Amount of Dentifrice and Fluoride Concentration Affect the pH and Inorganic Composition of Dual-Species Biofilms of Streptococcus mutans and Candida albicans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Artificial Saliva
2.2. Strains of Microorganisms, Growth Conditions
2.3. Treatment of the Biofilms
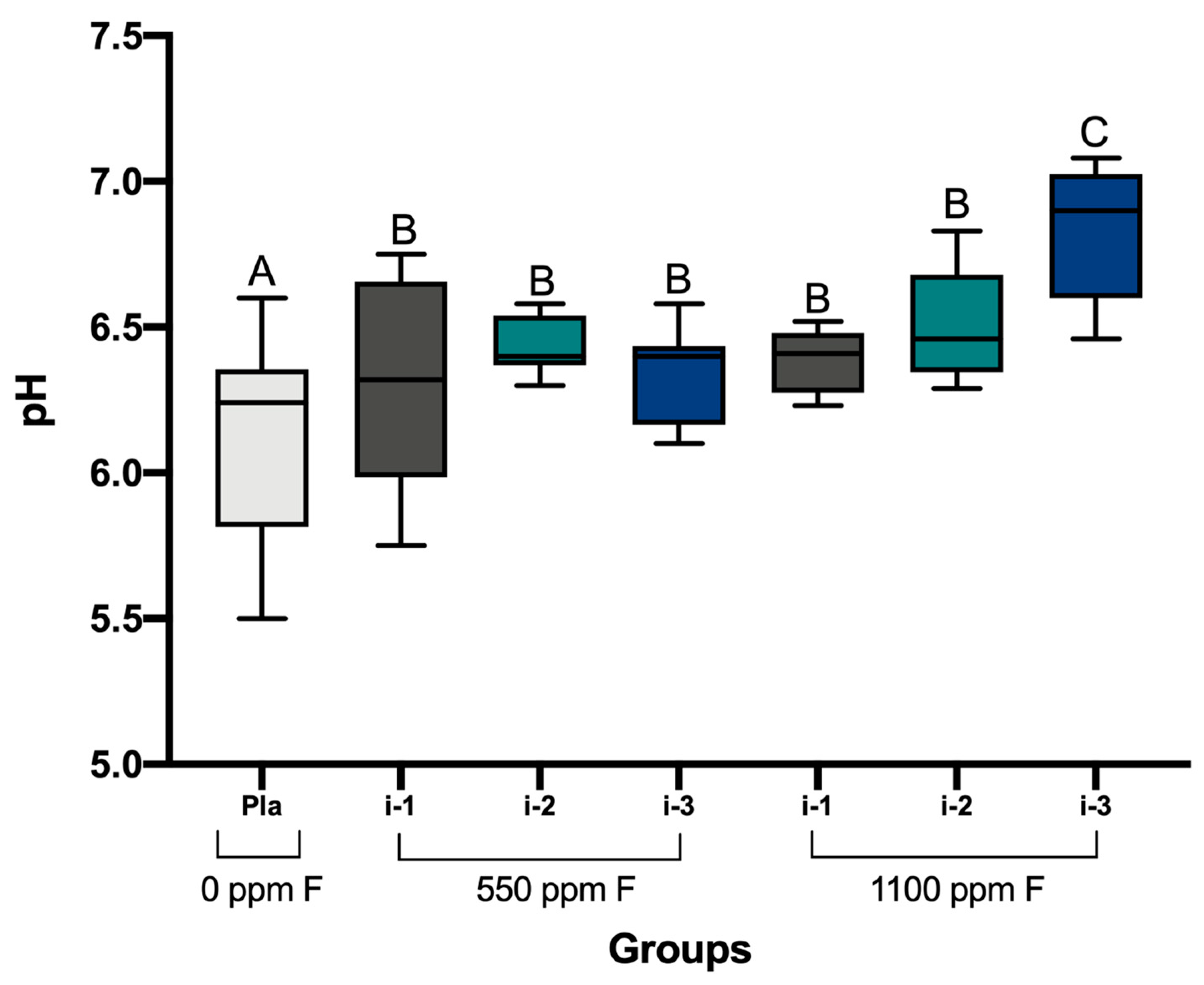
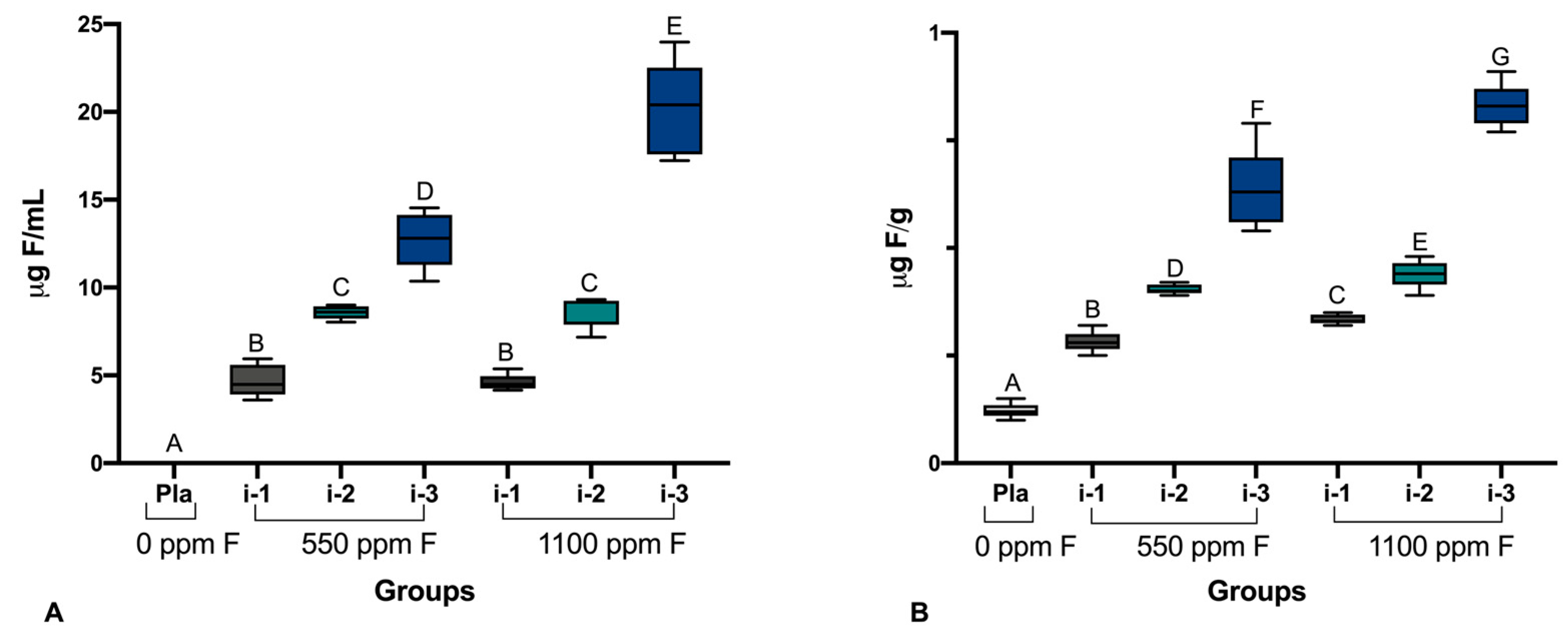
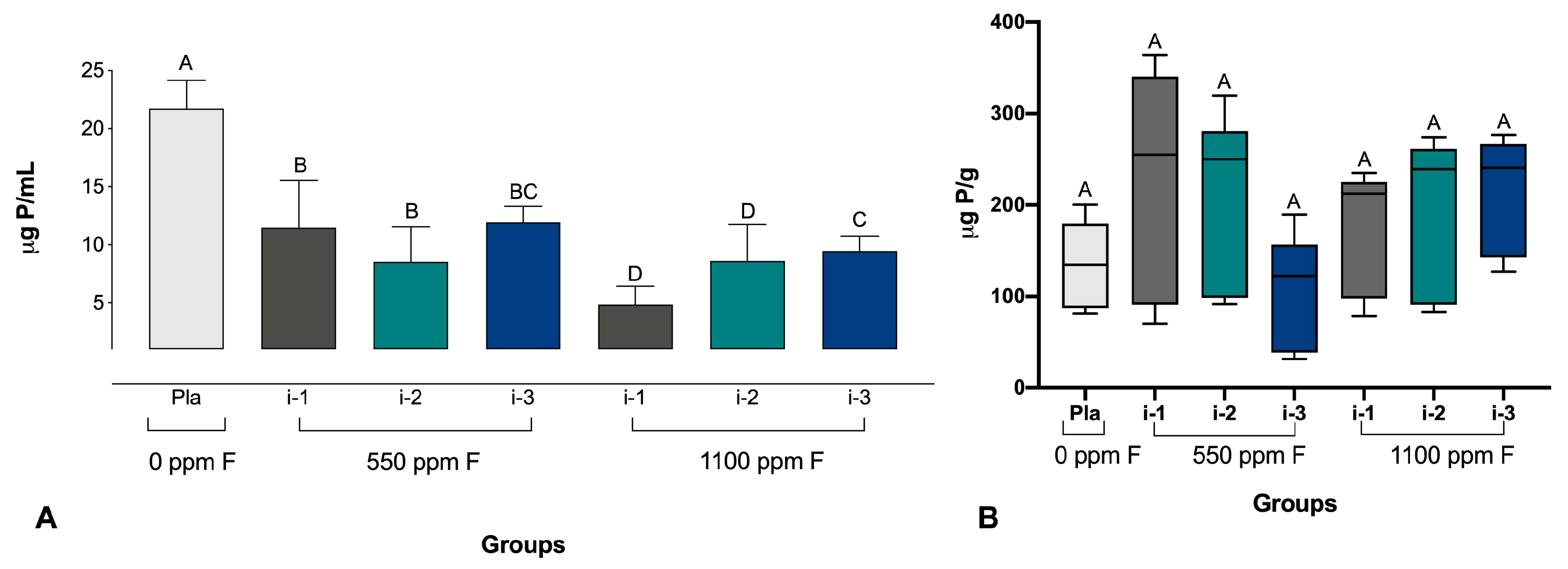
2.4. Determination of pH and Analysis of F, P, and Ca in Biofilm Fluid and Biomass
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C. EPS-Then and Now. Microorganisms 2016, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Margolis, H.C.; Moreno, E.C. Composition of pooled plaque fluid from cariesfree and caries-positive individuals following sucrose exposure. J. Dent. Res. 1992, 71, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Fejerskov, O. Changing paradigms in concepts on dental caries: Consequences for oral health care. Caries Res. 2004, 38, 182–191. [Google Scholar] [CrossRef]
- Fejerskov, O. Concepts of dental caries and their consequences for understanding the disease. Community Dent. Oral Epidemiol. 1997, 25, 5–12. [Google Scholar] [CrossRef]
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Trombini, J.; Scribante, A. Evaluation of Children Caries Risk Factors: A Narrative Review of Nutritional Aspects, Oral Hygiene Habits, and Bacterial Alterations. Children 2022, 9, 262. [Google Scholar] [CrossRef]
- Sheiham, A.; James, W.P. Diet and Dental Caries: The Pivotal Role of Free Sugars Reemphasized. J. Dent. Res. 2015, 94, 1341–1347. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef]
- Tomasin, L.; Pusinanti, L.; Zerman, N. The role of fluoride tablets in the prophylaxis of dental caries. A literature review. Ann. Stomatol. 2015, 18, 1–5. [Google Scholar] [CrossRef]
- Koulourides, T. Summary of session II: Fluoride and the caries process. J. Dent. Res. 1990, 69, 558. [Google Scholar] [CrossRef]
- Ten Cate, J.M. Review on fluoride, with special emphasis on calcium fluoride mechanisms in caries prevention. Eur. J. Oral Sci. 1997, 105, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Marquis, R.E.; Clock, S.A.; Mota-Meira, M. Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol. Rev. 2003, 26, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.; Glenny, A.M.; Tsang, B.W.; Lo, E.C.; Worthington, H.V.; Marinho, V.C. Topical fluoride as a cause of dental fluorosis in children. Cochrane Database Syst. Rev. 2010, 1, CD007693. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Worthington, H.V.; Glenny, A.M.; Marinho, V.C.; Jeroncic, A. Fluoride dentifrices of different concentrations for preventing dental caries. Cochrane Database Syst. Rev. 2019, 3, CD007868. [Google Scholar] [PubMed]
- Santos, A.P.; Oliveira, B.H.; Nadanovsky, P. Effects of low and standard fluoride dentifrices on caries and fluorosis: Systematic review and meta-analysis. Caries Res. 2013, 47, 382–390. [Google Scholar] [CrossRef]
- Coclete, G.E.G.; Delbem, A.C.B.; Sampaio, C.; Danelon, M.; Monteiro, D.R.; Pessan, J.P. Use of fluoridated dentifrices by children in Araçatuba, Brazil: Factors affecting brushing habits and amount applied on the brush. Eur. Arch. Paediatr. Dent. 2021, 22, 979–984. [Google Scholar] [CrossRef]
- dos Santos, A.P.; Nadanovsky, P.; de Oliveira, B.H. Inconsistencies in recommendations on oral hygiene practices for children by professional dental and paediatric organisations in ten countries. Int. J. Paediatr. Dent. 2011, 21, 223–231. [Google Scholar] [CrossRef]
- Zero, D.T.; Creeth, J.E.; Bosma, M.L.; Butler, A.; Guibert, R.G.; Karwal, R.; Lynch, R.J.; Martinez-Mier, E.A.; González-Cabezas, C.; Kelly, S.A. The effect of brushing time and dentifrice quantity on fluoride delivery in vivo and enamel surface microhardness in situ. Caries Res. 2010, 44, 90–100. [Google Scholar] [CrossRef]
- Duckworth, R.M.; Morgan, S.N.; Gilbert, R.J. Oral fluoride measurements for estimation of the anti-caries efficacy of fluoride treatments. J. Dent. Res. 1992, 71, 836–840. [Google Scholar] [CrossRef]
- Ashley, F.P.; Attrill, D.C.; Ellwood, R.P.; Worthington, H.V.; Davies, R.M. Toothbrushing habits and caries experience. Caries Res. 1999, 33, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, P.; Ko, H.S. Fluoride levels in whole saliva of preschool children after brushing with 0.25 g (pea-sized) as compared to 1.0 g (full-brush) of a fluoride dentifrice. Pediatr. Dent. 1996, 18, 277–280. [Google Scholar] [PubMed]
- Afflitto, J.; Schmid, R.; Esposito, A.; Toddywala, R.; Gaffar, A. Fluoride availability in human saliva after dentifrice use: Correlation with anticaries effects in rats. J. Dent. Res. 1992, 71, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.B.; Delbem, A.C.; Nagata, M.E.; Hosida, T.Y.; de Moraes, F.R.; Danelon, M.; Cunha, R.F.; Pessan, J.P. Influence of the amount of dentifrice and fluoride concentrations on salivary fluoride levels in children. Pediatr. Dent. 2016, 38, 379–384. [Google Scholar]
- Sampaio, C.; Delbem, A.C.B.; Paiva, M.F.; Zen, I.; Danelon, M.; Cunha, R.F.; Pessan, J.P. Amount of dentifrice and fluoride concentration influence salivary fluoride concentrations and fluoride intake by toddlers. Caries Res. 2020, 54, 234–241. [Google Scholar] [CrossRef]
- Pandit, S.; Kim, J.E.; Jung, K.H.; Chang, K.W.; Jeon, J.G. Effect of sodium fluoride on the virulence factors and composition of Streptococcus mutans biofilms. Arch. Oral Biol. 2011, 56, 643–649. [Google Scholar] [CrossRef]
- Pandit, S.; Kim, H.J.; Song, K.Y.; Jeon, J.G. Relationship between fluoride concentration and activity against virulence factors and viability of a cariogenic biofilm: In vitro study. Caries Res. 2013, 47, 539–547. [Google Scholar] [CrossRef]
- Pandit, S.; Cai, J.N.; Jung, J.E.; Jeon, J.G. Effect of 1-minute fluoride treatment on potential virulence and viability of a cariogenic biofilm. Caries Res. 2015, 49, 449–457. [Google Scholar] [CrossRef]
- Pandit, S.; Jung, J.E.; Choi, H.M.; Jeon, J.G. Effect of brief periodic fluoride treatments on the virulence and composition of a cariogenic biofilm. Biofouling 2018, 34, 53–61. [Google Scholar] [CrossRef]
- Lamfon, H.; Porter, S.R.; McCullough, M.; Pratten, J. Formation of Candida albicans biofilms on non-shedding oral surfaces. Eur. J. Oral Sci. 2003, 111, 465–471. [Google Scholar] [CrossRef]
- Hosida, T.Y.; Pessan, J.P.; Cavazana, T.P.; Sampaio, C.; de Morais, L.A.; Monteiro, D.R.; Delbem, A.C.B. Effects of Sodium Hexametaphosphate and Fluoride on the pH and Inorganic Components of Streptococcus mutans and Candida albicans Biofilm after Sucrose Exposure. Antibiotics 2022, 11, 1044. [Google Scholar] [CrossRef] [PubMed]
- Toumba, K.J.; Twetman, S.; Splieth, C.; Parnell, C.; van Loveren, C.; Lygidakis, N.A. Guidelines on the use of fluoride for caries prevention in children: An updated EAPD policy document. Eur. Arch. Paediatr. Dent. 2019, 20, 507–516. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatric Dentistry. Fluoride therapy. In The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2020; pp. 288–291. [Google Scholar]
- Vogel, G.L.; Chow, L.C.; Brown, W.E. A microanalytical procedure for the determination of calcium, phosphate and fluoride in enamel biopsy samples. Caries Res. 1983, 17, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Cury, J.A.; Rebelo, M.A.; Del Bel Cury, A.A.; Derbyshire, M.T.; Tabchoury, C.P. Biochemical composition and cariogenicity of dental plaque formed in the presence of sucrose or glucose and fructose. Caries Res. 2000, 34, 491–497. [Google Scholar] [CrossRef]
- Nobre dos Santos, M.; Melo dos Santos, L.; Francisco, S.B.; Cury, J.A. Relationship among dental plaque composition, daily sugar exposure and caries in the primary dentition. Caries Res. 2002, 36, 347–352. [Google Scholar] [CrossRef]
- Watson, P.S.; Pontefract, D.A.; Devine, D.A.; Shore, R.C.; Nattress, B.R.; Kirkham, J.; Robinson, C. Penetration of fluoride into natural plaque biofilms. J. Dent. Res. 2005, 84, 451–455. [Google Scholar] [CrossRef]
- Robinson, C.; Strafford, S.; Rees, G.; Brookes, S.J.; Kirkham, J.; Shore, R.C.; Watson, P.S.; Wood, S. Plaque biofilms: The effect of chemical environment on natural human plaque biofilm architecture. Arch. Oral Biol. 2006, 51, 1006–1014. [Google Scholar] [CrossRef]
- Pessan, J.P.; Silva, S.M.; Lauris, J.R.; Sampaio, F.C.; Whitford, G.M.; Buzalaf, M.A. Fluoride uptake by plaque from water and from dentifrice. J. Dent. Res. 2008, 87, 461–465. [Google Scholar] [CrossRef]
- Albahrani, M.M.; Alyahya, A.; Qudeimat, M.A.; Toumba, K.J. Salivary fluoride concentration following toothbrushing with and without rinsing: A randomised controlled trial. BMC Oral Health 2022, 22, 53. [Google Scholar] [CrossRef]
- Issa, A.I.; Toumba, K.J. Oral fluoride retention in saliva following toothbrushing with child and adult dentifrices with and without water rinsing. Caries Res. 2004, 38, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Chesters, R.K.; Huntington, E.; Burchell, C.K.; Stephen, K.W. Effect of oral care habits on caries in adolescents. Caries Res. 1992, 26, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Chestnutt, I.G.; Schafer, F.; Jacobsen, A.P.M.; Stephen, K.W. The influence of toothbrushing frequency and post-brushing rinsing on caries experience in a caries clinical trial. Community Dent. Oral Epidemiol. 1998, 26, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Ndokaj, A.; Bietolini, S.; Nisii, V.; Duś-Ilnicka, I.; Ottolenghi, L. Green dentistry: Organic toothpaste formulations. A literature review. Dent. Med. Probl. 2022, 59, 461–474. [Google Scholar] [CrossRef]
- Lucchese, M.; Bertacci, A.; Lo Giudice, A.; Polizzi, E.; Gherlone, E.; Manuelli, M.; Chersoni, S.; Moro, D.; Valdrè, G. Stannous Fluoride Preventive Effect on Enamel Erosion: An In Vitro Study. J. Clin. Med. 2020, 26, 2755. [Google Scholar] [CrossRef] [PubMed]

| Intensities | Amount of Dentifrice | Fluoride Concentration in the Dentifrice |
|---|---|---|
| i-1 | 0.08 g | 550 ppm F |
| i-2 | 0.16 g | |
| i-3 | 0.32 g | |
| i-1 | 0.04 g | 1100 ppm F |
| i-2 | 0.08 g | |
| i-3 | 0.16 g | |
| negative control | 0.32 g | Placebo (fluoride-free; 0 ppm F) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampaio, C.; Delbem, A.C.B.; Hosida, T.Y.; Fernandes, A.V.P.; do Amaral, B.; de Morais, L.A.; Monteiro, D.R.; Pessan, J.P. Amount of Dentifrice and Fluoride Concentration Affect the pH and Inorganic Composition of Dual-Species Biofilms of Streptococcus mutans and Candida albicans. Pharmaceutics 2024, 16, 562. https://doi.org/10.3390/pharmaceutics16040562
Sampaio C, Delbem ACB, Hosida TY, Fernandes AVP, do Amaral B, de Morais LA, Monteiro DR, Pessan JP. Amount of Dentifrice and Fluoride Concentration Affect the pH and Inorganic Composition of Dual-Species Biofilms of Streptococcus mutans and Candida albicans. Pharmaceutics. 2024; 16(4):562. https://doi.org/10.3390/pharmaceutics16040562
Chicago/Turabian StyleSampaio, Caio, Alberto Carlos Botazzo Delbem, Thayse Yumi Hosida, Ana Vitória Pereira Fernandes, Bruna do Amaral, Leonardo Antônio de Morais, Douglas Roberto Monteiro, and Juliano Pelim Pessan. 2024. "Amount of Dentifrice and Fluoride Concentration Affect the pH and Inorganic Composition of Dual-Species Biofilms of Streptococcus mutans and Candida albicans" Pharmaceutics 16, no. 4: 562. https://doi.org/10.3390/pharmaceutics16040562





