Impact of the COVID-19 Pandemic on Hepatitis C Treatment Initiation in British Columbia, Canada: An Interrupted Time Series Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Study Population and Design
2.3. Statistical Analysis
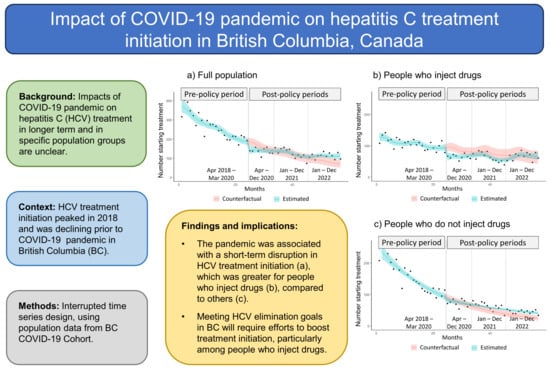
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021. Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact. Geneva: World Health Organization. 2021. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 26 August 2023).
- Feld, J.J.; Klein, M.B.; Rahal, Y.; Lee, S.S.; Mohammed, S.; King, A.; Smyth, D.; Gonzalez, Y.S.; Nugent, A.; Janjua, N.Z. Timing of elimination of hepatitis C virus in Canada’s provinces. Can. Liver J. 2022, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- The Canadian Network on Hepatitis C Blueprint Writing Committee and Working Groups. Blueprint to Inform Hepatitis C Elimination Efforts in Canada: The Canadian Network on Hepatitis C. 2019. Available online: https://www.canhepc.ca/sites/default/files/media/documents/blueprint_hcv_2019_05.pdf (accessed on 26 August 2023).
- Nuno Solinis, R.; Arratibel Ugarte, P.; Rojo, A.; Sanchez Gonzalez, Y. Value of Treating All Stages of Chronic Hepatitis C: A Comprehensive Review of Clinical and Economic Evidence. Infect. Dis. Ther. 2016, 5, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Sirpal, S.; Chandok, N. Barriers to hepatitis C diagnosis and treatment in the DAA era: Preliminary results of a community-based survey of primary care practitioners. Can. Liver J. 2022, 5, 96–100. [Google Scholar] [CrossRef]
- Binka, M.; Bartlett, S.; Velasquez Garcia, H.A.; Darvishian, M.; Jeong, D.; Adu, P.; Alvarez, M.; Wong, S.; Yu, A.; Samji, H.; et al. Impact of COVID-19-related public health measures on HCV testing in British Columbia, Canada: An interrupted time series analysis. Liver Int. 2021, 41, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Kondili, L.A.; Buti, M.; Riveiro-Barciela, M.; Maticic, M.; Negro, F.; Berg, T.; Craxì, A. Impact of the COVID-19 pandemic on hepatitis B and C elimination: An EASL survey. JHEP Rep. Innov. Hepatol. 2022, 4, 100531. [Google Scholar] [CrossRef] [PubMed]
- Mandel, E.; Peci, A.; Cronin, K.; Capraru, C.I.; Shah, H.; Janssen, H.L.A.; Tran, V.; Biondi, M.J.; Feld, J.J. The impact of the first, second and third waves of COVID-19 on hepatitis B and C testing in Ontario, Canada. J. Viral Hepat. 2022, 29, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Morales-Arráez, D.; Benítez-Zafra, F.; Díaz-Flores, F.; Medina-Alonso, M.J.; Santiago, L.G.; Pérez-Pérez, V.; Gutiérrez-Nicolás, F.; Hernández-Guerra, M. Hepatitis C diagnosis slowdown in high-prevalence groups and using decentralised diagnostic strategies during the COVID-19 pandemic. Rev. Esp. Enfermedades Dig. 2022, 115, 175–180. [Google Scholar] [CrossRef]
- Rehman, S.T.; Rehman, H.; Abid, S. Impact of coronavirus disease 2019 on prevention and elimination strategies for hepatitis B and hepatitis C. World J. Hepatol. 2021, 13, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Traeger, M.W.; van Santen, D.K.; Sacks-Davis, R.; Asselin, J.; Carter, A.; Doyle, J.S.; Pedrana, A.; Wilkinson, A.L.; Howell, J.; Thatcher, R.; et al. Impact of COVID-19 lockdown restrictions on hepatitis C testing in Australian primary care services providing care for people who inject drugs. J. Viral Hepat. 2022, 29, 908–918. [Google Scholar] [CrossRef]
- Trayner, K.M.; McAuley, A.; Palmateer, N.E.; Yeung, A.; Goldberg, D.J.; Glancy, M.; Hunter, C.; Ritchie, T.; Craik, J.; Raeburn, F.; et al. Examining the impact of the first wave of COVID-19 and associated control measures on interventions to prevent blood-borne viruses among people who inject drugs in Scotland: An interrupted time series study. Drug Alcohol Depend. 2022, 232, 109263. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Konstantelos, N.; Chu, C.; Antoniou, T.; Feld, J.; Suda, K.J.; Tadrous, M. Global Utilization Trends of Direct Acting Antivirals (DAAs) during the COVID-19 Pandemic: A Time Series Analysis. Viruses 2021, 13, 1314. [Google Scholar] [CrossRef] [PubMed]
- Levengood, T.W.; Aronsohn, A.I.; Chua, K.-P.; Conti, R.M. Dispensing of HIV and Hepatitis C Antivirals During COVID-19: An Interrupted Time-Series Analysis of U.S. National Data. Am. J. Prev. Med. 2022, 63, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Abramovitz, D.; Flores Ortega, R.E.; Martin, N.K.; Reau, N. Sustained Impact of the Coronavirus Disease 2019 Pandemic on Hepatitis C Virus Treatment Initiations in the United States. Clin. Infect. Dis. 2022, 75, e955–e961. [Google Scholar] [CrossRef] [PubMed]
- Konstantelos, N.; Shakeri, A.; McCormack, D.; Feld, J.J.; Gomes, T.; Tadrous, M. Impact of COVID-19 on Prescribing Trends of Direct-Acting Antivirals for the Treatment of Hepatitis C in Ontario, Canada. Am. J. Gastroenterol. 2021, 116, 1738–1740. [Google Scholar] [CrossRef] [PubMed]
- Aponte-Melendez, Y.; Mateu-Gelabert, P.; Fong, C.; Eckhardt, B.; Kapadia, S.; Marks, K. The impact of COVID-19 on people who inject drugs in New York City: Increased risk and decreased access to services. Harm Reduct. J. 2021, 18, 118. [Google Scholar] [CrossRef] [PubMed]
- Kesten, J.M.; Holland, A.; Linton, M.-J.; Family, H.; Scott, J.; Horwood, J.; Hickman, M.; Telfer, M.; Ayres, R.; Hussey, D.; et al. Living Under Coronavirus and Injecting Drugs in Bristol (LUCID-B): A qualitative study of experiences of COVID-19 among people who inject drugs. Int. J. Drug Policy 2021, 98, 103391. [Google Scholar] [CrossRef] [PubMed]
- May, T.; Dawes, J.; Fancourt, D.; Burton, A. A qualitative study exploring the impact of the COVID-19 pandemic on People Who Inject Drugs (PWID) and drug service provision in the UK: PWID and service provider perspectives. Int. J. Drug Policy 2022, 106, 103752. [Google Scholar] [CrossRef]
- Vasylyeva, T.I.; Smyrnov, P.; Strathdee, S.; Friedman, S.R. Challenges posed by COVID-19 to people who inject drugs and lessons from other outbreaks. J. Int. Aids Soc. 2020, 23, e25583. [Google Scholar] [CrossRef]
- British Columbia Ministry of Health. Discharge Abstract Database (Hospital Separations) Data Extract; Data Extract. MOH (2020); British Columbia Ministry of Health: Victoria, BC, Canada, 2021. [Google Scholar]
- British Columbia Ministry of Health. Population Grouper Methodology; Data Extract. MOH (2020); British Columbia Ministry of Health: Victoria, BC, Canada, 2021. [Google Scholar]
- British Columbia Ministry of Health. Health System Matrix; Data Extract. MOH (2020); British Columbia Ministry of Health: Victoria, BC, Canada, 2021. [Google Scholar]
- British Columbia Ministry of Health. 811 Calls; Data Extract. MOH (2020); British Columbia Ministry of Health: Victoria, BC, Canada, 2021. [Google Scholar]
- British Columbia Ministry of Health. Chronic Disease Registry; Data Extract. MOH (2020); British Columbia Ministry of Health: Victoria, BC, Canada, 2021. [Google Scholar]
- British Columbia Ministry of Health. National Ambulatory Care Reporting System; Data Extract. MOH (2020); British Columbia Ministry of Health: Victoria, BC, Canada, 2021. [Google Scholar]
- BC Vital Statistics Agency. Vital Statistics Deaths; Data Extract. BC Vital Statistics Agency (2020); BC Vital Statistics Agency: Vancouver, BC, Canada, 2021. [Google Scholar]
- British Columbia Ministry of Health. PharmaNet; Data Extract. MOH (2020); British Columbia Ministry of Health: Victoria, BC, Canada, 2021. [Google Scholar]
- British Columbia Ministry of Health. Medical Services Plan (MSP) Payment Information File; Data Extract. MOH (2020); British Columbia Ministry of Health: Victoria, BC, Canada, 2021. [Google Scholar]
- British Columbia Ministry of Health. Client Roster (Client Registry System/Enterprise Master Patient Index); Data Extract. MOH (2020); British Columbia Ministry of Health: Victoria, BC, Canada, 2021. [Google Scholar]
- British Columbia Centre for Disease Control. Respiratory Datamart; (2020); Public Health Reporting Data Warehouse, British Columbia Centre for Disease Control: Vancouver, BC, Canada, 2021. [Google Scholar]
- Provincial Health Services Authority. Provincial Laboratory Information Solution; (2020); Provincial Public Health Information Systems: Vancouver, BC, Canada, 2021. [Google Scholar]
- Provincial Health Services Authority. COVID-19 Vaccination Data; (2020); Provincial Immunizations Registry, Provincial Public Health Information Systems: Vancouver, BC, Canada, 2021. [Google Scholar]
- Provincial Health Services Authority. Provincial COVID-19 Monitoring Solution; (2020); Provincial Health Services Authority: Vancouver, BC, Canada, 2021. [Google Scholar]
- British Columbia Centre for Disease Control. Integrated COVID-19 Case Surveillance Data; (2020); British Columbia Centre for Disease Control: Victoria, BC, Canada, 2021. [Google Scholar]
- British Columbia Centre for Disease Control. Integrated COVID-19 Laboratory Dataset (SARS-CoV2 Tests from Private/Public Labs); (2020); Public Health Reporting Data Warehouse, British Columbia Centre for Disease Control: Victoria, BC, Canada, 2021. [Google Scholar]
- Federal Public Drug Benefit Programs Ottawa: Government of Canada; [Updated 11 April 2019–18 August 2023]. Available online: https://www.canada.ca/en/health-canada/services/health-care-system/pharmaceuticals/access-insurance-coverage-prescription-medicines/federal-public-drug-benefit-programs.html (accessed on 26 August 2023).
- FNHA Health Benefits Program Provides Status First Nations People Living in BC with a Comprehensive and Community-Driven Health Benefits Plan Vancouver, BC: First Nations Health Authority; [18 October 2023]. Available online: https://www.fnha.ca/benefits (accessed on 26 August 2023).
- Hepatitis C Coverage Expansion. BC PharmaCare Newsletter [Internet]. 18(005). Available online: https://www2.gov.bc.ca/assets/gov/health/health-drug-coverage/pharmacare/newsletters/pharmacare_newsletter_march_13_2018.pdf (accessed on 26 August 2023).
- Canadian COVID-19 Intervention Timeline: Canadian Institute of Health Information; [Updated 13 October 2022–18 August 2023]. Available online: https://www.cihi.ca/en/canadian-COVID-19-intervention-timeline (accessed on 26 August 2023).
- Jandoc, R.; Burden, A.M.; Mamdani, M.; Lévesque, L.E.; Cadarette, S.M. Interrupted time series analysis in drug utilization research is increasing: Systematic review and recommendations. J. Clin. Epidemiol. 2015, 68, 950–956. [Google Scholar] [CrossRef]
- Janjua, N.Z.; Islam, N.; Kuo, M.; Yu, A.; Wong, S.; Butt, Z.A.; Gilbert, M.; Buxton, J.; Chapinal, N.; Samji, H.; et al. Identifying injection drug use and estimating population size of people who inject drugs using healthcare administrative datasets. Int. J. Drug Policy 2018, 55, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, J.; Negrín, M. Non-Parametric Generalized Additive Models as a Tool for Evaluating Policy Interventions. Mathematics 2021, 9, 299. [Google Scholar] [CrossRef]
- Wood, S. Package ‘mgcv’. Mixed GAM Computation Vehicle with Automatic Smoothness Estimation [Updated 11 July–24 August 2023]. Available online: https://cran.r-project.org/web/packages/mgcv/mgcv.pdf (accessed on 26 August 2023).
- British Columbia Coroners Service. Illicit Drug Toxicity Deaths in BC: January 1, 2012–December 31, 2022: Ministry of Public Safety & Solicitor General; [Updated 31 January–25 April 2023]. Available online: https://www2.gov.bc.ca/assets/gov/birth-adoption-death-marriage-and-divorce/deaths/coroners-service/statistical/illicit-drug.pdf?trk=public_post_comment-text (accessed on 26 August 2023).
- Cousien, A.; Leclerc, P.; Morissette, C.; Bruneau, J.; Roy, É.; Tran, V.C.; Yazdanpanah, Y.; Cox, J. The need for treatment scale-up to impact HCV transmission in people who inject drugs in Montreal, Canada: A modelling study. BMC Infect. Dis. 2017, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.K.; Hickman, M.; Hutchinson, S.J.; Goldberg, D.J.; Vickerman, P. Combination interventions to prevent HCV transmission among people who inject drugs: Modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin. Infect. Dis. 2013, 57 (Suppl. 2), S39–S45. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.Z.; Kuo, M.; Yu, A.; Alvarez, M.; Wong, S.; Cook, D.; Wong, J.; Grebely, J.; Butt, Z.A.; Samji, H.; et al. The Population Level Cascade of Care for Hepatitis C in British Columbia, Canada: The BC Hepatitis Testers Cohort (BC-HTC). eBioMedicine 2016, 12, 189–195. [Google Scholar] [CrossRef]
- Popovic, N.; Williams, A.; Périnet, S.; Campeau, L.; Yang, Q.; Zhang, F.; Yan, P.; Feld, J.; Janjua, N.; Klein, M.; et al. National Hepatitis C estimates: Incidence, prevalence, undiagnosed proportion and treatment, Canada, 2019. Can. Commun. Dis. Rep. 2022, 48, 540–549. [Google Scholar] [CrossRef]



| Population | Rate Ratio (95% Confidence Interval) |
|---|---|
| (a) Overall | 0.74 (0.60, 0.91) |
| (b) Sex | |
| Female | 0.77 (0.64, 0.92) |
| Male | 0.81 (0.71, 0.93) |
| (c) Birth cohort | |
| Before 1945 | 0.70 (0.37, 1.32) |
| 1945–1964 | 0.81 (0.69, 0.94) |
| 1965–1974 | 0.60 (0.45, 0.82) |
| 1975 or later | 0.87 (0.72, 1.05) |
| (d) Injection drug use status | |
| People who inject drugs | 0.66 (0.50, 0.88) |
| People who do not inject drugs | 0.89 (0.77, 1.04) |
| Population | Absolute Difference, n (95% Confidence Interval) | Percentage Difference, % (95% Confidence Interval) | ||||
|---|---|---|---|---|---|---|
| April–December 2020 | January–December 2021 | January–December 2022 | April–December 2020 | January–December 2021 | January–December 2022 | |
| (a) Overall | −191.0 (−401.4, 8.4) | 77.8 (−227.1, 348.5) | 321.3 (9.7, 585.5) | −12.9 (−25.0, 0.6) | 6.4 (−13.3, 29.3) | 34.4 (0.6, 75.8) |
| (b) Sex | ||||||
| Female | −56.8 (−147.2, 26.7) | 70.1 (−52.8, 174.1) | 174.2 (55.3, 277.5) | −11.8 (−27.8, 6.7) | 18.5 (−9.7, 52.3) | 67.3 (13.3, 137.9) |
| Male | −126.5 (−274.5, 13.6) | 9.9 (−207.8, 200.4) | 143.4 (−91.3, 340.4) | −12.6 (−25.3, 1.4) | 2.0 (−17.5, 24.7) | 22.3 (−9.7, 62.1) |
| (c) Birth cohort | ||||||
| Before 1945 | −8.6 (−28.7, 7.8) | −6.9 (−35.5, 10.7) | −3.8 (−32.8, 12.2) | −22.5 (−61.0, 38.4) | −11.9 (−64.0, 81.9) | 8.2 (−71.7, 189.7) |
| 1945–1964 | −68.2 (−174.3, 32.1) | 68.9 (−64.9, 186.1) | 157.2 (33.4, 261.4) | −10.0 (−23.7, 5.4) | 13.2 (−9.4, 39.7) | 48.4 (7.7, 99.6) |
| 1965–1974 | −100.3 (−173.3, −32.4) | −95.0 (−214.6, 7.4) | −65.3 (−208.2, 49.5) | −25.5 (−39.1, −9.7) | −20.7 (−39.5, 2.3) | −15.5 (−42.3, 19.5) |
| 1975 or later | −29.4 (−113.4, 47.2) | 22.2 (−128.4, 146.6) | 86.2 (−112.7, 240.1) | −6.6 (−24.6, 14.4) | 6.8 (−20.8, 40.8) | 25.5 (−18.4, 84.5) |
| (d) Injection drug use status | ||||||
| PWID | −196.0 (−324.0, −77.1) | −164.4 (−384.8, 28.1) | −14.5 (−295.1, 214.3) | −24.5 (−36.4, −11.0) | −16.7 (−34.1, 3.7) | 0.5 (−27.3, 35.6) |
| Non-PWID | −12.3 (−126.1, 94.7) | 119.8 (−20.1, 242.4) | 188.1 (59.6, 296.7) | −1.3 (−16.2, 15.7) | 21.7 (−2.7, 50.2) | 55.4 (12.4, 109.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morrow, R.L.; Binka, M.; Li, J.; Irvine, M.; Bartlett, S.R.; Wong, S.; Jeong, D.; Makuza, J.D.; Wong, J.; Yu, A.; et al. Impact of the COVID-19 Pandemic on Hepatitis C Treatment Initiation in British Columbia, Canada: An Interrupted Time Series Study. Viruses 2024, 16, 655. https://doi.org/10.3390/v16050655
Morrow RL, Binka M, Li J, Irvine M, Bartlett SR, Wong S, Jeong D, Makuza JD, Wong J, Yu A, et al. Impact of the COVID-19 Pandemic on Hepatitis C Treatment Initiation in British Columbia, Canada: An Interrupted Time Series Study. Viruses. 2024; 16(5):655. https://doi.org/10.3390/v16050655
Chicago/Turabian StyleMorrow, Richard L., Mawuena Binka, Julia Li, Mike Irvine, Sofia R. Bartlett, Stanley Wong, Dahn Jeong, Jean Damascene Makuza, Jason Wong, Amanda Yu, and et al. 2024. "Impact of the COVID-19 Pandemic on Hepatitis C Treatment Initiation in British Columbia, Canada: An Interrupted Time Series Study" Viruses 16, no. 5: 655. https://doi.org/10.3390/v16050655