standards-rspms/ (accessed on 18 June 2018).
- European Plant Protection Organization (EPPO). Available online: https://www.eppo.int/ (accessed on 18 June 2018).
- International Plant Protection Convention (IPPC). ISPM 2—Framework for Pest Risk Analysis. ISPM No. 2. In International Standards for Phytosanitary Measures; FAO: Rome, Italy, 2007. [Google Scholar]
- International Plant Protection Convention (IPPC). ISPM 11—Pest risk analysis for quarantine pests, including analysis of environmental risks and living modified organisms. ISPM No. 11. In International Standards for Phytosanitary Measures; FAO: Rome, Italy, 2004. [Google Scholar]
- International Plant Protection Convention (IPPC). ISPM 5. Glossary of phytosanitary terms. ISPM No. 5. In International Standards for Phytosanitary Measures; FAO: Rome, Italy, 2007. [Google Scholar]
- EPPO Standards. Available online: https://www.eppo.int/STANDARDS/standards.htm (accessed on 18 June 2018).
- EPPO—Computer Assisted Pest Risk Analysis—CAPRA. Available online: http://capra.eppo.org (accessed on 18 June 2018).
- Gilioli, G.; Schrader, G.; Grégoire, J.C.; MacLeod, A.; Mosbach-Schulz, O.; Rafoss, T.; Rossi, V.; Urek, G.; Werf, W. The EFSA quantitative approach to pest risk—methodological aspects and case studies. EPPO Bull. 2017, 47, 213–219. [Google Scholar] [CrossRef]
- Jeger, M.; Caffier, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen-Schmutz, K.; Gilioli, G.; Grégoire, J.C.; Miret, J.; Anton, A.; MacLeod, A.; et al. Pest risk assessment of Atropellis spp. for the EU territory. FSA J. 2017, 15, 4877. [Google Scholar] [CrossRef]
- Jeger, M.; Bragard, C.; Chatzivassiliou, E.; Dehnen-Schmutz, K.V.; Gilioli, G.; Anton, J.; Miret, J.; Macleod, A.; Navajas, M.; et al. Risk assessment and reduction options for Cryphonectria parasitica in the EU. EFSA J. 2016, 14, 4641. [Google Scholar] [CrossRef]
- International Standards for Phytosanitary Measures ISPM 9. Generic elements for contingency plans. EPPO Bull. 2009, 39, 471–474. [Google Scholar] [CrossRef]
- EPPO Global Database. Commodity-specific Phytosanitary Measures PM8. Available online: https://gd.eppo.int/standards/PM8/ (accessed on 18 June 2018).
- EPPO Global Database. Available online: https://gd.eppo.int/ (accessed on 18 June 2018).
- EFSA. Available online: https://www.efsa.europa.eu (accessed on 18 June 2018).
- Klapwijk, M.J.; Bylund, H.; Schroeder, M.; Björkman, C. Forest management and natural biocontrol of insect pests. Forestry 2016, 89, 253–262. [Google Scholar] [CrossRef] [Green Version]
- EPPO Quarantine Pest. Data Sheets on Quarantine Pests: Anoplophora malasiaca and Anoplophora chinensis. 2018. Available online: https://gd.eppo.int/taxon/ANOLCN/documents (accessed on 18 June 2018).
- EPPO Quarantine Pest. Data Sheets on Quarantine Pests: Data Sheet on Anoplophora glabripennis. 2018. Available online: https://gd.eppo.int/taxon/ANOLGL/documents (accessed on 18 June 2018).
- EPPO Quarantine Pest. Data Sheets on Quarantine Pests: Data Sheet on Bursaphelenchus xylophilus. 2018. Available online: https://gd.eppo.int/taxon/BURSXY/documents (accessed on 18 June 2018).
- Figueiredo, J.; Simoes, M.J.; Gomes, P.; Barroso, C.; Pinho, D.; Conceicao, L.; Fonseca, L.; Abrantes, I.; Pinheiro, M.; Egas, C.; et al. Assessment of the geographic origins of pinewood nematode isolates via single nucleotide polymorphism in effector genes. PLoS ONE 2013, 8, e83542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EPPO. Mini Data Sheet on Phytophthora ramorum. 2013. Available online: https://gd.eppo.int/taxon/PHYTRA/documents (accessed on 18 June 2018).
- Brasier, C.; Webber, J. Sudden larch death. Nature 2010, 466, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Parke, J.L.; Rizzo, D.M. Phytophthora ramorum. For. Phytophthoras 2011, 1, 1. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D.; Coutinho, T.A. Pitch canker caused by Fusarium circinatum—A growing threat to pine plantations and forests worldwide. Australas. Plant Pathol. 2008, 37, 319–334. [Google Scholar] [CrossRef]
- Gordon, T.R.; Okamoto, D.; Storer, A.J.; Wood, D.L. Susceptibility of five landscape pines to pitch canker disease, caused by Fusarium subglutinans f. sp. pini. Hortscience 1998, 33, 868–871. [Google Scholar] [CrossRef]
- Gordon, T.R.; Wikler, K.R.; Clark, L.; Okamoto, D.; Storer, A.J.; Bonello, P. Resistance to pitch canker disease, caused by Fusarium subglutinans f. sp. pini, in Monterey pine (Pinus radiata). Plant Pathol. 1998, 47, 706–711. [Google Scholar] [CrossRef]
- Viljoen, A.; Wingfield, M.J.; Marasas, W.F.O. First report of Fusarium subglutinans f. sp. pini on pine seedlings in South Africa. Plant Dis. 1994, 78, 309–312. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.; Landeras, E.; León, M.; Berbegal, M.; García-Jiménez, J.; Armengol, J. Characterization of Fusarium circinatum from Pinus spp. in northern Spain. Mycol. Res. 2007, 111, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Iturritxa, E.; Mesanza, N.; Elvira-Recuenco, M.; Serrano, Y.; Quintana, E.; Raposo, R. Evaluation of genetic resistance in Pinus to pitch canker in Spain. Australas. Plant Pathol. 2012, 41, 601–607. [Google Scholar] [CrossRef]
- Bragança, H.; Diogo, E.V.; Moniz, F.V.; Amaro, P. First report of pitch canker on pines caused by Fusarium circinatum in Portugal. Plant Dis. 2009, 93, 1079. [Google Scholar] [CrossRef]
- Carlucci, A.; Colatruglio, L.; Frisullo, S. First report of Pitch Canker caused by Fusarium circinatum on Pinus halepensis and P. pinea in Apulia (southern Italy). Plant Dis. 2007, 91, 1683. [Google Scholar] [CrossRef]
- Chapin, E.; Chauvel, G. Phytosanitary overview for 2006 of tree plantations, shrubs and clumps in green spaces. In PHM Revue Horticole; P.H.M. Revue Horticole: Paris, France, 2007; Volume 490, pp. 40–44. [Google Scholar]
- Landeras, E.; García, P.; Fernández, Y.; Braña, M.; Fernández-Alonso, O.; Méndez-Lodos, S.; Pérez-Sierra, A.; León, M.; Abad-Campos, P.; Berbegal, M.; et al. Outbreak of pitch canker caused by Fusarium circinatum on Pinus spp. in Northern Spain. Plant Dis. 2005, 89, 1015. [Google Scholar] [CrossRef]
- EPPO. Available online: https://gd.eppo.int/taxon/GIBBCI (accessed on 18 June 2018).
- Ganley, R.J.; Watt, M.S.; Manning, L.; Iturritxa, E. A global climatic risk assessment of pitch canker disease. Can. J. For. Res. 2009, 39, 2246–2256. [Google Scholar] [CrossRef]
- Dvořák, M.; Rotkova, G.; Botello, L. Detection of airborne inoculum of Hymenoscyphus fraxineus and H.albidus during seasonal fluctuations associated with absence of apothecia. Forests 2016, 7, 1. [Google Scholar] [CrossRef]
- Dvorák, M.; Janoš, P.; Botella, L.; Rotková, G.; Zas, R. Spore Dispersal Patterns of Fusarium circinatum on an Infested Monterey Pine Forest in North-Western Spain. Forests 2017, 8, 432. [Google Scholar] [CrossRef]
- Bezos, D.; Martínez-Alvarez, P.; Fernández, M.; Diez, J.J. Epidemiology and Management of Pine Pitch Canker Disease in Europe—A Review. Balt. For. 2017, 23, 279–293. [Google Scholar]
- Elvira-Recuenco, M.; Iturritxa, E.; Raposo, R. Impact of seed transmission on the infection and development of pitch canker disease in Pinus radiata. Forests 2015, 6, 9. [Google Scholar]
- Serrano, Y.; Iturritxa, E.; Elvira-Recuenco, M.; Raposo, R. Survival of Fusarium circinatum in soil and Pinusradiata needle and branch segments. Plant Pathol. 2017, 66, 934–940. [Google Scholar] [CrossRef]
- Forestry Commission. Contingency-plan-Pitch-canker-of-pine. DEFRA 2016. Available online: https://www.forestry.gov.uk (accessed on 18 June 2018).
- European Commission. Regulation (EU) No 1143/2014 of the European Parliament and of the Council 22 October 2014 on the Prevention and Management of the Introduction and Spread of Invasive Alien Species, Brussels, Belgium, 22 October 2014; Official Journal of the European Union, European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Roques, A.; Fan, J.T.; Courtial, B.; Zhang, Y.Z.; Yart, A.; Auger-Rozenberg, M.A.; Denux, O.; Kenis, M.; Barker, R.; Sun, J. Planting Sentinel European Trees in Eastern Asia as a Novel Method to Identify Potential Insect Pest Invaders. PLoS ONE 2015, 10, e0120864. [Google Scholar] [CrossRef] [PubMed]
- Vettraino, A.; Roques, A.; Yart, A.; Fan, J.T.; Sun, J.H.; Vannini, A. Sentinel Trees as a Tool to Invasions of Alien Plant Pathogens. PLoS ONE 2015, 10, e0120571. [Google Scholar] [CrossRef] [PubMed]
- Vettraino, A.M.; Li, H.M.; Eschen, R.; Morales-Rodriguez, C.; Vannini, A. The sentinel tree nursery as an early warning system for pathway risk assessment: Fungal pathogens associated with Chinese woody plants commonly shipped to Europe. PLoS ONE 2017, 12, e0188800. [Google Scholar] [CrossRef] [PubMed]
- Eschen, R.; Rigaux, L.; Sukovata, L.; Vettraino, A.M.; Marzano, M.; Grégoire, J.C. Phytosanitary inspection of woody plants for planting at European Union entry points—A practical enquiry. Biol. Invasions 2015, 17, 2403–2413. [Google Scholar] [CrossRef]




| EPPO | Documents | Commodity/Pests |
|---|---|---|
| Standards | PM 8/2(2) | Coniferae |
| PM 8/4(1) | Castanea | |
| PM 8/5(1) | Quercus | |
| PM 8/6(1) | Betula | |
| PM 8/7(1) | Populus | |
| PM 8/8(1) | Salix | |
| Diagnostic Protocols | PM 7/14(2) | Ceratocystis platani |
| PM 7/45(1) | Cryphonectria parasitica | |
| PM 7/46(3) | Lecanosticta acicola | |
| PM 7/73(1) | Gymnosporangium spp. (non-European) | |
| PM 7/91(1) | Fusarium circinatum | |
| PM7/119(1) | Bursaphelenchus xylophilus (nematode extraction) | |
| PM7/123(1) | Phytophthora lateralis | |
| PM7/112(1) | P. kernoviae | |
| PM7/66(1) | P. ramorum | |
| Final Decision | ||
| Pest Risk Analysis | A1-2011 | Agrilus anxius |
| A1-2013 | Apriona spp. | |
| A1-2014 | Aromia bungii | |
| A1-2013 | Oemona hirta | |
| A2-2014 | Polygraphus proximus | |
| A2-2015 | Geosmithia morbida | |
| A2-2015 | Heterobasidion irregulare | |
| A1—transferred to A2 in 2011 | Phytophthora lateralis | |
| A2 in 2013 | P. kernoviae and P. ramorum | |
| A2-2017 | Thekopsora minima | |
| A1—transferred to A2 in 2010 | Bursaphelenchus xylophilus | |
| Pest/Pathogen | Group | Outbreak Areas | Description | EU Decision | |
|---|---|---|---|---|---|
| Present | Transient, under Eradication | ||||
| Anoplophora chinensis | Insect | China (Anhui, Aomen (Macau), Fujian, Gansu, Guangdong, Guizhou, Hebei, Hubei, Hunan, Jiangsu, Jiangxi, Liaoning, Shaanxi, Sichuan, Xinjiang, Xizhang, Yunnan, Zhejiang), EU (Italy), Indonesia, Japan (Hokkaido, Honshu, Japan Kyushu, Ryukyu Archipelago, Shikoku), Korea Dem. People’s Republic, Malaysia, Myanmar, Philippines, Taiwan, Vietnam. | EU (Croatia, Germany), Switzerland, Turkey | The life cycle can be one or two generations per year, depending on the climatic and feeding conditions. Adults feed on leaves, petioles, and young bark of various tree species. The eggs are deposited under the bark. In international trade, these insects are most likely to move as eggs, larvae, or pupae hidden in woody plants and packing material. Larvae and adults of A. glabripennis have been intercepted in the United Kingdom on packaging material, while individuals of A. chinensis entered Europe on bonsai plants. Damage to fruit, and ornamental and amenity trees, results in serious economic loss. Moreover, attacks on urban plants poses hazards to pedestrians and vehicles from structural weakening and falling branches [53,54]. | 2012/138/EU |
| Anoplophora glabripennis | Insect | China (Anhui, Fujian, Gansu, Guangdong, Guangxi, Guizhou, Hebei, Heilongjiang, Henan, Hubei, Hunan, Jiangsu, Jiangxi, Jilin, Liaoning, Neimenggu, Ningxia, Qinghai, Shaanxi, Shandong, Shanxi, Sichuan, Xinjiang, Xizhang, Yunnan, Zhejiang), EU (France—Corsica, Finland) Korea Dem. People’s Republic, Lebanon, Russia Far East, United States of America (Ohio, New York). | EU (Austria, France, Germany, Italy), Canada (Ontario), Montenegro, Switzerland, United Kingdom (England) | 2015/893/EU | |
| Bursaphelenchus xylophilus (Pine Wood Nematode—PWN) | Nematode | Canada (Alberta, British Columbia, Manitoba, New Brunswick, Newfoundland, Northwest Territories, Nova Scotia, Nunavut, Ontario, Québec, Saskatchewan, Yukon Territory), China (Anhui, Chongqing, Fujian, Gansu, Guangdong, Guangxi, Guizhou, Hubei, Hunan, Jiangxi, Liaoning, Shandong, Sichuan, Yunnan, Zhejiang, Xianggang), EU (Portugal), Japan (Honshu, Kyushu, Ryukyu Archipelago, Shaanxi, Shikoku, Jiangsu), Korea Republic, Mexico, Taiwan, USA (Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Jersey, New York, North Carolina, Ohio, Oklahoma, Oregon, Pennsylvania, South Carolina, Tennessee, Texas, Vermont, Virginia, West Virginia, Wisconsin). | EU (Portugal, Spain) | Bursaphelenchus xylophilus is the causal agent of the pine wilt disease. It is transmitted from one host to the next by insect vectors, mainly belonging to the genus Monochamus. It enters the tree through wounds caused by the insect feeding on the twig bark or wounds by the vector to lay its eggs. Once inside the tree, nematodes feed on the hyphae of fungi (usually Ceratocystis spp.) also transmitted to the wood by ovipositing beetles. They rapidly multiple in the resin canals leading to tree death within a few months [55,56]. | |
| Fusarium circinatum | Fungus | Chile, EU (Portugal, Spain), Haiti, Japan (Kyushu, Ryukyu Archipelago), Korea Republic, Mexico, South Africa, Uruguay, USA (Alabama, Arkansas, California, Florida, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, Texas, Virginia). | EU (Portugal, Spain) | See section “The case of Fusarium circinatum”. | 2007/433/EC |
| Phytophtora ramorum | Chromista | EU (Denmark, Germany, Greece, Poland, Portugal, Serbia, Spain, Belgium, Croatia, France, Ireland, Netherlands, Norway, Sweden, United Kingdom—Channel Islands, England, Scotland) Switzerland, Canada—British Columbia, USA (Florida, Georgia, Louisiana, South Carolina, Tennessee, Washington, Virginia, California, Oregon). | EU (Czech Republic, Finland, Italy, Slovenia). | Phytophthora ramorum is an oomycete pathogen known as responsible agent of sudden oak disease (SOD). The disease causes extensive damage and death to a wide range of trees and ornamental plants. It resulted in significant losses of trees, mainly oaks in California and Oregon. By contrast, in Europe, the pathogen affects mainly ornamental shrubs. However, recently, P. ramorum was unexpectedly detected on Japanese larches (Larix kaempferi), causing widespread tree mortality in England. P. ramorum produces several types of structures (zoospores, sporangia, and chlamydospores) specialised for survival, dispersal, or infection. Movement of infected ornamental shrubs is a significant mode of dispersal. The disease can be transmitted by infected plants and soil, and dispersal through vectors and air/water is still poorly understood [57,58,59]. | 2002/757/EC |
| United Kingdom | Spain |
|---|---|
| Obligations within Demarcated Areas | |
| Trees felled to eradicate F. circinatum should be destroyed in situ. | All susceptible plants in the infected zone should be destroyed in situ. |
| For nurseries in the area, authorization for Pinus and Douglas fir plants suspended until the presence of F. circinatum within the nursery and within the demarcated area is determined. | For forest reproductive material, all fields and facilities that use this material will be declared as possibly contaminated and, therefore, susceptible plant material is to be eliminated and facilities decontaminated. Particularly for seeds, the affected batch will be destroyed, and all other batches that share facilities will be declared as possibly contaminated and immobilised until presence of F. circinatum is determined. |
| After immediate measures taken, possible preventive measures to be applied are described. | |
| Tracing Backwards | |
| If the infected trees have been planted within the previous two years, the source of the plants must be traced back to the supplying nursery, and the nursery inspected for the presence of F. circinatum. | The origin of affected plants within a planted forest will be investigated to determine the possible source of plants. Suppliers of infected forest reproductive material will provide a list with users of that material in the last two years. Material will be immobilised and analysis will be done to determine the presence of F. circinatum. |
| If the infected trees have been planted within the previous two years, the source of the plants must be traced back to the supplying nursery, and the nursery inspected for the presence of F. circinatum. | Origin of affected plants within a planted forest will be investigated to determine the possible source of plants. Suppliers of infected forest reproductive material will provide a list with users of that material in the last two years. Material will be immobilised and analysis will be done to determine the presence of F. circinatum. |
| Disposal of Felled Trees (Including Branches and Round Wood) | |
| By chipping, composting or burning. Regulations for burning are explained | By burning or any other accepted method. |
| Plant material for decorative purposes, particularly material used for Christmas trees, should be preferably buried or composted. | |
| In nurseries, infected plants and seedlings should be uprooted and burned. | Infected plants and seedlings have to be eliminated; way not specified. |
| Movement of Plant Material from Demarcated Areas | |
| It is not recommended that logs and firewood cut in infested areas be moved from the demarcated area. | It is forbidden to move plants and plant material (including wood) out from the demarcated areas. |
| If logs must be moved, debarking is recommended. They should be transported, with a protective covering ensuring that all material is contained, to a licensed incinerator. Merchantable logs may be sold to an authorised processing plant within the demarcated area for conversion to products such as pulp or fibreboard. Their use as saw logs is not allowed. | An exception is made for wood and wood products (first transformation) if it is completely debarked, a heat treatment is applied in a way that inner wood reaches at least 56 °C for 30 min, and it has its phytosanitary passport. If the wood requires transportation because there are no facilities to treat it within the demarcated area, it has to be done under supervision. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vettraino, A.M.; Potting, R.; Raposo, R. EU Legislation on Forest Plant Health: An Overview with a Focus on Fusarium circinatum. Forests 2018, 9, 568. https://doi.org/10.3390/f9090568
Vettraino AM, Potting R, Raposo R. EU Legislation on Forest Plant Health: An Overview with a Focus on Fusarium circinatum. Forests. 2018; 9(9):568. https://doi.org/10.3390/f9090568
Chicago/Turabian StyleVettraino, Anna Maria, Roel Potting, and Rosa Raposo. 2018. "EU Legislation on Forest Plant Health: An Overview with a Focus on Fusarium circinatum" Forests 9, no. 9: 568. https://doi.org/10.3390/f9090568