Effects of Forest Management Practices on Moth Communities in a Japanese Larch (Larix kaempferi (Lamb.) Carrière) Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Functional Groups
2.3. Data Analyses
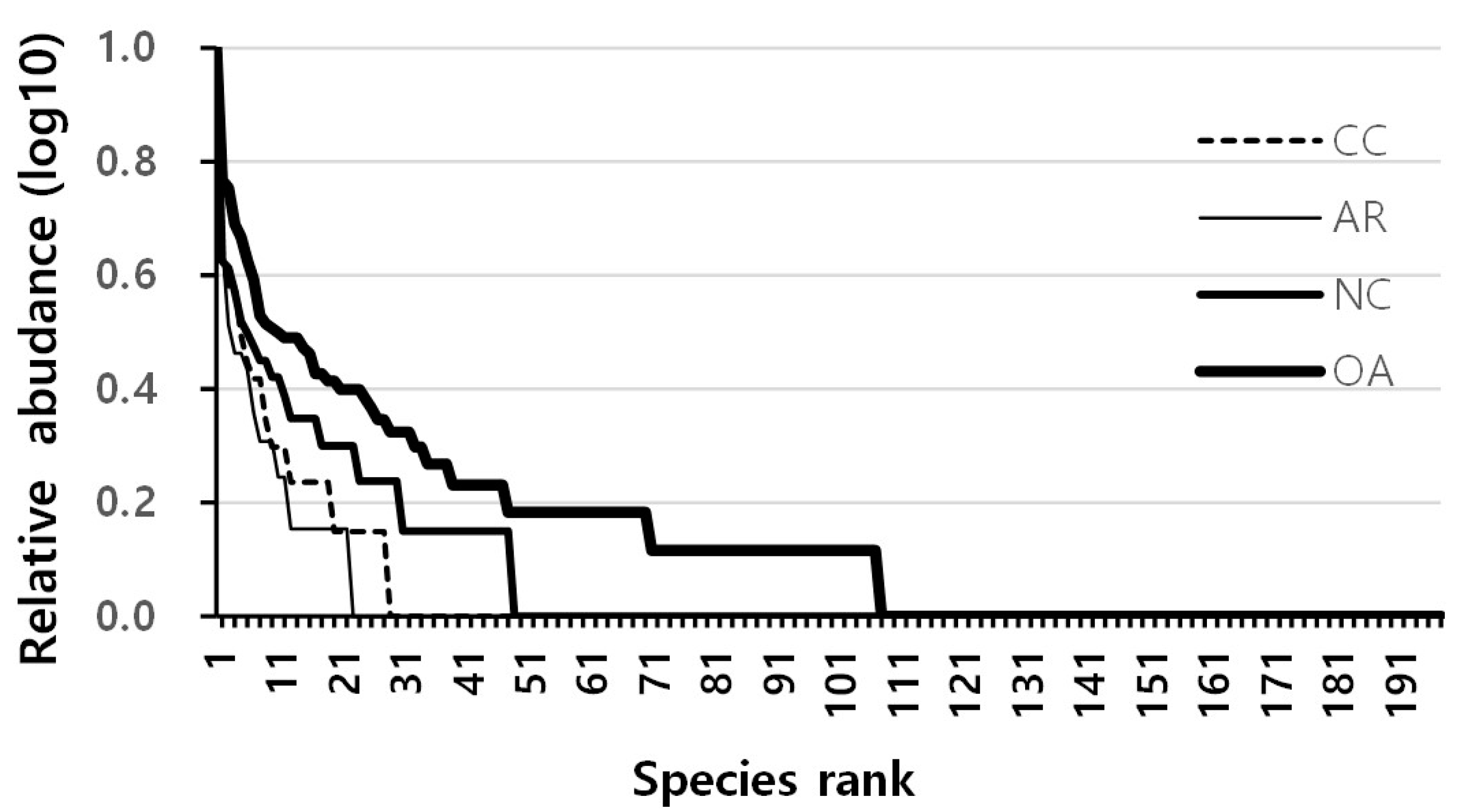
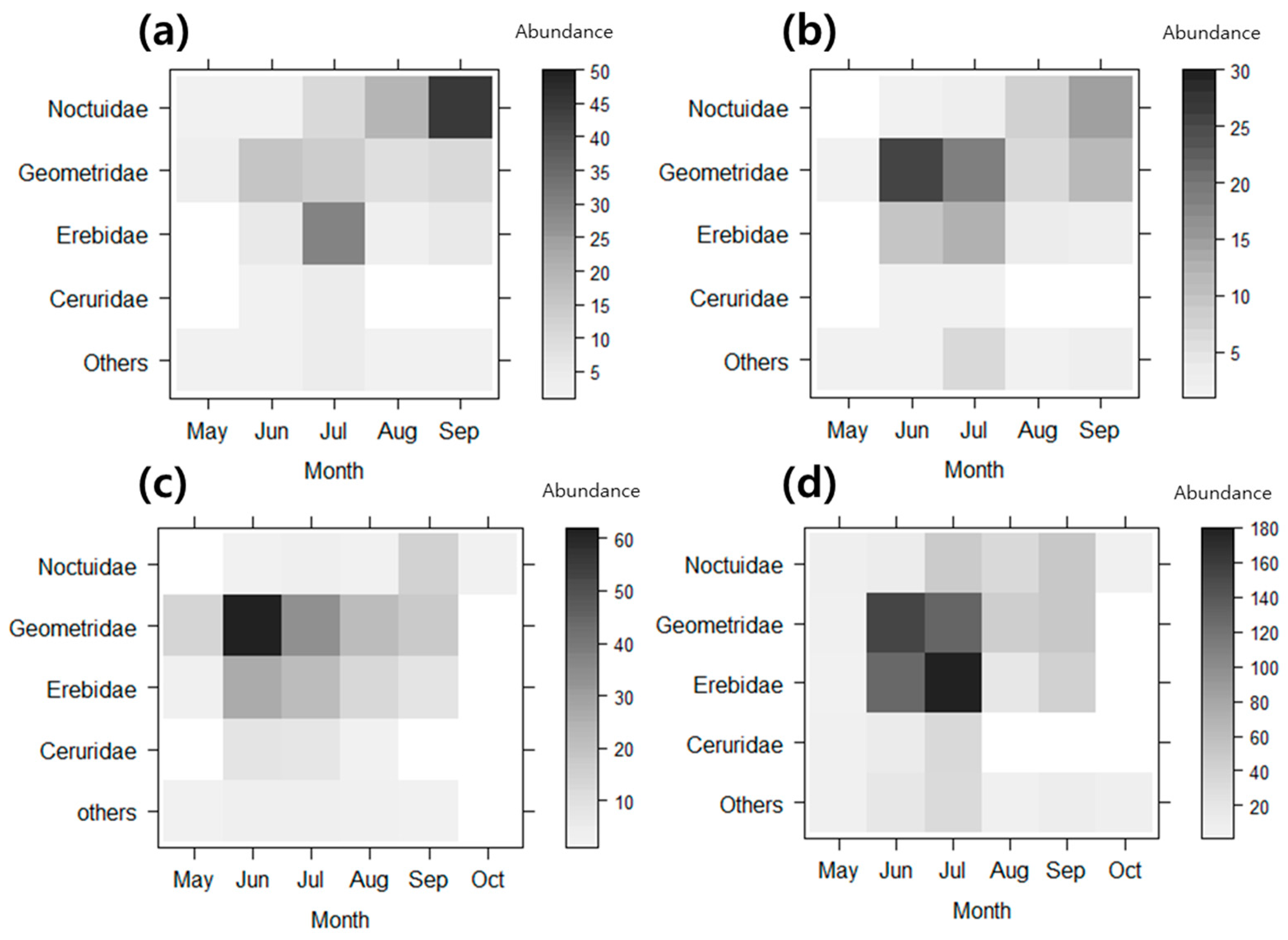
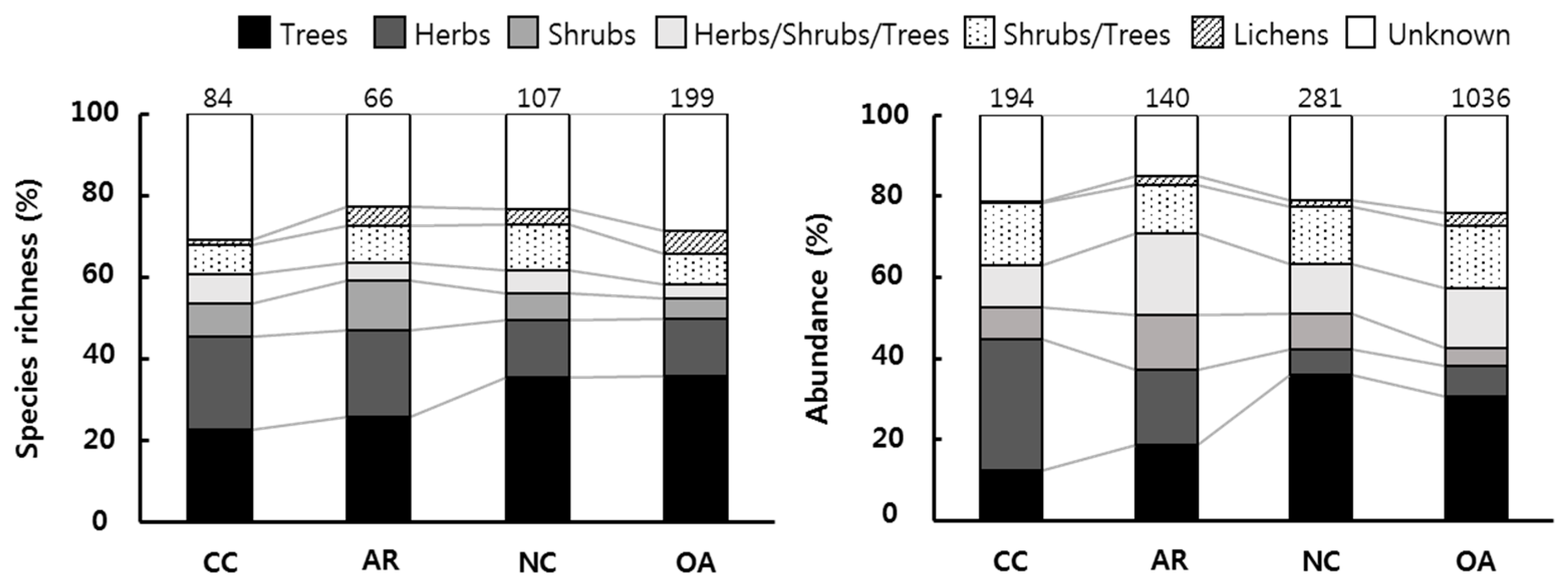
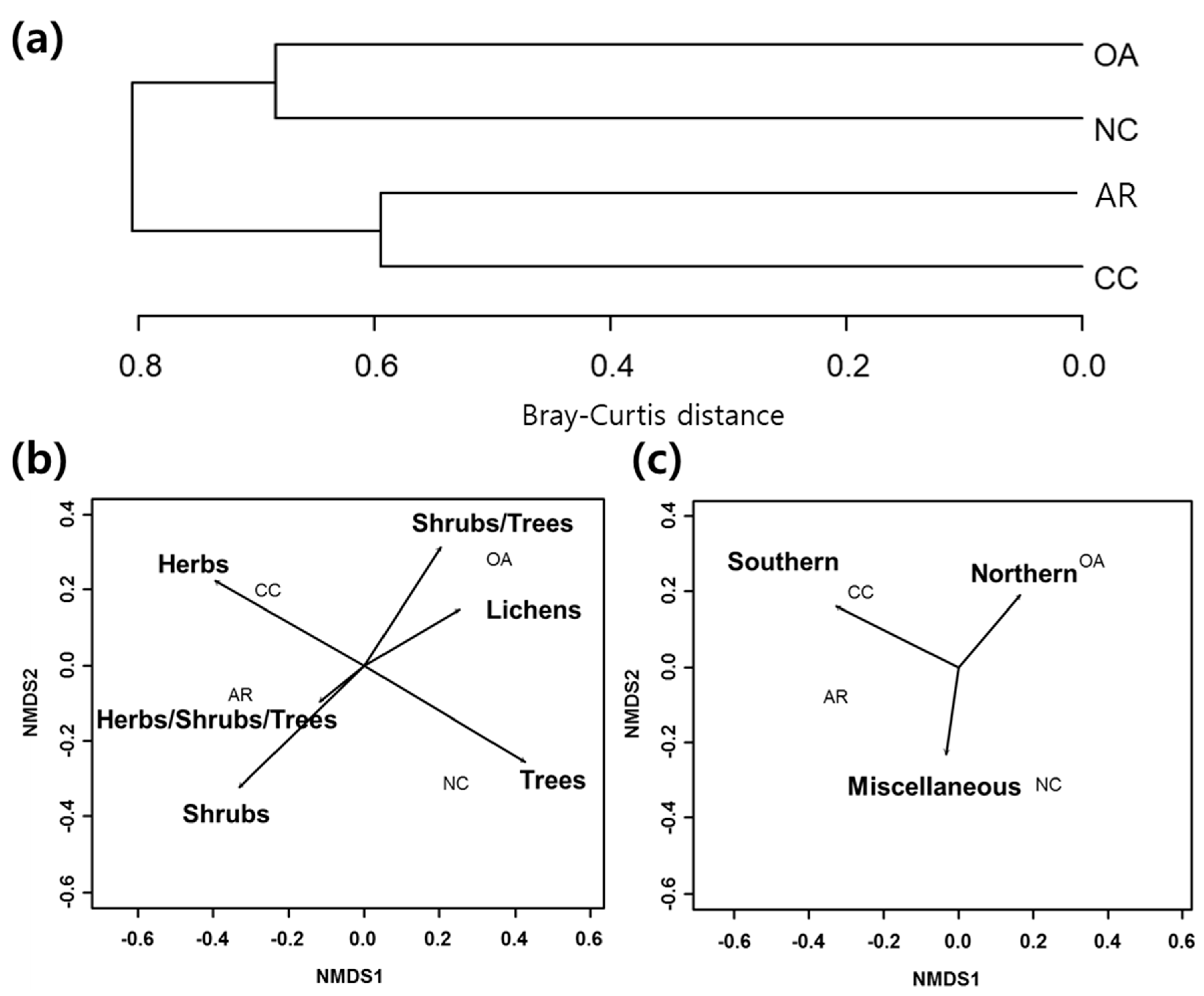
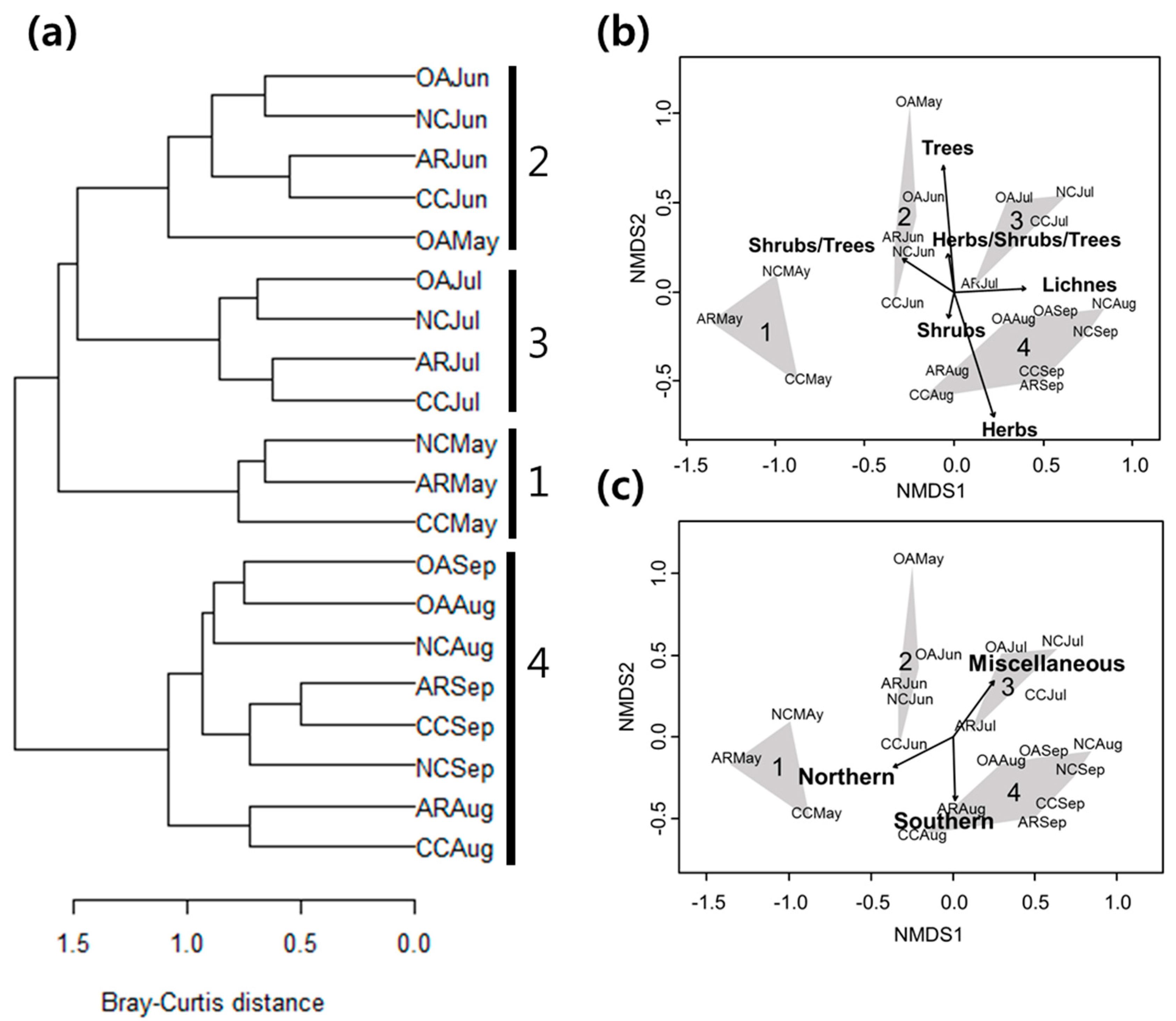
3. Results
3.1. Differences in Community Composition
3.2. Functional Groups
3.3. Forest Management Practices and Functional Groups
4. Discussion
4.1. Changes in Community Structure and Function Due to the Forest Management Practices
4.2. Aggregated Retention and Biodiversity
4.3. Long-Term Effects of Forest Management Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lindenmayer, D.B.; Margules, C.R.; Botkin, D.B. Indicators of biodiversity for ecologically sustainable forest management. Conserv. Biol. 2000, 14, 941–950. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergès, L.; HjÄltén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.J.; De Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity differences between managed and unmanaged forests: Meta-analysis of species richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Wilson, L. An economic perspective on clearcut harvesting. For. Chron. 2001, 77, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Bliss, J.C. Public perceptions of clearcutting. J. For. 2000, 98, 4–10. [Google Scholar]
- Escobar, M.A.H.; Uribe, S.V.; Chiappe, R.; Estades, C.F. Effect of Clearcutting Operations on the Survival Rate of a Small Mammal. PLoS ONE 2015, 10, e0118883. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-I.; Spence, J.R.; Langor, D.W. Combinations of aggregated and dispersed retention improve conservation of saproxylic beetles in boreal white spruce stands. For. Ecol. Manag. 2017, 385, 116–126. [Google Scholar] [CrossRef]
- Heliövaara, K.; Väisänen, R. Effects of modern forestry on northwestern European forest invertebrates: A synthesis. Acta For. Fenn. 1984, 189, 1–32. [Google Scholar] [CrossRef]
- Park, J.H. Environmental changes after timber harvesting in (Mt.) Paekunsan. J. Korean For. Soc. 1995, 84, 465–478. [Google Scholar]
- Kovács, B.; Tinya, F.; Guba, E.; Németh, C.; Sass, V.; Bidló, A.; Ódor, P. The short-term effects of experimental forestry treatments on site conditions in an Oak–hornbeam forest. Forests 2018, 9, 406. [Google Scholar] [CrossRef]
- Okuda, T.; Suzuki, M.; Adachi, N.; Quah, E.S.; Hussein, N.A.; Manokaran, N. Effect of selective logging on canopy and stand structure and tree species composition in a lowland dipterocarp forest in peninsular Malaysia. For. Ecol. Manag. 2003, 175, 297–320. [Google Scholar] [CrossRef]
- Putz, F.E.; Blate, G.M.; Redford, K.H.; Fimbel, R.; Robinson, J. Tropical forest management and conservation of biodiversity: An overview. Conserv. Biol. 2001, 15, 7–20. [Google Scholar] [CrossRef]
- Schmidt, W. Herb layer species as indicators of biodiversity of managed and unmanaged beech forests. For. Snow Landsc. Res. 2005, 125, 111–125. [Google Scholar]
- Kim, J.S. A Study on Stand Structure, Environmental Factor and Understory Vegetation for Variable Retention in Quercus Mongolica Forest. Ph.D. Thesis, Kyungpook National University, Daegu, Korea, 2014. [Google Scholar]
- Ming, Z. Effect of Aggregated Retention on Understory Vegetation and Micro-Environment in Quercus Mongolica Forest. Master’s Thesis, Kyungpook National University, Sangju, Korea, 2012. [Google Scholar]
- Jeon, J.H.; Roh, S.J.; Shin, S.B.; Shin, Y.M.; Son, J.D.; Park, S.W.; Byun, B.K. Insect fauna of the scheduled regeneration forests in Samcheok, Gangwon-do, Korea in summer. J. Asia-Pac. Biodivers. 2014, 7, e72–e75. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Cho, Y.C.; Byun, B.K.; Kim, H.J.; Bae, K.H.; Kim, H.S.; Kim, J.S. Initial responses of vegetation regeneration after strip clear cutting in secondary Korean red pine (Pinus densiflora) forest in Samcheok, Gangwon-do, South Korea. Korean J. Environ. Ecol. 2015, 29, 785–790. [Google Scholar] [CrossRef]
- Roh, S.J.; Son, J.D.; Jeon, J.H. Initial change of Coleopteran insect community affected by anthropogenic disturbances within pine tree forest. Korean J. Environ. Ecol. 2015, 29, 539–551. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, S.W.; Roh, S.J.; Jeon, J.H.; Yoo, T.-H.; Yoon, H.-K.; Kim, H.-S.; Byun, B.-K. Responses of bark beetle (Coleoptera: Curculionidae: Scolytinae) community structure to green-tree retention in pine tree forest from Korea. J. Asia-Pac. Biodivers. 2016, 9, 443–447. [Google Scholar] [CrossRef]
- Niemelä, J.; Spence, J.R.; Langor, D.W.; Haila, Y.; Tukia, H. Logging and boreal ground-beetle assemblages on two continents: Implications for conservation. In Perspectives in Insect Conservation; Intercept Ltd.: Andover, UK, 1993; pp. 29–50. [Google Scholar]
- Hill, J.K.; Hamer, K.C.; Lace, L.A.; Banham, W.M.T. Effects of selective logging on tropical forest butterflies on Buru, Indonesia. J. Appl. Ecol. 1995, 32, 754–760. [Google Scholar] [CrossRef]
- Chey, V.K.; Holloway, J.D.; Speight, M.R. Diversity of moths in forest plantations and natural forests in Sabah. Bull. Entomol. Res. 1997, 87, 371–385. [Google Scholar] [CrossRef]
- Park, Y.-S.; Park, Y.K.; Yang, H.M. Effects of clear-cutting on forest arthropod communities at two different vertical levels (Crown and Ground Surface). Korean J. Ecol. Environ. 2016, 49, 271–278. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Tahvonen, O.; Aakala, T. Even-aged and uneven-aged forest management in boreal fennoscandia: A review. Ambio 2012, 41, 720–737. [Google Scholar] [CrossRef] [PubMed]
- Kitching, R.L.; Orr, A.G.; Thalib, L.; Mitchell, H.; Hopkins, M.S.; Graham, A.W. Moth assemblages of environmental as indicators in remnants of upland Australian rain forest quality. J. Appl. Ecol. 2000, 37, 284–297. [Google Scholar] [CrossRef]
- Summerville, K.S.; Ritter, L.M.; Crist, T.O. Forest moth taxa as indicators of lepidopteran richness and habitat disturbance: A preliminary assessment. Biol. Conserv. 2004, 116, 9–18. [Google Scholar] [CrossRef]
- Summerville, K.S. Do smaller forest fragments contain a greater abundance of lepidopteran crop and forage consumers? Environ. Entomol. 2004, 33, 234–241. [Google Scholar] [CrossRef]
- Hilt, N.; Brehm, G.; Fiedler, K. Diversity and ensemble composition of geometrid moths along a successional gradient in the Ecuadorian Andes. J. Trop. Ecol. 2006, 22, 155–166. [Google Scholar] [CrossRef]
- Summerville, K.S.; Crist, T.O. Structure and conservation of lepidopteran communities in managed forests of northeastern North America: A review. Can. Entomol. 2008, 140, 475–494. [Google Scholar] [CrossRef]
- Sayama, K.; Ito, M.; Tabuchi, K.; Ueda, A.; Ozaki, K.; Hironaga, T. Seasonal trends of forest moth assemblages in Central Hokkaido, Northern Japan. J. Lepidopterists’ Soc. 2012, 66, 11–26. [Google Scholar] [CrossRef]
- Sanyal, A.K.; Uniyal, V.P.; Chandra, K.; Bhardwaj, M. Diversity, distribution pattern and seasonal variation in moth assemblages along altitudinal gradient in Gangotri landscape area, Western Himalaya, Uttarakhand, India. J. Threat. Taxa 2013, 5, 3646–3653. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K. Seasonal fluctuation of moth community in Tomakomai experimental forest of Hokkaido University. Res. Bull. Coll. Exp. For. Coll. Agric. Hokkaido Univ. 1980, 37, 675–685. [Google Scholar]
- Yela, J.L.; Herrera, C.M. Seasonality and life cycles of woody plant-feeding noctuid moths (Lepidoptera: Noctuidae) in Mediterranean habitats. Ecol. Entomol. 1993, 18, 259–269. [Google Scholar] [CrossRef]
- Butler, L.; Kondo, V.; Strazanac, J. Light trap catches of Lepidoptera in two central Appalachian forests. Proc. Entomol. Soc. Wash. 2001, 103, 879–902. [Google Scholar]
- Feeny, P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 1970, 51, 565–581. [Google Scholar] [CrossRef]
- Niemela, P.; Haukioja, E. Seasonal patterns in species richness of herbivores: Macrolepidopteran larvae on Finnish deciduous trees. Ecol. Entomol. 1982, 7, 169–175. [Google Scholar] [CrossRef]
- Butler, L.; Strazanc, J. Occurrence of Lepidoptera on selected host trees in two central Appalachian national forests. Ann. Entomol. Soc. Am. 2000, 93, 500–511. [Google Scholar] [CrossRef]
- Summerville, K.S.; Crist, T.O.; Kahn, J.K.; Gering, J.C. Community structure of arboreal caterpillars within and among four tree species of the eastern deciduous forest. Ecol. Entomol. 2003, 28, 747–757. [Google Scholar] [CrossRef]
- Murakami, M.; Yoshida, K.; Hara, H.; Toda, M.J. Spatio-temporal variation in Lepidopteran larval assemblages associated with oak, Quercus crispula: The importance of leaf quality. Ecol. Entomol. 2005, 30, 521–531. [Google Scholar] [CrossRef]
- Hunter, M.D.; McNeil, J.N. Host-plant quality influences diapause and voltinism in a polyphagous insect herbivore. Ecology 1997, 78, 977–986. [Google Scholar] [CrossRef]
- Nock, C.A.; Vogt, R.J.; Beisner, B.E. Functional Traits. ELS; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–8. [Google Scholar]
- Brown, L.E.; Khamis, K.; Wilkes, M.; Blaen, P.; Brittain, J.E.; Carrivick, J.L.; Fell, S.; Friberg, N.; Füreder, L.; Gislason, G.M.; et al. Functional diversity and community assembly of river invertebrates show globally consistent responses to decreasing glacier cover. Nat. Ecol. Evol. 2018, 2, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Navas, M.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Díaz, S.; Purvis, A.; Cornelissen, J.H.C.; Mace, G.M.; Donoghue, M.J.; Ewers, R.M.; Jordano, P.; Pearse, W.D. Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecol. Evol. 2013, 3, 2958–2975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gossner, M.M.; Lachat, T.; Brunet, J.; Isacsson, G.; Bouget, C.; Brustel, H.; Brandl, R.; Weisser, W.W.; Müller, J. Current near-to-nature forest management effects on functional trait composition of saproxylic beetles in beech forests. Conserv. Biol. 2013, 27, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Choi, K.-S.; Lyu, D.-P.; Lee, J.-S.; Lim, J.; Lee, S.; Shin, S.-C.; Chung, Y.-J.; Park, Y.-S. Seasonal changes of functional groups in coleopteran communities in pine forests. Biodivers. Conserv. 2010, 19, 2291–2305. [Google Scholar] [CrossRef]
- Schmidt, B.C.; Roland, J. Moth diversity in a fragmented habitat: Importance of functional groups and landscape scale in the boreal forest. Ann. Entomol. Soc. Am. 2006, 99, 1110–1120. [Google Scholar] [CrossRef]
- Baek, W.; Park, W.; Lee, W. Flora and vegetation of resources plants in the Mt. Kariwang (Kangwon-do). Korean J. Plant Res. 1998, 11, 217–243. [Google Scholar]
- Shin, Y.H. Coloured Illustrations of the Moths of Korea; Academybook: Seoul, Korea, 2001. [Google Scholar]
- Heo, U.H. Guide Book of Moth Larvae; Econature Publishing Co.: Seoul, Korea, 2012. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Science: Oxford, UK, 2004. [Google Scholar]
- Everitt, B.S. Cluster analysis: A brief discussion of some of the problems. Br. J. Psychiatr. 1972, 120, 143–145. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Ward, J.; Joe, H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Kruskal, J.B. Nonmetric multidimensional scaling: A numerical method. Psychometrika 1964, 29, 115–129. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package, Version 2.5-2, 2018, The Comprehensive R Archive Network. Available online: http://cran.r-project.org/web/packages/vegan (accessed on 17 May 2018).
- Intachat, J.; Holloway, J.D.; Speight, M.R. The effects of different forest management practices on geometroid moth populations and their diversity in Peninsular Malaysia. J. Trop. For. Sci. 1997, 9, 411–430. [Google Scholar]
- Willott, S.J. The effects of selective logging on the distribution of moths in a Bornean rainforest. Philos. Trans. R. Soc. Lond. 1999, 354, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Lack, D. The numbers of bird species on islands. Bird Study 1969, 16, 193–209. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.S., Jr. Conservation of neotropical environments: Insects as indicators. In The Conservation of Insects and Their Habitats; Collins, N.M., Thomas, J.A., Eds.; Academic Press: London, UK, 1991; pp. 349–404. [Google Scholar]
- Brubaker, L.B. Long-term forest dynamics. In Forest Succession; West, D.C., Shugart, H.H., Botkin, D.B., Eds.; Springer: New York, NY, USA, 1981; pp. 95–106. [Google Scholar]
- Janzen, D.H.; Waterman, P.G. A seasonal census of Phenolics, Fiber and Alkaloids in foliage of forest trees in Costa-Rica: Some factors influencing their distribution and relation to host selection by Sphingidae and Saturniidae. Biol. J. Linn. Soc. 1984, 21, 439–454. [Google Scholar] [CrossRef]
- Murakami, M.; Ichie, T.; Hirao, T. Beta-diversity of lepidopteran larval communities in a Japanese temperate forest: Effects of phenology and tree species. Ecol. Res. 2008, 23, 179–187. [Google Scholar] [CrossRef]
- Gustafsson, L.; Baker, S.C.; Bauhus, J.; Beese, W.J.; Brodie, A.; Kouki, J.; Lindenmayer, D.B.; Lõhmus, A.; Martínez Pastur, G.; Messier, C.; et al. Retention forestry to maintain multifunctional forests: A world perspective. Bioscience 2012, 62, 633–645. [Google Scholar] [CrossRef]
- Fedrowitz, K.; Koricheva, J.; Baker, S.C.; Lindenmayer, D.B.; Palik, B.; Rosenvald, R.; Beese, W.; Franklin, J.F.; Kouki, J.; Macdonald, E.; et al. Can retention forestry help conserve biodiversity? A meta-analysis. J. Appl. Ecol. 2014, 51, 1669–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halaj, J.; Halpern, C.B.; Yi, H. Effects of green-tree retention on abundance and guild composition of corticolous arthropods. For. Ecol. Manag. 2009, 258, 850–859. [Google Scholar] [CrossRef]
- Axmacher, J.C.; Tünte, H.; Schrumpf, M.; Müller-hohenstein, K.; Lyaruu, H.V.M.; Fiedler, K. Diverging diversity patterns of vascular plants and geometrid moths during forest regeneration on Mt Kilimanjaro, Tanzania. J. Biogeogr. 2004, 31, 895–904. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Ostertag, R. Neotropical secondary forest succession: Changes in structural and functional characteristics. For. Ecol. Manag. 2001, 148, 185–206. [Google Scholar] [CrossRef]


| Study Site (Stand) | Acronym | Altitude (m) | Area (ha) | Dominant Vegetation |
|---|---|---|---|---|
| Clearcutting | CC | 980 | 5 | Actinidia polygama, Persicaria nepalensis |
| Aggregated retention | AR | 950 | 5 | Magnolia sieboldii K. Koch, Actinidia polygama, Persicaria nepalensis, |
| Japanese larch (no cutting) | NC | 910 | 5 | Larix kaempferi, Acer pseudosieboldianum var. ambiguum Nakai, Commelina communis L., Actinidia polygama |
| Mongolian oak (no cutting) | OA | 950 | 5 | Quercus dentate Thunb., Actinidia polygama, Ainsliaea acerifolia var. subapoda Nakai |
| Community Index | Treatment | May | June | July | August | September | October | Total |
|---|---|---|---|---|---|---|---|---|
| Species richness | CC a | 8 | 20 | 32 | 21 | 24 | 0 | 86 |
| AR | 2 | 16 | 28 | 17 | 22 | 0 | 67 | |
| NC | 9 | 36 | 33 | 24 | 30 | 2 | 108 | |
| OA | 13 | 57 | 87 | 54 | 44 | 4 | 201 | |
| Overall | 24 | 84 | 122 | 85 | 74 | 6 | 301 | |
| Abundance | CC | 8 | 40 | 96 | 71 | 86 | 0 | 301 |
| AR | 3 | 52 | 67 | 52 | 56 | 0 | 230 | |
| NC | 24 | 135 | 91 | 74 | 57 | 2 | 383 | |
| OA | 36 | 418 | 576 | 171 | 232 | 11 | 1444 | |
| Overall | 71 | 645 | 830 | 368 | 431 | 13 | 2358 | |
| Shannon diversity | CC | 2.08 | 2.46 | 2.57 | 2.01 | 2.53 | 0.00 | 3.19 |
| AR | 0.64 | 2.14 | 2.55 | 1.76 | 2.33 | 0.00 | 2.95 | |
| NC | 1.90 | 2.89 | 2.96 | 2.23 | 3.05 | 0.69 | 3.69 | |
| OA | 1.93 | 2.64 | 3.1 | 2.87 | 2.83 | 1.16 | 3.65 | |
| Overall | 2.56 | 2.95 | 3.24 | 2.86 | 3.20 | 1.52 | 3.84 |
| Family | Species | Dominant Species a | Biological Trait | ||||
|---|---|---|---|---|---|---|---|
| CC b | AR | NC | OA | Host Plant c | Distribution Pattern d | ||
| Noctuidae | Xestia fuscostigma | 2 | 5 | HR | M | ||
| Athetis gluteosa | 3 | HR | N | ||||
| Sineugraphe exusta | 4 | Unknown | N | ||||
| Geometridae | Idaea biselata | 5 | 1 | 3 | HR/TS | M | |
| Eustroma melancholicum | 2 | SR | M | ||||
| Alcis angulifera | 4 | 1 | 4 | HR/SR/TS/LC | M | ||
| Duliophyle agitata | 4 | SR/TS | M | ||||
| Erebidae | Paracolax tristalis | 1 | 3 | 5 | 2 | SR/TS | N |
| Hydrillodes pacifica | 2 | 1 | Unknown | S | |||
| Zanclognatha Griselda | 3 | TS | N | ||||
| Zanclognatha lunalis (Scopoil) | 5 | SR/TS | N | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, M.Y.; Kim, S.-S.; Lee, D.-S.; Yang, H.M.; Park, C.-W.; Kim, H.S.; Park, Y.-S. Effects of Forest Management Practices on Moth Communities in a Japanese Larch (Larix kaempferi (Lamb.) Carrière) Plantation. Forests 2018, 9, 574. https://doi.org/10.3390/f9090574
Moon MY, Kim S-S, Lee D-S, Yang HM, Park C-W, Kim HS, Park Y-S. Effects of Forest Management Practices on Moth Communities in a Japanese Larch (Larix kaempferi (Lamb.) Carrière) Plantation. Forests. 2018; 9(9):574. https://doi.org/10.3390/f9090574
Chicago/Turabian StyleMoon, Mi Young, Sung-Soo Kim, Dae-Seong Lee, Hee Moon Yang, Chan-Woo Park, Hyun Seop Kim, and Young-Seuk Park. 2018. "Effects of Forest Management Practices on Moth Communities in a Japanese Larch (Larix kaempferi (Lamb.) Carrière) Plantation" Forests 9, no. 9: 574. https://doi.org/10.3390/f9090574





