Outbreaks of Foot-and-Mouth Disease in Burundi, East Africa, in 2016, Caused by Different Serotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Serological Tests
2.2.1. Non-Structural Proteins (NSP) Antibody ELISA
2.2.2. Structural Proteins (SP) Antibody ELISA
2.3. Virus Identification and Characterization
2.3.1. Real-Time RT-PCR (rRT-PCR)
2.3.2. Virus Isolation and Antigen ELISA
2.3.3. Conventional RT-PCR, Cloning, and Sequencing
2.3.4. VP1 Phylogenetic Analysis
3. Results
3.1. Serological Analyses
3.1.1. Detection of Antibodies against Non-Structural Proteins of FMDV
3.1.2. Detection of Antibodies against Structural Proteins of FMDV
3.2. Virological Analyses
3.2.1. Real-Time RT-PCR, Virus Isolation, and Antigen ELISA
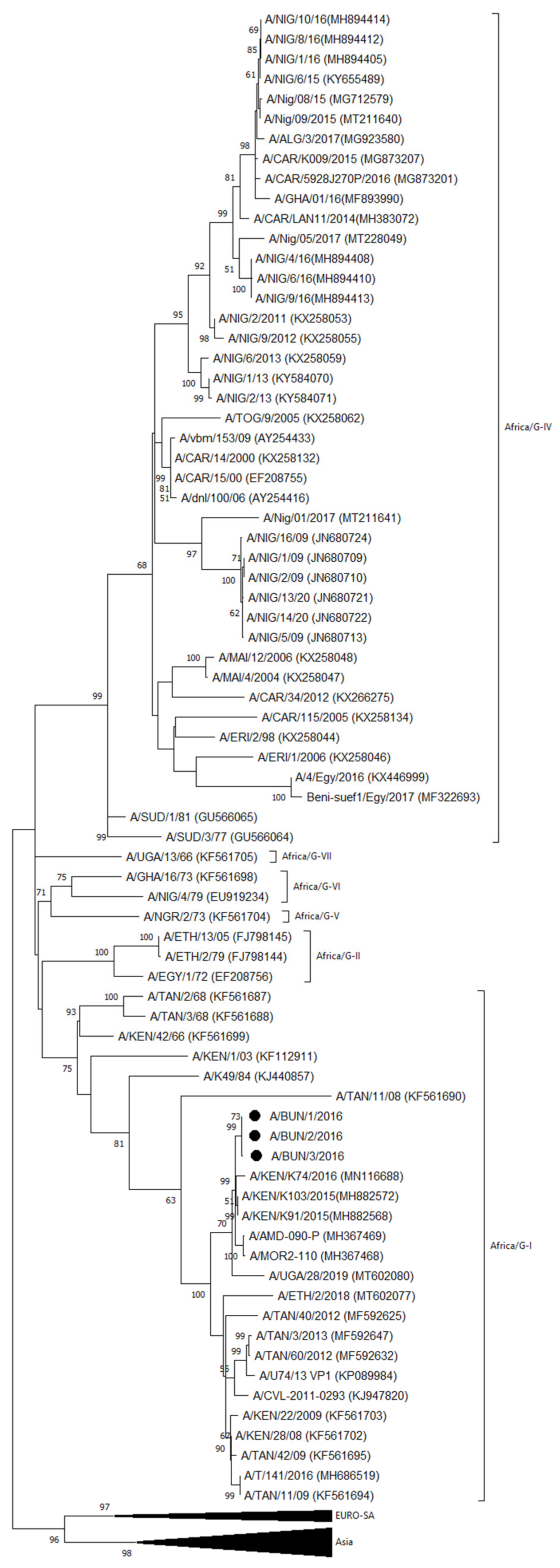
3.2.2. VP1 Sequencing and Phylogenetic Analysis
- Serotype A
- Serotype SAT2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamal, S.M.; Belsham, G.J. Foot-and-mouth disease: Past, present and future. Vet. Res. 2013, 44, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Organization for Animal Health (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Chapter 3.1.8: Foot and Mouth Disease (Infection with Foot and Mouth Disease Virus). Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.08_FMD.pdf (accessed on 17 March 2022).
- Mwiine, F.N.; Ayebazibwe, C.; Alexandersen, S.; Olaho-Mukani, W.; Okurut, A.R.A.; Tjørnehøj, K. Seroepidemiological investigation of foot-and-mouth disease virus serotypes in cattle around Lake Mburo National Park in South-Western Uganda. J. Vet. Med. Anim. Health 2010, 2, 46–54. [Google Scholar]
- Mwiine, F.N.; Ayebazibwe, C.; Olaho-Mukani, W.; Alexandersen, S.; Balinda, S.N.; Masembe, C.; Okurut, A.R.A.; Christensen, L.S.; Sørensen, K.J.; Tjørnehøj, K. Serotype specificity of antibodies against foot-and-mouth disease virus in cattle in selected districts in Uganda. Transbound. Emerg. Dis. 2010, 57, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, A.; Libeau, G.; Chardonnet, B.; Chardonnet, P.; Kock, R.A.; Parekh, K.; Hamblin, P.; Li, Y.; Parida, S.; Sumption, K.J. Serological profile of foot-and-mouth disease in wildlife populations of West and Central Africa with special reference to Syncerus caffer subspecies. Vet. Res. 2015, 46, 77. [Google Scholar] [CrossRef] [Green Version]
- Namatovu, A.; Belsham, G.J.; Ayebazibwe, C.; Dhikusooka, M.T.; Wekesa, S.N.; Siegismund, H.R.; Muwanika, V.B.; Tjørnehøj, K. Challenges for Serology-Based Characterization of Foot-and-Mouth Disease Outbreaks in Endemic Areas; Identification of Two Separate Lineages of Serotype O FMDV in Uganda in 2011. Transbound. Emerg. Dis. 2015, 62, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Paton, D.J.; Sumption, K.J.; Charleston, B. Options for control of foot-and-mouth disease: Knowledge, capability and policy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2657–2667. [Google Scholar] [CrossRef]
- FAO. Foot-and-Mouth Disease, April–June 2021, Quarterly Report. FAST Reports: Foot-and-Mouth and Similar Transboundary Animal Diseases. Rome. Available online: http://www.fao.org/3/cb5998en/cb5998en.pdf (accessed on 17 March 2022).
- Namatovu, A.; Tjørnehøj, K.; Belsham, G.J.; Dhikusooka, M.T.; Wekesa, S.N.; Muwanika, V.B.; Ayebazibwe, C. Characterization of Foot-And-Mouth Disease Viruses (FMDVs) from Ugandan Cattle Outbreaks during 2012-2013: Evidence for Circulation of Multiple Serotypes. PLoS ONE 2015, 10, e0114811. [Google Scholar] [CrossRef] [Green Version]
- Ularamu, H.G.; Lefebvre, D.J.; Haegeman, A.; Wungak, Y.S.; Ehizibolo, D.O.; Lazarus, D.D.; De Vleeschauwer, A.R.; De Clercq, K. Complex Circulation of Foot-and-Mouth Disease Virus in Cattle in Nigeria. Front. Vet. Sci. 2020, 7, 466. [Google Scholar] [CrossRef]
- Mwiine, F.N.; Velazquez-Salinas, L.; Ahmed, Z.; Ochwo, S.; Munsey, A.; Kenney, M.; Lutwama, J.J.; Maree, F.F.; Lobel, L.; Perez, A.M.; et al. Serological and phylogenetic characterization of foot and mouth disease viruses from Uganda during cross-sectional surveillance study in cattle between 2014 and 2017. Transbound. Emerg. Dis. 2019, 66, 2011–2024. [Google Scholar] [CrossRef]
- WRLFMD—Website of the World Reference Laboratory (WRL) for FMD. Available online: https://www.wrlfmd.org/country-reports (accessed on 17 March 2022).
- Sangula, A.K.; Siegismund, H.R.; Belsham, G.J.; Balinda, S.N.; Masembe, C.; Muwanika, V.B. Low diversity of foot-and-mouth disease serotype C virus in Kenya: Evidence for probable vaccine strain re-introductions in the field. Epidemiol. Infect. 2011, 139, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Paton, D.J.; Di Nardo, A.; Knowles, N.J.; Wadsworth, J.; Pituco, E.M.; Cosivi, O.; Rivera, A.M.; Kassimi, L.B.; Brocchi, E.; de Clercq, K.; et al. The history of foot-and-mouth disease virus serotype C: The first known extinct serotype? Virus Evol. 2021, 7, veab009. [Google Scholar] [CrossRef] [PubMed]
- AU-IBAR. The Livestock Development Strategy for Africa 2015–2035. Nairobi, Kenya. 2015. Available online: http://repository.au-ibar.org/bitstream/handle/123456789/540/2015-LiDeSA.pdf?sequence=1&isAllowed=y (accessed on 17 March 2022).
- FAO. Subregional Report on Animal Genetic Resources: East Africa. Annex to The State of the World’s Animal Genetic Resources for Food and Agriculture. Rome. 2007. Available online: http://www.fao.org/3/a1250e/annexes/Subregional%20Reports/Africa/East%20Africa.pdf (accessed on 17 March 2022).
- Cochet, H. Agrarian dynamics, population growth and resource management: The case of Burundi. GeoJournal 2004, 60, 111–122. [Google Scholar] [CrossRef]
- McElwain, T.F.; Thumbi, S.M. Animal pathogens and their impact on animal health, the economy, food security, food safety and public health. Rev. Sci. Tech. 2017, 36, 423–433. [Google Scholar] [CrossRef]
- Hatungumukama, G.; Hornick, J.L.; Detilleux, J. Aspects zootechniques de l’élevage bovin laitier au Burundi: Présent et futur. Ann. Méd. Vét. 2007, 151, 150–165. [Google Scholar]
- Sebushahu, T. Poultry Sector Country Review. Burundi. FAO. 2008. Version of 15th September 2011. Available online: https://www.fao.org/3/a-ak087e.pdf (accessed on 18 March 2022).
- Namatovu, A.; Wekesa, S.N.; Tjørnehøj, K.; Dhikusooka, M.T.; Muwanika, V.B.; Siegsmund, H.R.; Ayebazibwe, C. Laboratory capacity for diagnosis of foot-and-mouth disease in Eastern Africa: Implications for the progressive control pathway. BMC Vet. Res. 2013, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Burundi Eco. 423 Vaches Atteintes de Fièvre Aphteuse. 7 April 2016. Available online: http://burundi-eco.com/423-vaches-atteintes-de-fievre-aphteuse/#.WbMdZrpuIe1 (accessed on 19 March 2022).
- OIE-WAHIS (OIE World Animal Health Information System). Available online: https://wahis.oie.int/#/dashboards/qd-dashboard (accessed on 3 May 2022).
- Infos Grands Lacs. La Fièvre Aphteuse Frappe Certaines Provinces. 3 March 2016. Available online: https://www.infosgrandslacs.info/productions/la-fievre-aphteuse-frappe-certaines-provinces (accessed on 19 March 2022).
- Vosloo, W.; Bastos, A.D.S.; Sangare, O.; Hargreaves, S.K.; Thomson, G.R. Review of the status and control of foot and mouth disease in sub-Saharan Africa. Rev. Sci. Tech. 2002, 21, 437–449. [Google Scholar] [CrossRef]
- Sørensen, K.J.; De Stricker, K.; Dyrting, K.C.; Grazioli, S.; Haas, B. Differentiation of foot-and-mouth disease virus infected animals from vaccinated animals using a blocking ELISA based on baculovirus expressed FMDV 3ABC antigen and a 3ABC monoclonal antibody. Arch. Virol. 2005, 150, 805–814. [Google Scholar] [CrossRef]
- Goris, N.; De Clercq, K. Quality assurance/quality control of foot and mouth disease solid phase competition enzyme-linked immunosorbent assay—Part I. Quality assurance: Development of secondary and working standards. Rev. Sci. Tech. 2005, 24, 995–1004. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Lefebvre, D.J.; De Leeuw, I.; Van Borm, S.; De Clercq, K. Laboratory validation of two real-time RT-PCR methods with 5′-tailed primers for an enhanced detection of foot-and-mouth disease virus. J. Virol. Methods 2017, 246, 90–94. [Google Scholar] [CrossRef]
- Ehizibolo, D.O.; Haegeman, A.; De Vleeschauwer, A.R.; Umoh, J.U.; Kazeem, H.M.; Okolocha, E.C.; Van Borm, S.; De Clercq, K. Detection and molecular characterization of foot and mouth disease viruses from outbreaks in some states of Northern Nigeria 2013–2015. Transbound. Emerg. Dis. 2017, 64, 1979–1990. [Google Scholar] [CrossRef]
- Knowles, N.J.; Wadsworth, J.; Bachanek-Bankowska, K.; King, D.P. VP1 sequencing protocol for foot and mouth disease virus molecular epidemiology. Rev. Sci. Tech. 2016, 35, 741–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatusova, T.A.; Madden, T.L. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 1999, 174, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Kishino, H.; Yano, T. Dating the human-ape split by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- WRLFMD—Website of the World Reference Laboratory (WRL) for FMD. Available online: https://www.wrlfmd.org/sites/world/files/quick_media/WRLFMD-2021-00005-BAR-GTR-O-A_001.pdf (accessed on 29 March 2022).
- Palinski, R.M.; Sangula, A.; Gakuya, F.; Bertram, M.R.; Pauszek, S.J.; Hartwig, E.J.; Smoliga, G.R.; Obanda, V.; Omondi, G.; VanderWaal, K.; et al. Foot-and-Mouth Disease Virus Serotype A Genome Sequence from Kenya in 2016. Microbiol. Resour. Announc. 2019, 8, e00987-19. [Google Scholar] [CrossRef] [Green Version]
- Velazquez-Salinas, L.; Mwiine, F.N.; Ahmed, Z.; Ochwo, S.; Munsey, A.; Lutwama, J.J.; Perez, A.M.; VanderWaal, K.; Rieder, E. Genetic Diversity of Circulating Foot and Mouth Disease Virus in Uganda Cross-Sectional Study During 2014-2017. Front. Vet. Sci. 2020, 7, 162. [Google Scholar] [CrossRef]
- Sallu, R.S.; Kasanga, C.J.; Mathias, M.; Yongolo, M.; Mpelumbe-Ngeleja, C.; Mulumba, M.; Ranga, E.; Wambura, P.; Rweyemamu, M.; Knowles, N.; et al. Molecular survey for foot-and-mouth disease virus in livestock in Tanzania, 2008–2013. Onderstepoort J. Vet. Res. 2014, 81, E1–E6. [Google Scholar] [CrossRef]
- Casey-Bryars, M.; Reeve, R.; Bastola, U.; Knowles, N.J.; Auty, H.; Bachanek-Bankowska, K.; Fowler, V.L.; Fyumagwa, R.; Kazwala, R.; Kibona, T.; et al. Waves of endemic foot-and-mouth disease in eastern Africa suggest feasibility of proactive vaccination approaches. Nat. Ecol. Evol. 2018, 2, 1449–1457. [Google Scholar] [CrossRef]
- Palinski, R.M.; Sangula, A.; Gakuya, F.; Bertram, M.R.; Pauszek, S.J.; Hartwig, E.J.; Smoliga, G.R.; Obanda, V.; Omondi, G.; VanderWaal, K.; et al. Genome Sequences of Foot-and-Mouth Disease Virus SAT1 and SAT2 Strains from Kenya in 2014 to 2016. Microbiol. Resour. Announc. 2019, 8, e00809-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Hurtle, W.; Rowland, J.M.; Casteran, K.A.; Bucko, S.M.; Grau, F.R.; Valdazo-González, B.; Knowles, N.J.; King, D.P.; Beckham, T.R.; et al. Development of a universal RT-PCR for amplifying and sequencing the leader and capsid-coding region of foot-and-mouth disease virus. J. Virol. Methods 2013, 189, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.M.; Pierce, K.E.; Mistry, R.; Bharya, S.; Dukes, J.P.; Volpe, C.; Wangh, L.J.; King, D.P. Pan-serotypic detection of foot-and-mouth disease virus by RT linear-after-the-exponential PCR. Mol. Cell. Probes. 2010, 24, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.L.; Handel, I.G.; Tanya, V.N.; Hamman, S.M.; Nfon, C.; Bergman, I.E.; Malirat, V.; Sorensen, K.J.; Bronsvoort, B.M.d.C. Accuracy of herdsmen reporting versus serologic testing for estimating foot-and-mouth disease prevalence. Emerg. Infect. Dis. 2014, 20, 2048–2054. [Google Scholar] [CrossRef] [Green Version]
- FAO. Risk-Based Disease Surveillance—A Manual for Veterinarians on the Design and Analysis of Surveillance for Demonstration of Freedom from Disease; FAO Animal Production and Health Manual: Rome, Italy, 2014; No. 17. [Google Scholar]
- OIE; FAO. The Global Foot and Mouth Disease Control. Strategy—Strengthening Animal Health Systems through Improved Control of Major Diseases. Available online: https://www.oie.int/en/document/the-global-foot-and-mouth-disease-control-strategy-strengthening-animal-health-systems-through-improved-control-of-major-diseases/ (accessed on 4 May 2022).
- Armson, B.; Di Nardo, A.; Nyaguthii, D.M.; Sanz-Bernardo, B.; Kitala, P.M.; Chepkwony, E.; Mioulet, V.; King, D.P.; Lyons, N.A. Utilizing milk from pooling facilities as a novel approach for foot-and-mouth disease surveillance. Transbound. Emerg. Dis. 2020, 67, 1532–1542. [Google Scholar] [CrossRef] [Green Version]
- Garner, G.; Vosloo, W.; Tapsuwan, S.; Bradhurst, R.; Seitzinger, A.H.; Breed, A.C.; Capon, T. Comparing surveillance approaches to support regaining free status after a foot-and-mouth disease outbreak. Prev. Vet. Med. 2021, 194, 105441. [Google Scholar] [CrossRef]
- Mathijs, E.; Vandenbussche, F.; Van Borm, S. Using genomics for surveillance of veterinary infectious agents. Rev. Sci. Tech. 2016, 35, 143–157. [Google Scholar] [CrossRef]
- Fana, E.M.; Mpoloka, S.W.; Leteane, M.; Seoke, L.; Masoba, K.; Mokopasetso, M.; Rapharing, A.; Kabelo, T.; Made, P.; Hyera, J. A Five-Year Retrospective Study of Foot-and-Mouth Disease Outbreaks in Southern Africa, 2014 to 2018. Vet. Med. Int. 2021, 2021, 7438809. [Google Scholar] [CrossRef]
- Teye, M.V.; Sebunya, T.K.; Fana, E.M.; King, D.P.; Seoke, L.; Knowles, N.J.; Awuni, J.A.; Matlho, G.; Leteane, M.; Hyera, J.M.K. Foot-and-mouth disease in Southern Ghana: Occurrence and molecular characterization of circulating viruses. Trop. Anim. Health. Prod. 2019, 51, 1667–1677. [Google Scholar] [CrossRef]
- King, D.P.; Madi, M.; Mioulet, V.; Wadsworth, J.; Wright, C.F.; Valdazo-González, B.; Ferris, N.P.; Knowles, N.J.; Hammond, J. New technologies to diagnose and monitor infectious diseases of livestock: Challenges for sub-Saharan Africa. Onderstepoort J. Vet. Res. 2012, 79, 456. [Google Scholar] [CrossRef] [Green Version]
- Kitching, R.P. Clinical variation in foot and mouth disease: Cattle. Rev. Sci. Tech. 2002, 21, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Foglia, E.A.; Lembo, T.; Kazwala, R.; Ekwem, D.; Shirima, G.; Grazioli, S.; Brocchi, E.; Pezzoni, G. Combining Multiple Assays Improves Detection and Serotyping of Foot-and-Mouth Disease Virus. A Practical Example with Field Samples from East Africa. Viruses 2021, 13, 1583. [Google Scholar] [CrossRef]
- Hamblin, C.; Barnett, I.T.; Hedger, R.S. A new enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against foot-and-mouth disease virus. I. Development and method of ELISA. J. Immunol. Methods 1986, 93, 115–121. [Google Scholar] [CrossRef]
- Dhikusooka, M.T.; Tjørnehøj, K.; Ayebazibwe, C.; Namatovu, A.; Ruhweza, S.; Siegismund, H.R.; Wekesa, S.N.; Normann, P.; Belsham, G.J. Foot-and-mouth disease virus serotype SAT 3 in long-horned Ankole calf, Uganda. Emerg Infect. Dis. 2015, 21, 111–114. [Google Scholar] [CrossRef]
- Rweyemamu, M.; Roeder, P.; Mackay, D.; Sumption, K.; Brownlie, J.; Leforban, Y.; Valarcher, J.F.; Knowles, N.J.; Saraiva, V. Epidemiological patterns of foot-and-mouth disease worldwide. Transbound. Emerg. Dis. 2008, 55, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Tekleghiorghis, T.; Moormann, R.J.; Weerdmeester, K.; Dekker, A. Foot-and-mouth Disease Transmission in Africa: Implications for Control, a Review. Transbound. Emerg. Dis. 2016, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Maree, F.; Kasanga, C.; Scott, K.; Opperman, P.; Chitray, M.; Sangula, A.; Sallu, R.; Sinkala, Y.; Wambura, P.; King, D.; et al. Challenges and prospects for the control of foot-and-mouth disease: An African perspective. Vet. Med. 2014, 5, 119–138. [Google Scholar] [CrossRef] [Green Version]
- Di Nardo, A.; Knowles, N.J.; Paton, D.J. Combining livestock trade patterns with phylogenetics to help understand the spread of foot and mouth disease in sub-Saharan Africa, the Middle East and Southeast Asia. Rev. Sci. Tech. 2011, 30, 63–85. [Google Scholar] [CrossRef]
- Hammond, J.M.; Maulidi, B.; Henning, N. Targeted FMD Vaccines for Eastern Africa: The AgResults Foot and Mouth Disease Vaccine Challenge Project. Viruses 2021, 13, 1830. [Google Scholar] [CrossRef]
- GF-TADs FMD. Final Report of the 3rd East African Roadmap Meeting on the Foot-and-Mouth Disease Progressive Control Pathway (FMD-PCP), 3–5 July 2018. Available online: https://www.fao.org/3/CA1479EN/ca1479en.pdf (accessed on 6 April 2022).



| Type of Sample | Total | Provinces | |||||
|---|---|---|---|---|---|---|---|
| Bubanza | Bururi | Cankuzo | Cibitoke | Mwaro | Rutana | ||
| Saliva | 86 | 56 | 18 | 0 | 6 | 6 | 0 |
| Oral lesions * | 84 | 0 | 3 | 10 | 30 | 9 | 32 |
| Foot lesions | 23 | 0 | 0 | 17 | 2 | 1 | 3 |
| Total of tissue and/or saliva samples | 193 | 56 | 21 | 27 | 38 | 16 | 35 |
| Total of serum samples | 172 | 58 | 19 | 29 | 33 | 0 | 33 |
| Province | # Serum Samples Tested | # NSP Positive Samples | # SP Positive Samples per Serotype * | |||||
|---|---|---|---|---|---|---|---|---|
| O | A | C | SAT1 | SAT2 | SAT3 | |||
| Bubanza | 58 | 45 | 30 | 20 | 9 | 22 | 15 | 6 |
| Bururi | 19 | 19 | 13 | 7 | 2 | 4 | 19 | 5 |
| Cankuzo | 29 | 29 | 17 | 8 | 0 | 12 | 29 | 12 |
| Cibitoke | 33 | 28 | 12 | 23 | 12 | 4 | 5 | 5 |
| Rutana | 33 | 28 | 5 | 8 | 6 | 12 | 19 | 6 |
| Total | 172 | 149 | 77 | 66 | 29 | 54 | 87 | 34 |
| Province | # Positive/Animals Tested | Serotype in Antigen ELISA | ||||||
|---|---|---|---|---|---|---|---|---|
| rRT-PCR | Virus Isolation * | A | SAT2 | |||||
| saliva | oral lesion | foot lesion | saliva * | oral lesion * | foot lesion * | |||
| Bubanza | 2/56 | — | — | 0/0 | — | — | — | — |
| Bururi | 0/18 | 0/3 | — | 0/0 | — | — | — | — |
| Cankuzo | — | 3/10 | 5/17 | — | 0/0 | 0/1 | — | — |
| Cibitoke | 5/6 | 23/30 | 2/2 | 0/0 | 6/8 | 0/1 | 3/6 | 3/6 |
| Mwaro | 4/6 | 6/9 | 0/1 | 0/0 | 3/3 | 0/0 | 0/3 | 3/3 |
| Rutana | — | 26/32 | 3/3 | — | 9/19 | 0/3 | 0/9 | 9/9 |
| Total | 11/86 | 58/84 | 10/23 | 0/0 | 18/30 | 0/5 | 3/18 | 15/18 |
| 79/193 | 18/35 | |||||||
| Sample ID | Community | Province | FMDV Serotype/Topotype | GenBank Accession # |
|---|---|---|---|---|
| A/BUN/1/2016 | Rugombo | Cibitoke | A/Africa/G-I | OM817501 |
| A/BUN/2/2016 | Rugombo | Cibitoke | A/Africa/G-I | OM817502 |
| A/BUN/3/2016 | Rugombo | Cibitoke | A/Africa/G-I | OM817503 |
| SAT2/BUN/1/2016 | Rusaka | Mwaro | SAT2/IV | OM817504 |
| SAT2/BUN/2/2016 | Rusaka | Mwaro | SAT2/IV | OM817505 |
| SAT2/BUN/3/2016 | Rusaka | Mwaro | SAT2/IV | OM817506 |
| SAT2/BUN/4/2016 | Rugombo | Cibitoke | SAT2/IV | OM817507 |
| SAT2/BUN/5/2016 | Rugombo | Cibitoke | SAT2/IV | OM817508 |
| SAT2/BUN/6/2016 | Rugombo | Cibitoke | SAT2/IV | OM817509 |
| SAT2/BUN/7/2016 | Mpinga-Kayove | Rutana | SAT2/IV | OM817510 |
| SAT2/BUN/8/2016 | Mpinga-Kayove | Rutana | SAT2/IV | OM817511 |
| SAT2/BUN/9/2016 | Mpinga-Kayove | Rutana | SAT2/IV | OM817512 |
| SAT2/BUN/10/2016 | Mpinga-Kayove | Rutana | SAT2/IV | OM817513 |
| SAT2/BUN/11/2016 | Mpinga-Kayove | Rutana | SAT2/IV | OM817514 |
| SAT2/BUN/12/2016 | Mpinga-Kayove | Rutana | SAT2/IV | OM817515 |
| SAT2/BUN/13/2016 | Mpinga-Kayove | Rutana | SAT2/IV | OM817516 |
| SAT2/BUN/14/2016 | Mpinga-Kayove | Rutana | SAT2/IV | OM817517 |
| SAT2/BUN/15/2016 | Mpinga-Kayove | Rutana | SAT2/IV | OM817518 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevez Garcia, A.I.; Lefebvre, D.J.; Nyabongo, L.; Haegeman, A.; Nkundwanayo, C.; De Vleeschauwer, A.; Ntakirutimana, D.; De Leeuw, I.; Nsanganiyumwami, D.; Niyokwizera, P.; et al. Outbreaks of Foot-and-Mouth Disease in Burundi, East Africa, in 2016, Caused by Different Serotypes. Viruses 2022, 14, 1077. https://doi.org/10.3390/v14051077
Estevez Garcia AI, Lefebvre DJ, Nyabongo L, Haegeman A, Nkundwanayo C, De Vleeschauwer A, Ntakirutimana D, De Leeuw I, Nsanganiyumwami D, Niyokwizera P, et al. Outbreaks of Foot-and-Mouth Disease in Burundi, East Africa, in 2016, Caused by Different Serotypes. Viruses. 2022; 14(5):1077. https://doi.org/10.3390/v14051077
Chicago/Turabian StyleEstevez Garcia, Andrea Isabel, David J. Lefebvre, Lionel Nyabongo, Andy Haegeman, Canesius Nkundwanayo, Annebel De Vleeschauwer, Désiré Ntakirutimana, Ilse De Leeuw, Deogratias Nsanganiyumwami, Pascal Niyokwizera, and et al. 2022. "Outbreaks of Foot-and-Mouth Disease in Burundi, East Africa, in 2016, Caused by Different Serotypes" Viruses 14, no. 5: 1077. https://doi.org/10.3390/v14051077







