Description of a Natural Infection with Decapod Iridescent Virus 1 in Farmed Giant Freshwater Prawn, Macrobrachium rosenbergii
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. DNA and RNA Extraction
2.3. Pathogen Detection
2.4. Histopathological Sections
2.5. In Situ Digoxigenin-Labeled Loop-Mediated Isothermal Amplification (ISDL)
2.6. Transmission Electron Microscopy (TEM)
2.7. Quantitative Detection of DIV1 in Different Tissues of Naturally Infected M. rosenbergii
2.8. Relative Abundance of DIV1 in Different Tissues
3. Results
3.1. Observation of Clinical Signs of Diseaesd M. rosenbergii
3.2. Pathogen Detection of Samples
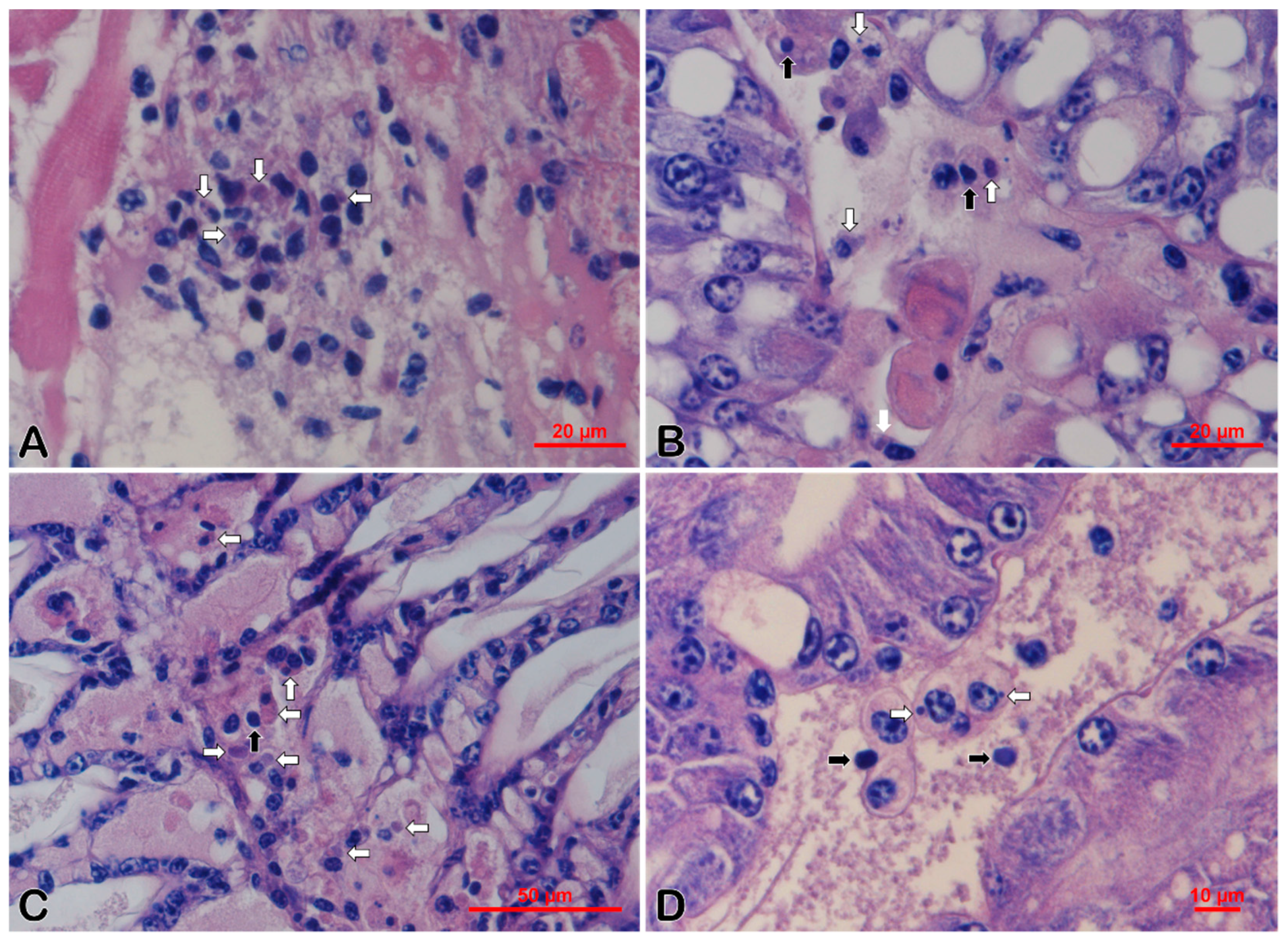
3.3. Histopathology
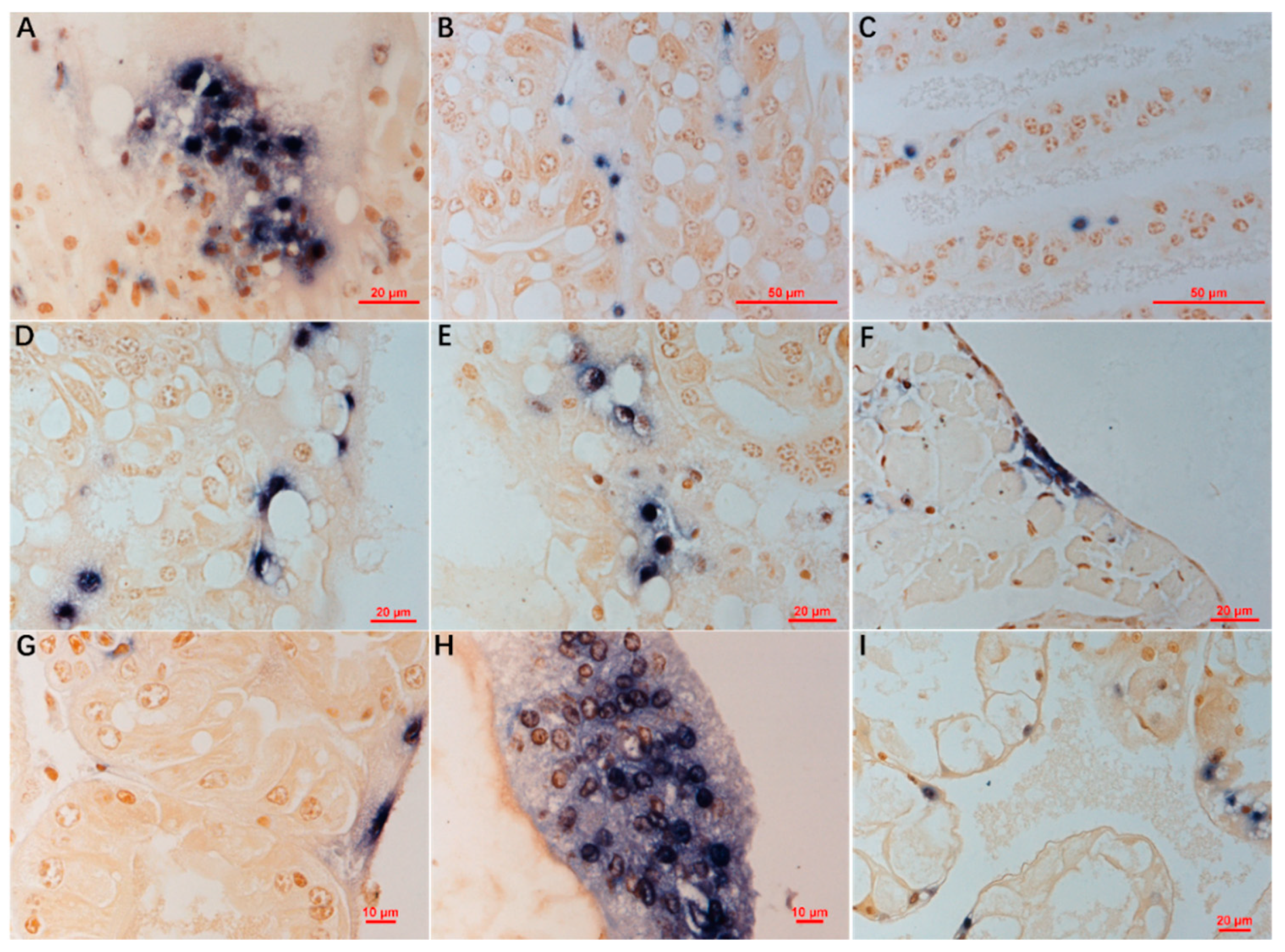
3.4. ISDL
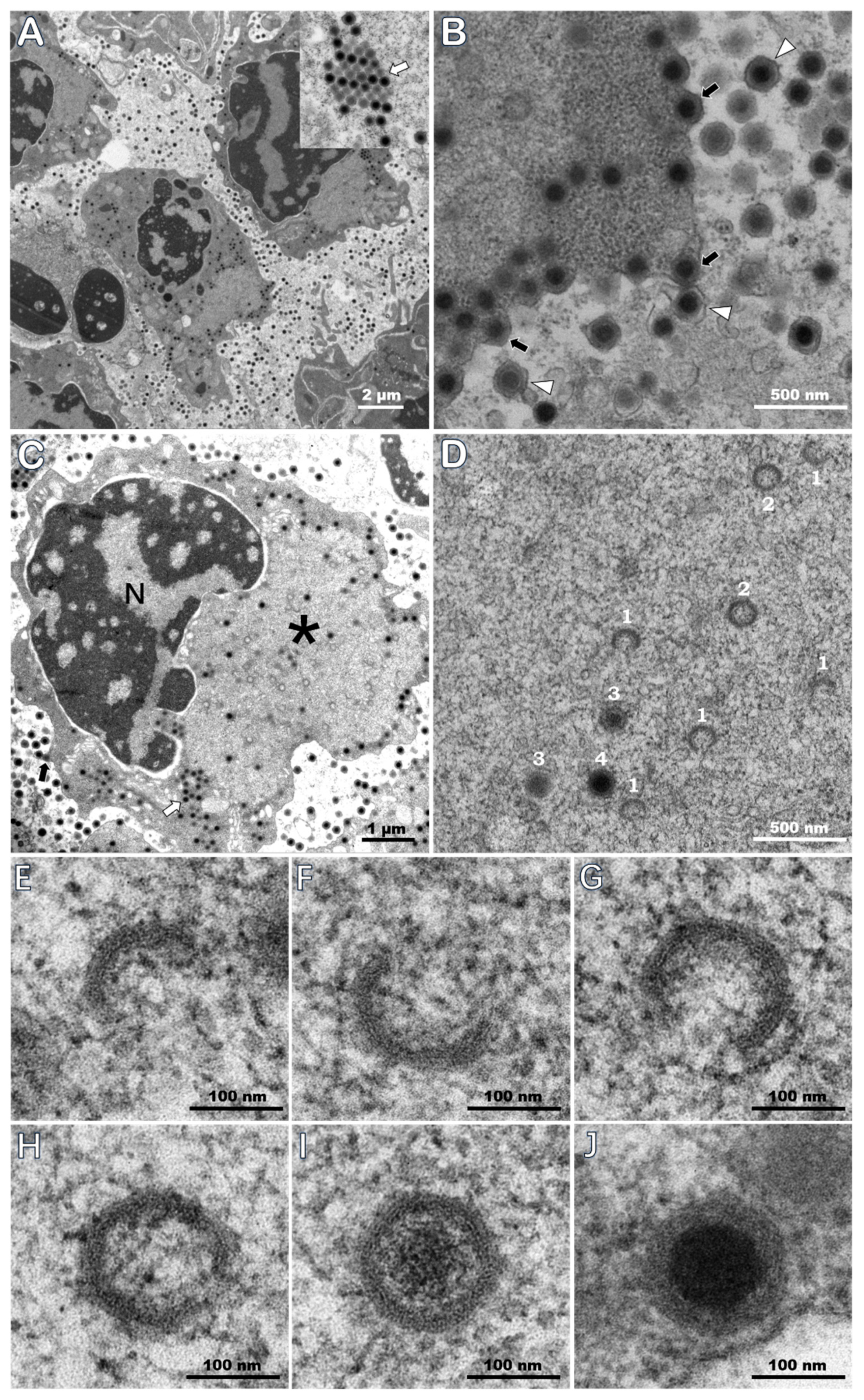
3.5. TEM of Ultrathin Sections
3.6. Quantitative Detection of DIV1 in Different Tissues of Naturally Infected M. rosenbergii
3.7. Relative Abundance of DIV1 in Different Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, L.; Wang, T.; Li, F.; Yang, F. Isolation and preliminary characterization of a new pathogenic iridovirus from redclaw crayfish Cherax quadricarinatus. Dis. Aquat. Organ. 2016, 120, 17–26. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, M.M.; Wan, X.Y.; Li, C.; Zhang, Q.L.; Wang, R.Y.; Cheng, D.Y.; Dong, X.; Yang, B.; Wang, X.H.; et al. Characterization of a new member of Iridoviridae, Shrimp hemocyte iridescent virus (SHIV), found in white leg shrimp (Litopenaeus vannamei). Sci. Rep. 2017, 19, 11834. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, M.M.; Wang, R.Y.; Wan, X.Y.; Li, C.; Zhang, Q.L.; Dong, X.; Yang, B.; Xiang, J.H.; Huang, J. Complete genome sequence of shrimp hemocyte iridescent virus (SHIV) isolated from white leg shrimp, Litopenaeus vannamei. Arch. Virol. 2018, 163, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, L.; Yang, F. Genomic characterization of a novel iridovirus from redclaw crayfish Cherax quadricarinatus: Evidence for a new genus within the family Iridoviridae. J. Gen. Virol. 2017, 98, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Chen, M.M.; Wan, X.Y.; Zhang, Q.L.; Li, C.; Dong, X.; Yang, B.; Huang, J. Detection and quantification of shrimp hemocyte iridescent virus by TaqMan probe based real-time PCR. J. Invertebr. Pathol. 2018, 154, 95–101. [Google Scholar] [CrossRef] [PubMed]
- One New Genus with One New Species in the Subfamily Betairidovirinae. Available online: https://talk.ictvonline.org/files/ictv_official_taxonomy_updates_since_the_8th_report/m/animal-dna-viruses-and-retroviruses/8051 (accessed on 10 March 2019).
- Qiu, L.; Dong, X.; Wan, X.Y.; Huang, J. Analysis of iridescent viral disease of shrimp (SHID) in 2017. In Analysis of Important Diseases of Aquatic Animals in China in 2017; Fishery and Fishery Administration Bureau under the Ministry of Agriculture and Rural Affairs, National Fishery Technical Extension Center, Eds.; China Agriculture Press: Beijing, China, 2018; pp. 187–204. ISBN 978-7-109-24522-8. (In Chinese) [Google Scholar]
- Rao, R.; Bhassu, S.; Bing, R.Z.; Alinejad, T.; Hassan, S.S.; Wang, J. A transcriptome study on Macrobrachium rosenbergii hepatopancreas experimentally challenged with white spot syndrome virus (WSSV). J. Invertebr. Pathol. 2016, 136, 10–22. [Google Scholar] [CrossRef]
- Hameed, A.S.S. Viral infections of Macrobrachium spp.: Global status of outbreaks, diagnosis, surveillance, and research. Isr. J. Aquac. 2009, 61, 240–247. [Google Scholar]
- Ho, K.L.; Gabrielsen, M.; Beh, P.L.; Kueh, C.L.; Thong, Q.X.; Streetley, J.; Tan, W.S.; Bhella, D. Structure of the Macrobrachium rosenbergii nodavirus: A new genus within the Nodaviridae? PLoS Biol. 2018, 16, e3000038. [Google Scholar] [CrossRef]
- Macrobrachium rosenbergii (De Man, 1879). Available online: http://www.fao.org/fishery/culturedspecies/Macrobrachium_rosenbergii/en (accessed on 9 January 2019).
- Bonami, J.R.; Sri Widada, J. Viral diseases of the giant fresh water prawn Macrobrachium rosenbergii: A review. J. Invertebr. Pathol. 2011, 106, 131–142. [Google Scholar] [CrossRef]
- Sahul Hameed, A.S.; Bonami, J.R. White tail disease of freshwater prawn, Macrobrachium rosenbergii. Indian J. Virol. 2012, 23, 134–140. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Xu, T.T.; Wan, X.Y.; Liu, S.; Wang, X.H.; Li, X.P.; Dong, X.; Yang, B.; Huang, J. Prevalence and distribution of Covert mortality nodavirus (CMNV) in cultured crustacean. Virus. Res. 2017, 233, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Who Is the Ringleader of the “White Spot” Disease in Macrobrachium rosenbergii. Available online: http://www.shuichan.cc/article_view-41757.html (accessed on 9 January 2019). (In Chinese).
- Huang, S. Polyculture of Macrobrachium rosenbergii and Litopenaeus vannamei Will Become the Main Mixed Culture Mode in Chaozhou and Shantou of Guangdong Province. Chinese Aquaculture Gateway Web. 2015. Available online: http://www.bbwfish.com/article.asp?artid=173520 (accessed on 9 January 2019). (In Chinese).
- Manual of Diagnostic Tests for Aquatic Animals. Available online: http://www.oie.int/en/standard-setting/aquatic-manual/access-online/ (accessed on 11 September 2018).
- Zhang, Q.L.; Liu, Q.; Liu, S.; Yang, H.L.; Liu, S.; Zhu, L.L.; Yang, B.; Jin, J.T.; Ding, L.X.; Wang, X.H.; et al. A new nodavirus is associated with covert mortality disease of shrimp. J. Gen. Virol. 2014, 95, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.A.; Lightner, D.V. A Handbook of Normal Penaeid Shrimp Histology; World Aquaculture Society: Tucson, AZ, USA, 1988. [Google Scholar]
- Chen, X.; Qiu, L.; Wang, H.L.; Zou, P.Z.; Dong, X.; Li, F.H.; Huang, J. Susceptibility of Exopalaemon carinicauda to the infection with Shrimp hemocyte iridescent virus (SHIV 20141215), a strain of Decapod iridescent virus 1 (DIV1). Viruses 2019. accepted. [Google Scholar]
- Huang, C.H.; Shi, Z.L.; Zhang, J.H.; Zhang, L.R.; Chen, D.H.; Bonami, J.R. Establishment of a model for proliferating White spot syndrome virus in vivo. Virol. Sin. 1999, 14, 358–363. [Google Scholar]
- Corteel, M.; Dantas-Lima, J.J.; Tuan, V.V.; Thuong, K.V.; Wille, M.; Alday-Sanz, V.; Pensaert, M.B.; Sorgeloos, P.; Nauwynck, H.J. Susceptibility of juvenile Macrobrachium rosenbergii to different doses of high and low virulence strains of white spot syndrome virus (WSSV). Dis. Aquat. Org. 2012, 100, 211–218. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Fu, H.T.; Sun, S.M.; Qiao, H.; Zhang, W.Y.; Jin, S.B.; Jiang, S.F.; Xiong, Y.W.; Gong, Y.S. Experimental inoculation of oriental river prawn Macrobrachium nipponense with white spot syndrome virus (WSSV). Dis. Aquat. Org. 2017, 126, 125–134. [Google Scholar] [CrossRef]
- Sarathi, M.; Nazeer Basha, A.; Ravi, M.; Venkatesan, C.; Senthil Kumar, B.; Sahul Hameed, A.S. Clearance of white spot syndrome virus (WSSV) and immunological changes in experimentally WSSV-injected Macrobrachium rosenbergii. Fish Shellfish Immunol. 2008, 25, 222–230. [Google Scholar] [CrossRef]
- Shen, Y.J.; Zhu, B.K.; Xu, K.C.; Xu, J.F. Pond culture experiment of polyculture with white leg shrimp and Macrobrachium rosenbergii. Xiandai Nongye Keji 2017, 17, 231–241. (In Chinese) [Google Scholar]
- Azim, M.E.; Mazid, M.A.; Alam, M.J.; Nurullah, M. The potential of mixed culture of freshwater giant prawn Macrobrachium rosenbergii de Man and tiger shrimp Penaeus monodon Fab. at Khulna region, Bangladesh. Bangladesh J. Fish. Res. 2001, 5, 67–74. [Google Scholar]
- Ali, H.; Rahman, M.M.; Rico, A.; Jaman, A.; Basak, S.K.; Islam, M.M.; Khan, N.; Keus, H.J.; Mohan, C.V. An assessment of health management practices and occupational health hazards in tiger shrimp (Penaeus monodon) and freshwater prawn (Macrobrachium rosenbergii) aquaculture in Bangladesh. Vet. Ani. Sci. 2018, 5, 10–19. [Google Scholar] [CrossRef]
- Sadek, S.; Moreau, J. Performance of Macrobrachium rosenbergii and Penaeus semisulcatus under mono and mixed culture systems, and their suitability for polyculture with Florida Red Tilapia, in Egypt. J. Aquacult. Trop. 2000, 15, 97–107. [Google Scholar]
- Diseases of Farmed Shrimp and Their Biosecurity Control Technologies. Available online: http://www.shuichan.cc/news_view-268040.html (accessed on 9 February 2019). (In Chinese).
- Escobedo-Bonilla, C.M.; Alday-Sanz, V.; Wille, M.; Sorgeloos, P.; Pensaert, M.B.; Nauwynck, H.J. A review on the morphology, molecular characterization, morphogenesis and pathogenesis of White spot syndrome virus. J. Fish. Dis. 2008, 31, 1–18. [Google Scholar] [CrossRef]
- Lightner, D.V.; Redman, R.M. A putative iridovirus from the penaeid shrimp Protrachypene precipua Burkenroad (Crustacea: Decapoda). J. Invertebr. Pathol. 1993, 62, 107–109. [Google Scholar] [CrossRef]
- Tang, K.F.J.; Redman, R.M.; Pantoja, C.R.; Groumellec, M.L.; Duraisamy, P.; Lightner, D.V. Identification of an iridovirus in Acetes erythraeus, (Sergestidae) and the development of in situ, hybridization and PCR method for its detection. J. Invertebr. Pathol. 2007, 96, 255–260. [Google Scholar] [CrossRef]
- Mahardika, K.; Yamamoto, A.; Miyazaki, T. Susceptibility of juvenile humpback grouper Cromileptes altivelis to grouper sleepy disease iridovirus (GSDIV). Dis. Aquat. Org. 2004, 59, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sudthongkong, C.; Miyata, M.; Miyazaki, T. Iridovirus disease in two ornamental tropical freshwater fishes: African lampeye and dwarf gourami. Dis. Aquat. Org. 2002, 48, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G.; Hick, P.; Ince, I.A.; Jancovich, J.K.; Marschang, R.; Qin, Q.; Subramaniam, K.; Waltzek, T.B.; Whittington, R.; Williams, T.; et al. ICTV Virus Taxonomy Profile: Iridoviridae. J. Gen. Virol. 2017, 98, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G.; Hyatt, A.; Miyazaki, T.; Williams, T. Family Iridoviridae: Poor viral relations no longer. Curr. Top. Microbiol. Immunol. 2009, 328, 123–170. [Google Scholar] [CrossRef]
- Williams, T.; Barbosa-Solomieu, V.; Chinchar, V.G. A decade of advances in iridovirus research. Adv. Virus Res. 2005, 65, 173–248. [Google Scholar] [CrossRef]
- Jitrakorn, S.; Arunrut, N.; Sanguanrut, P.; Flegel, T.W.; Kiatpathomchai, W.; Saksmerprome, V. In situ DIG-labeling, loop-mediated DNA amplification (ISDL) for highly sensitive detection of infectious hypodermal and hematopoietic necrosis virus (IHHNV). Aquaculture 2016, 456, 36–43. [Google Scholar] [CrossRef]
- Liu, Y.; Tran, B.N.; Wang, F.; Ounjai, P.; Wu, J.; Hew, C.L. Visualization of assembly intermediates and budding vacuoles of Singapore grouper iridovirus in grouper embryonic cells. Sci. Rep. 2016, 6, 18696. [Google Scholar] [CrossRef] [PubMed]


| Species | Positive Samples | Total Samples | Geometric Mean (Copies/μg-DNA) | DIV1 Range (Copies/μg-DNA) |
|---|---|---|---|---|
| M. rosenbergii | 5 | 5 | 10(8.65 ± 0.21) | 3.16 × 108–9.83 × 108 |
| P. vannamei | 3 | 3 | 10(5.96 ± 0.79) | 4.56 × 105–7.19 × 106 |
| M. nipponense | 3 | 3 | 10(4.17 ± 1.68) | 1.30 × 103–1.30 × 106 |
| Pr. clarkii | 5 | 5 | 10(3.82 ± 0.36) | 2.20 × 103–1.57 × 104 |
| Cladocera | 3 | 3 | 10(1.10 ± 0.06) | 1.09 × 101–1.43 × 101 |
| M. superbum | 1 | 1 | 10(1.04 ± 0.05) | 1.00 × 101–1.18 × 101 |
| Samples | n | Geometric Mean (Copies/μg-DNA) | Range (Copies/µg-DNA) |
|---|---|---|---|
| Hematopoietic tissue | 15 | 10(7.92 ± 0.91) | 8.11 × 104–6.33 × 108 |
| Antenna | 15 | 10(7.84 ± 0.70) | 1.44 × 106–1.03 × 109 |
| Uropods | 15 | 10(7.74 ± 0.71) | 6.45 × 105–6.04 × 108 |
| Pleopods | 15 | 10(7.77 ± 0.69) | 4.77 × 105–5.30 × 108 |
| Gills | 15 | 10(7.69 ± 0.66) | 4.45 × 105–4.10 × 108 |
| Pereiopod | 15 | 10(7.62 ± 0.69) | 6.10 × 105–1.05 × 109 |
| Muscle | 15 | 10(6.96 ± 0.57) | 1.87 × 105–4.36 × 107 |
| Hepatopancreas | 15 | 10(6.85 ± 0.72) | 2.35 × 104–3.18 × 107 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Chen, X.; Zhao, R.-H.; Li, C.; Gao, W.; Zhang, Q.-L.; Huang, J. Description of a Natural Infection with Decapod Iridescent Virus 1 in Farmed Giant Freshwater Prawn, Macrobrachium rosenbergii. Viruses 2019, 11, 354. https://doi.org/10.3390/v11040354
Qiu L, Chen X, Zhao R-H, Li C, Gao W, Zhang Q-L, Huang J. Description of a Natural Infection with Decapod Iridescent Virus 1 in Farmed Giant Freshwater Prawn, Macrobrachium rosenbergii. Viruses. 2019; 11(4):354. https://doi.org/10.3390/v11040354
Chicago/Turabian StyleQiu, Liang, Xing Chen, Ruo-Heng Zhao, Chen Li, Wen Gao, Qing-Li Zhang, and Jie Huang. 2019. "Description of a Natural Infection with Decapod Iridescent Virus 1 in Farmed Giant Freshwater Prawn, Macrobrachium rosenbergii" Viruses 11, no. 4: 354. https://doi.org/10.3390/v11040354
APA StyleQiu, L., Chen, X., Zhao, R.-H., Li, C., Gao, W., Zhang, Q.-L., & Huang, J. (2019). Description of a Natural Infection with Decapod Iridescent Virus 1 in Farmed Giant Freshwater Prawn, Macrobrachium rosenbergii. Viruses, 11(4), 354. https://doi.org/10.3390/v11040354






