Ingested Microplastics Can Act as Microbial Vectors of Ichthyofauna
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens and Exposure
2.2. Microbiota Recovery
2.3. Taxonomic Identification
2.4. Polymer Analysis
2.4.1. Differential Scanning Calorimetry Analysis
2.4.2. Fourier-Transform Infrared Spectroscopy
3. Results
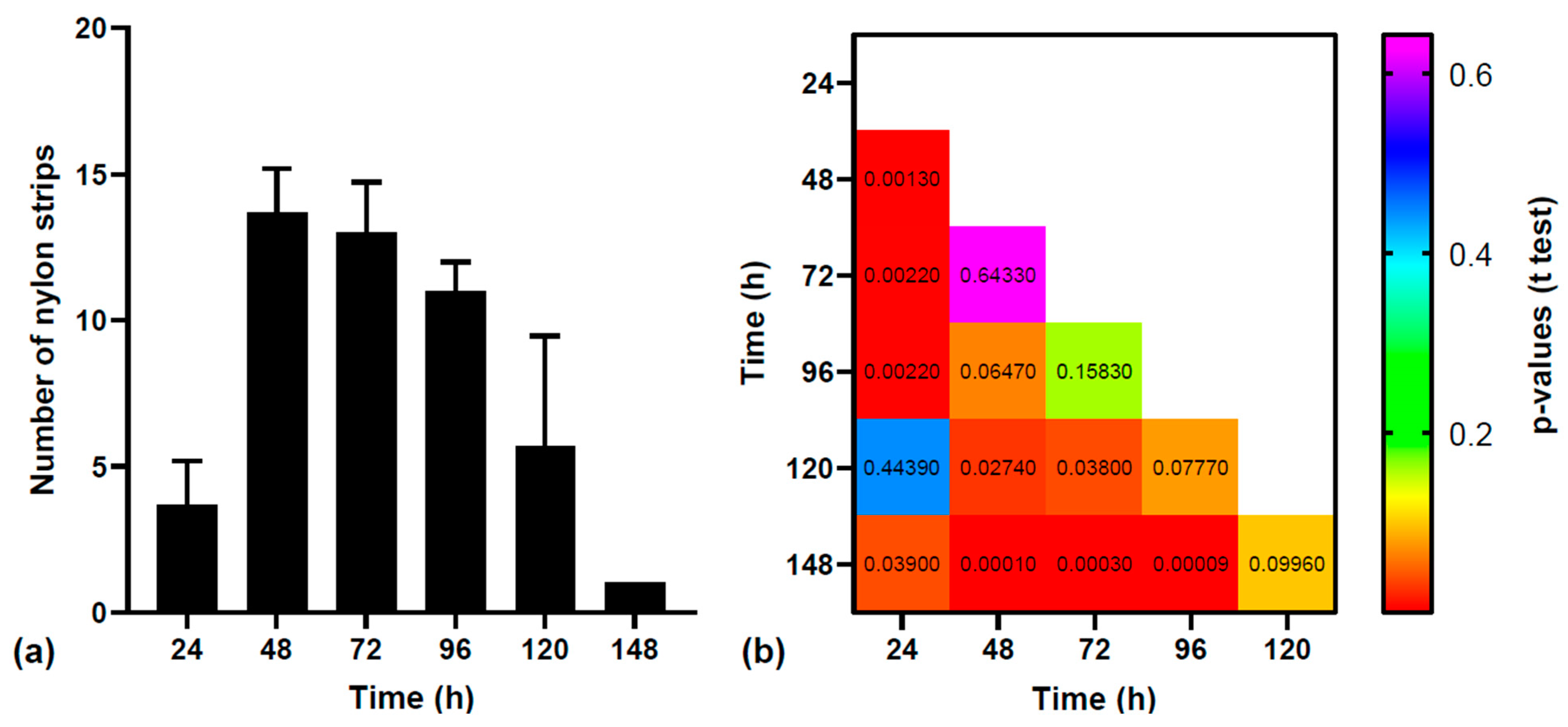
3.1. Statistical Analysis of MPs Excretion
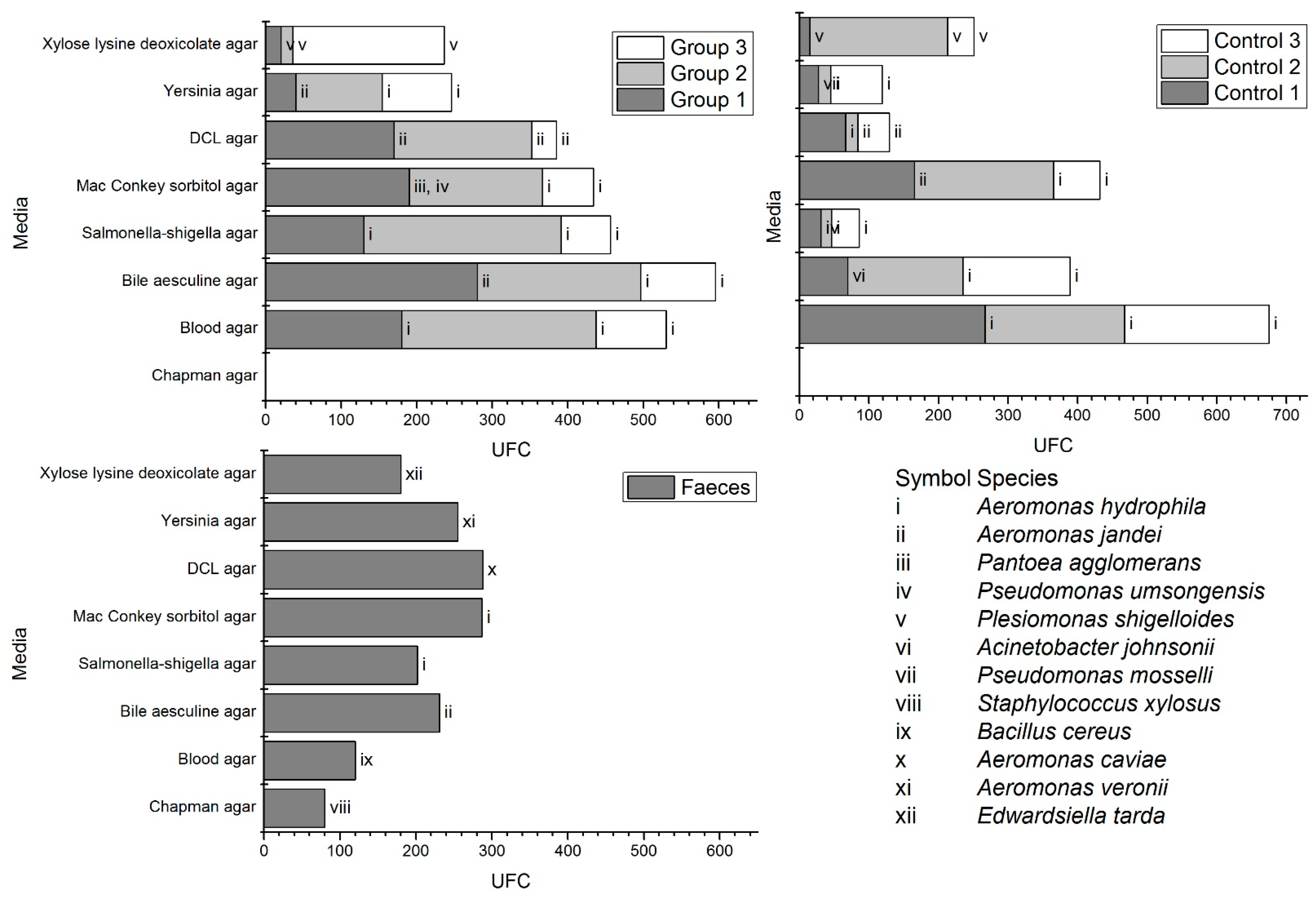
3.2. Microbial Diversity
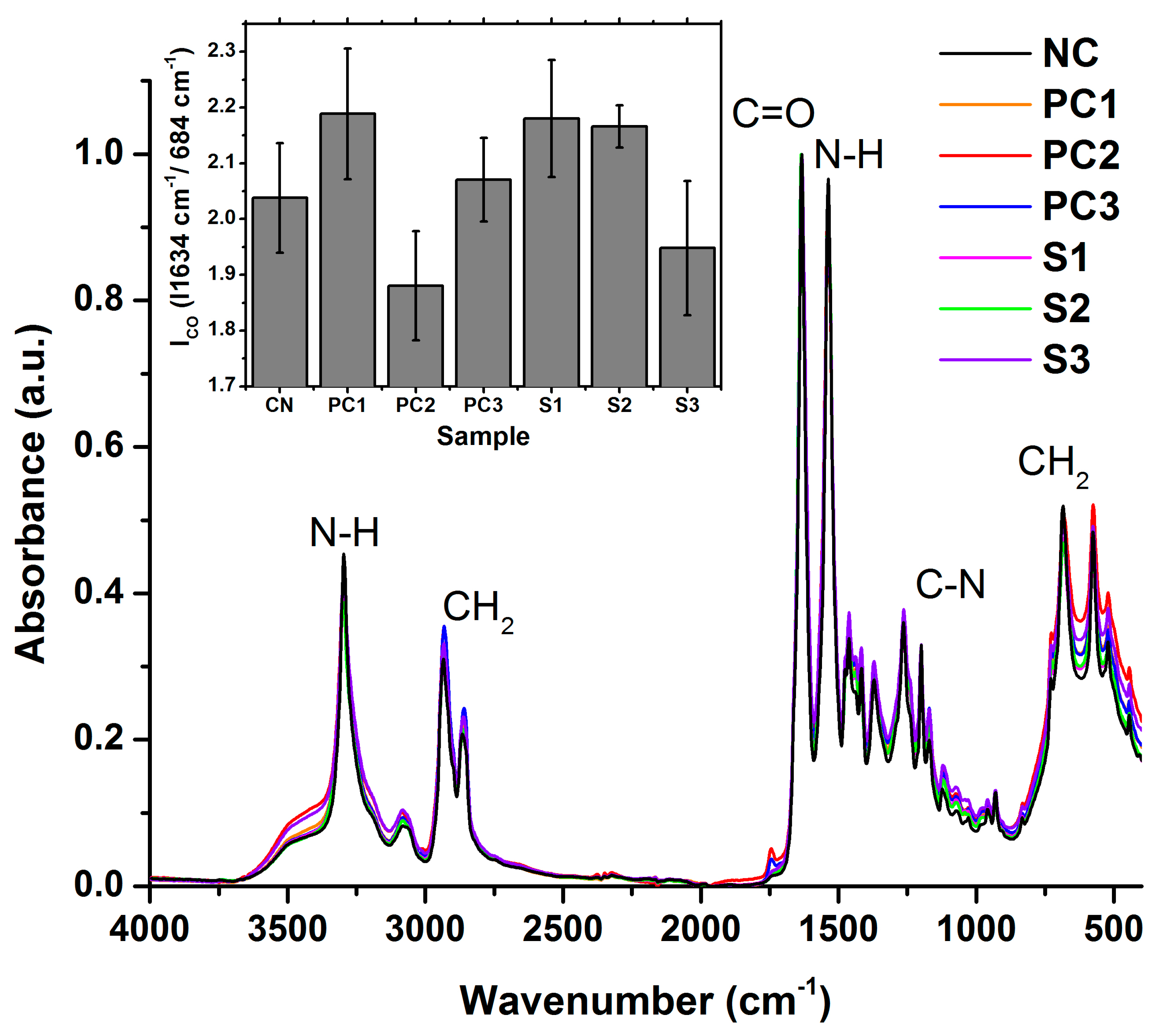
3.3. Polymer Structural Integrity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Vries, A.N.; Govoni, D.; Árnason, S.H.; Carlsson, P. Microplastic ingestion by fish: Body size, condition factor and gut fullness are not related to the amount of plastics consumed. Mar. Pollut. Bull. 2020, 151, 110827. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liang, W.; Liu, Q.-X.; Fu, S.; Ma, C.; Chen, Q.; Su, L.; Craig, N.J.; Shi, H. Fish Ingest Microplastics Unintentionally. Environ. Sci. Technol. 2021, 55, 10471–10479. [Google Scholar] [CrossRef] [PubMed]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the mass of microplastics ingested—A pivotal first step towards human health risk assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef] [PubMed]
- Hofstede, L.T.; Vasse, G.F.; Melgert, B.N. Microplastics: A threat for developing and repairing organs? Camb. Prism. Plast. 2023, 1, e19. [Google Scholar] [CrossRef]
- Weingrill, R.B.; Lee, M.-J.; Benny, P.; Riel, J.; Saiki, K.; Garcia, J.; Oliveira, L.F.A.d.M.; Fonseca, E.J.d.S.; Souza, S.T.d.; D’Amato, F.d.O.S.; et al. Temporal trends in microplastic accumulation in placentas from pregnancies in Hawaii. Environ. Int. 2023, 180, 108220. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard Mater. 2021, 416, 126124. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.S.; Tuan Anuar, S.; Azmi, A.A.; Wan Mohd Khalik, W.M.A.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open 2021, 5, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Ahmad, S.; Guo, X.; Ullah, S.; Ullah, S.; Nabi, G.; Wanghe, K. A review of the endocrine disrupting effects of micro and nano plastic and their associated chemicals in mammals. Front. Endocrinol. 2022, 13, 1084236. [Google Scholar] [CrossRef] [PubMed]
- EndocrineSociety. Plastics Pose Threat to Human Health. Available online: https://www.endocrine.org/news-and-advocacy/news-room/2020/plastics-pose-threat-to-human-health (accessed on 8 March 2024).
- Pop, C.-E.; Miu, B.A.; Németh, D.; Wolff, R.; Mihăilescu, D.F.; Avramescu, S.M.; Mernea, M. Bisphenol A analysis and quantification inconsistencies via HPLC-UV: A systematic review with technical notes. Discov. Appl. Sci. 2024, 6, 171. [Google Scholar] [CrossRef]
- Stabnikova, O.; Stabnikov, V.; Marinin, A.; Klavins, M.; Klavins, L.; Vaseashta, A. Microbial Life on the Surface of Microplastics in Natural Waters. Appl. Sci. 2021, 11, 11692. [Google Scholar] [CrossRef]
- D’Avignon, G.; Gregory-Eaves, I.; Ricciardi, A. Microplastics in lakes and rivers: An issue of emerging significance to limnology. Environ. Rev. 2022, 30, 228–244. [Google Scholar] [CrossRef]
- Issac, M.N.; Kandasubramanian, B. Effect of microplastics in water and aquatic systems. Environ. Sci. Pollut. Res. 2021, 28, 19544–19562. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.-S.; Wu, Y.-S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Esmaili, Z.; Khan, F.R. Plastic debris and microplastics along the beaches of the Strait of Hormuz, Persian Gulf. Mar. Pollut. Bull. 2017, 114, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics generated when opening plastic packaging. Sci. Rep. 2020, 10, 4841. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.T.; Perumal, S. Trophic Transfer and Accumulation of Microplastics in Freshwater Ecosystem: Risk to Food Security and Human Health. Int. J. Ecol. 2022, 2022, 1234078. [Google Scholar] [CrossRef]
- Garrido Gamarro, E.; Ryder, J.; Elvevoll, E.O.; Olsen, R.L. Microplastics in fish and shellfish–a threat to seafood safety? J. Aquat. Food Prod. Technol. 2020, 29, 417–425. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.-C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Walkinshaw, C.; Lindeque, P.K.; Thompson, R.; Tolhurst, T.; Cole, M. Microplastics and seafood: Lower trophic organisms at highest risk of contamination. Ecotoxicol. Environ. Saf. 2020, 190, 110066. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, I.F.; Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Worldwide contamination of fish with microplastics: A brief global overview. Mar. Pollut. Bull. 2020, 160, 111681. [Google Scholar] [CrossRef] [PubMed]
- Justino, A.K.S.; Ferreira, G.V.B.; Fauvelle, V.; Schmidt, N.; Lenoble, V.; Pelage, L.; Martins, K.; Travassos, P.; Lucena-Frédou, F. From prey to predators: Evidence of microplastic trophic transfer in tuna and large pelagic species in the southwestern Tropical Atlantic. Environ. Pollut. 2023, 327, 121532. [Google Scholar] [CrossRef] [PubMed]
- Mizraji, R.; Ahrendt, C.; Perez-Venegas, D.; Vargas, J.; Pulgar, J.; Aldana, M.; Patricio Ojeda, F.; Duarte, C.; Galbán-Malagón, C. Is the feeding type related with the content of microplastics in intertidal fish gut? Mar. Pollut. Bull. 2017, 116, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, C.R.; Searle, C.L.; Schaber, J.; Höök, T.O. Microplastics impact simple aquatic food web dynamics through reduced zooplankton feeding and potentially releasing algae from consumer control. Sci. Total Environ. 2023, 904, 166691. [Google Scholar] [CrossRef] [PubMed]
- Clere, I.K.; Ahmmed, F.; Remoto, P.I.I.I.J.G.; Fraser-Miller, S.J.; Gordon, K.C.; Komyakova, V.; Allan, B.J.M. Quantification and characterization of microplastics in commercial fish from southern New Zealand. Mar. Pollut. Bull. 2022, 184, 114121. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, A.M.; Morrison, L.; Croot, P.L.; Allcock, A.L.; MacLoughlin, E.; Savard, O.; Brownlow, H.; Doyle, T.K. Frequency of Microplastics in Mesopelagic Fishes from the Northwest Atlantic. Front. Mar. Sci. 2018, 5, 39. [Google Scholar] [CrossRef]
- Gregory, M.R. Environmental implications of plastic debris in marine settings—Entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Uy, C.A.; Johnson, D.W. Effects of microplastics on the feeding rates of larvae of a coastal fish: Direct consumption, trophic transfer, and effects on growth and survival. Mar. Biol. 2022, 169, 27. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O.; Igwaran, A. Microplastics in seafood: Implications for food security, safety, and human health. J. Sea Res. 2023, 194, 102410. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene spherules in coastal waters. Science 1972, 178, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Souza-Silva, T.G.d.; Oliveira, I.A.; Silva, G.G.d.; Giusti, F.C.V.; Novaes, R.D.; Paula, H.A.d.A. Impact of microplastics on the intestinal microbiota: A systematic review of preclinical evidence. Life Sci. 2022, 294, 120366. [Google Scholar] [CrossRef] [PubMed]
- Parsaeimehr, A.; Miller, C.M.; Ozbay, G. Microplastics and their interactions with microbiota. Heliyon 2023, 9, e15104. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Bañares, M.A.; Fernández, J.F.; Moreno-Arribas, M.V. PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Sci. Rep. 2022, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Marathe, N.P.; Bank, M.S. The Microplastic-Antibiotic Resistance Connection. In Microplastic in the Environment: Pattern and Process; Bank, M.S., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 311–322. [Google Scholar]
- Gaylarde, C.C.; de Almeida, M.P.; Neves, C.V.; Neto, J.A.B.; da Fonseca, E.M. The Importance of Biofilms on Microplastic Particles in Their Sinking Behavior and the Transfer of Invasive Organisms between Ecosystems. Micro 2023, 3, 320–337. [Google Scholar] [CrossRef]
- Mincer, T.J.; Bos, R.P.; Zettler, E.R.; Zhao, S.; Asbun, A.A.; Orsi, W.D.; Guzzetta, V.S.; Amaral-Zettler, L.A. Sargasso Sea Vibrio bacteria: Underexplored potential pathovars in a perturbed habitat. Water Res. 2023, 242, 120033. [Google Scholar] [CrossRef]
- Nikolopoulou, I.; Piperagkas, O.; Moschos, S.; Karayanni, H. Bacteria Release from Microplastics into New Aquatic Environments. Diversity 2023, 15, 115. [Google Scholar] [CrossRef]
- Harrison, J.P.; Schratzberger, M.; Sapp, M.; Osborn, A.M. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 2014, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Viršek, M.K.; Lovšin, M.N.; Koren, Š.; Kržan, A.; Peterlin, M. Microplastics as a vector for the transport of the bacterial fish pathogen species Aeromonas salmonicida. Mar. Pollut. Bull. 2017, 125, 301–309. [Google Scholar] [CrossRef]
- Kruglova, A.; Muñoz-Palazón, B.; Gonzalez-Martinez, A.; Mikola, A.; Vahala, R.; Talvitie, J. The dangerous transporters: A study of microplastic-associated bacteria passing through municipal wastewater treatment. Environ. Pollut. 2022, 314, 120316. [Google Scholar] [CrossRef]
- Beans, C. Are microplastics spreading infectious disease? Proc. Natl. Acad. Sci. USA 2023, 120, e2311253120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hong, X.; Chai, J.; Wan, B.; Zhao, K.; Han, C.; Zhang, W.; Huan, H. Interaction between Microplastics and Pathogens in Subsurface System: What We Know So Far. Water 2024, 16, 499. [Google Scholar] [CrossRef]
- Zhong, H.; Wu, M.; Sonne, C.; Lam, S.S.; Kwong, R.W.M.; Jiang, Y.; Zhao, X.; Sun, X.; Zhang, X.; Li, C.; et al. The hidden risk of microplastic-associated pathogens in aquatic environments. Eco-Environ. Health 2023, 2, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Esangbedo, M.O.; Abifarin, J.K. Determination and managerial implications of machine conditions for high-grade industrial polycaprolactam (nylon 6). Sci. Rep. 2023, 13, 10779. [Google Scholar] [CrossRef] [PubMed]
- Information, N.C.f.B. PubChem Compound Summary for, Nylon 6. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nylon-6 (accessed on 21 March 2024).
- Society, A.C. Nylon 6. Available online: https://commonchemistry.cas.org/detail?cas_rn=25038-54-4 (accessed on 21 March 2024).
- Close, B.; Banister, K.; Baumans, V.; Bernoth, E.-M.; Bromage, N.; Bunyan, J.; Erhardt, W.; Flecknell, P.; Gregory, N.; Hackbarth, H. Recommendations for euthanasia of experimental animals: Part 2. Lab. Anim. 1997, 31, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [PubMed]
- Welker, M. Proteomics for routine identification of microorganisms. Proteomics 2011, 11, 3143–3153. [Google Scholar] [CrossRef]
- Valentine, N.; Wunschel, S.; Wunschel, D.; Petersen, C.; Wahl, K. Effect of culture conditions on microorganism identification by matrix-assisted laser desorption ionization mass spectrometry. Appl. Environ. Microbiol. 2005, 71, 58–64. [Google Scholar] [CrossRef]
- Carbonnelle, E.; Beretti, J.L.; Cottyn, S.; Quesne, G.; Berche, P.; Nassif, X.; Ferroni, A. Rapid identification of Staphylococci isolated in clinical microbiology laboratories by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2007, 45, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Benítez, A.; Sánchez, J.J.; Arnal, M.L.; Müller, A.J.; Rodríguez, O.; Morales, G. Abiotic degradation of LDPE and LLDPE formulated with a pro-oxidant additive. Polym. Degrad. Stab. 2013, 98, 490–501. [Google Scholar] [CrossRef]
- UserCom. Information for users of Mettler Toledo thermal analysis systems. 2000; 11.
- Bhullar, S.K.; Rana, D.; Ozsel, B.K.; Orhan, M.; Jun, M.B.G.; Buttar, H.S.; Ostrovidov, S.; Ramalingam, M. Development of Silver-Based Bactericidal Composite Nanofibers by Airbrushing. J. Nanosci. Nanotechnol. 2018, 18, 2951–2955. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Vázquez-Vélez, E.; Martinez, H.; Torres, A. Superficial Surface Treatment using Atmospheric Plasma on Recycled Nylon 6,6. J. Nucl. Phys. Mater. Sci. Radiat. Appl. 2021, 8, 191–196. [Google Scholar] [CrossRef]
- Polyamide (Nylon 6). Available online: https://spectra.chem.ut.ee/textile-fibres/polyamide/ (accessed on 9 March 2024).
- Pakbin, B.; Brück, W.M.; Rossen, J.W.A. Virulence Factors of Enteric Pathogenic Escherichia coli: A Review. Int. J. Mol. Sci. 2021, 22, 9922. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, Y.; Boraschi, D. Association between Microorganisms and Microplastics: How Does It Change the Host-Pathogen Interaction and Subsequent Immune Response? Int. J. Mol. Sci. 2023, 24, 4065. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.N.; Clark, L.; Li, M. Microplastics as hubs enriching antibiotic-resistant bacteria and pathogens in municipal activated sludge. J. Hazard. Mater. Lett. 2021, 2, 100014. [Google Scholar] [CrossRef]
- Cholewińska, P.; Moniuszko, H.; Wojnarowski, K.; Pokorny, P.; Szeligowska, N.; Dobicki, W.; Polechoński, R.; Górniak, W. The Occurrence of Microplastics and the Formation of Biofilms by Pathogenic and Opportunistic Bacteria as Threats in Aquaculture. Int. J. Environ. Res. Public Health 2022, 19, 8137. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, S.; Gkoutselis, G.; Hink, L.; Weig, A.R.; Obst, M.; Diekmann, A.; Ho, A.; Rambold, G.; Horn, M.A. Microplastic polymer properties as deterministic factors driving terrestrial plastisphere microbiome assembly and succession in the field. Environ. Microbiol. 2023, 25, 2681–2697. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Ji, C.; Li, F.; Shan, X.; Wu, H. The legacy effect of microplastics on aquatic animals in the depuration phase: Kinetic characteristics and recovery potential. Environ. Int. 2022, 168, 107467. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, S.H. 17—Examination of faeces for bacterial pathogens. In Medical Microbiology Illustrated; Gillespie, S.H., Ed.; Butterworth-Heinemann: Oxford, UK, 1994; pp. 192–210. [Google Scholar]
- Desmond, E.; Janda, J.M. Growth of Aeromonas species on enteric agars. J. Clin. Microbiol. 1986, 23, 1065–1067. [Google Scholar] [CrossRef]
- Arcos, M.L.; Vicente, A.d.; Moriñigo, M.A.; Romero, P.; Borrego, J.J. Evaluation of several selective media for recovery of Aeromonas hydrophila from polluted waters. Appl. Environ. Microbiol. 1988, 54, 2786–2792. [Google Scholar] [CrossRef]
- Furmanczyk, E.M.; Kaminski, M.A.; Dziembowski, A.; Lipinski, L.; Sobczak, A. Draft Genome Sequence of the Type Strain Pseudomonas umsongensis DSM 16611. Genome Announc. 2017, 5, 39. [Google Scholar] [CrossRef]
- Ellner, P.D.; McCarthy, L.R. Aeromonas shigelloides Bacteremia: A Case Report. Am. J. Clin. Pathol. 1973, 59, 216–218. [Google Scholar] [CrossRef]
- Pereira, C.S.; Amorim, S.D.; Santos, A.F.; Siciliano, S.; Moreno, I.B.; Ott, P.H.; Rodrigues Ddos, P. Plesiomonas shigelloides and Aeromonadaceae family pathogens isolated from marine mammals of Southern and Southeastern Brazilian coast. Braz. J. Microbiol. 2008, 39, 749–755. [Google Scholar] [CrossRef]
- Holmberg, S.D.; Farmer, J.J., III. Aeromonas hydrophila and Plesiomonas shigelloides as Causes of Intestinal Infections. Rev. Infect. Dis. 1984, 6, 633–639. [Google Scholar] [CrossRef]
- Alabi, S.A.; Odugbemi, T. Occurrence of Aeromonas species and Plesiomonas shigelloides in patients with and without diarrhoea in Lagos, Nigeria. J. Med. Microbiol. 1990, 32, 45–48. [Google Scholar] [CrossRef]
- Pitarangsi, C.; Echeverria, P.; Whitmire, R.; Tirapat, C.; Formal, S.; Dammin, G.J.; Tingtalapong, M. Enteropathogenicity of Aeromonas hydrophila and Plesiomonas shigelloides: Prevalence among individuals with and without diarrhea in Thailand. Infect. Immun. 1982, 35, 666–673. [Google Scholar] [CrossRef]
- Duman, M.; García Valdés, E.; Ay, H.; Altun, S.; Saticioglu, I.B. Description of a Novel Fish Pathogen, Plesiomonas shigelloides subsp. oncorhynchi, Isolated from Rainbow Trout (Oncorhynchus mykiss): First Genome Analysis and Comparative Genomics. Fishes 2023, 8, 179. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L.; McIver, C.J. Plesiomonas shigelloides Revisited. Clin. Microbiol. Rev. 2016, 29, 349–374. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Sulaiman, B.; Woodward, J.C.; Shiels, H.A. Riverine microplastics and their interaction with freshwater fish. Water Biol. Secur. 2023, 2, 100192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jawdhari, A.; Deák, G.; Mihăilescu, D.F.; Crăciun, N.; Staicu, A.C.; Stanca, I.; Cozorici, D.; Fendrihan, S.; Pop, C.-E.; Mernea, M. Ingested Microplastics Can Act as Microbial Vectors of Ichthyofauna. Microbiol. Res. 2024, 15, 614-625. https://doi.org/10.3390/microbiolres15020040
Jawdhari A, Deák G, Mihăilescu DF, Crăciun N, Staicu AC, Stanca I, Cozorici D, Fendrihan S, Pop C-E, Mernea M. Ingested Microplastics Can Act as Microbial Vectors of Ichthyofauna. Microbiology Research. 2024; 15(2):614-625. https://doi.org/10.3390/microbiolres15020040
Chicago/Turabian StyleJawdhari, Abdulhusein, György Deák, Dan Florin Mihăilescu, Nicolai Crăciun, Andrea Cristina Staicu, Ioana Stanca, Derniza Cozorici, Sergiu Fendrihan, Cristian-Emilian Pop, and Maria Mernea. 2024. "Ingested Microplastics Can Act as Microbial Vectors of Ichthyofauna" Microbiology Research 15, no. 2: 614-625. https://doi.org/10.3390/microbiolres15020040





