Secondary Metabolites and Their Antioxidant Activity Enhance the Tolerance to Water Deficit on Clover Lotus corniculatus L. through Different Seasonal Times
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geographic Location
2.2. Experimental Setup
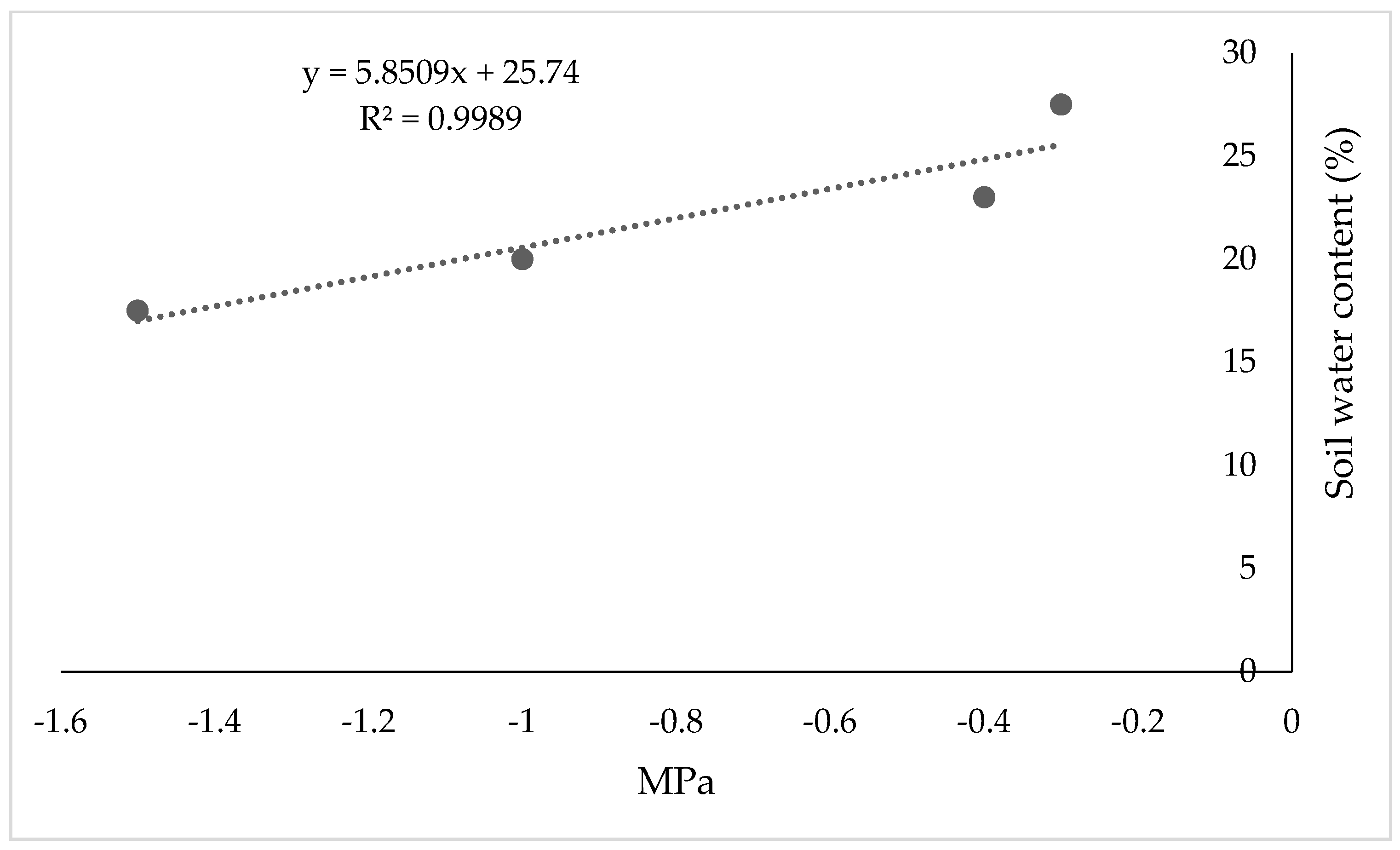
2.3. Irrigation
2.4. Measured Variables
2.5. Data Analysis
3. Results
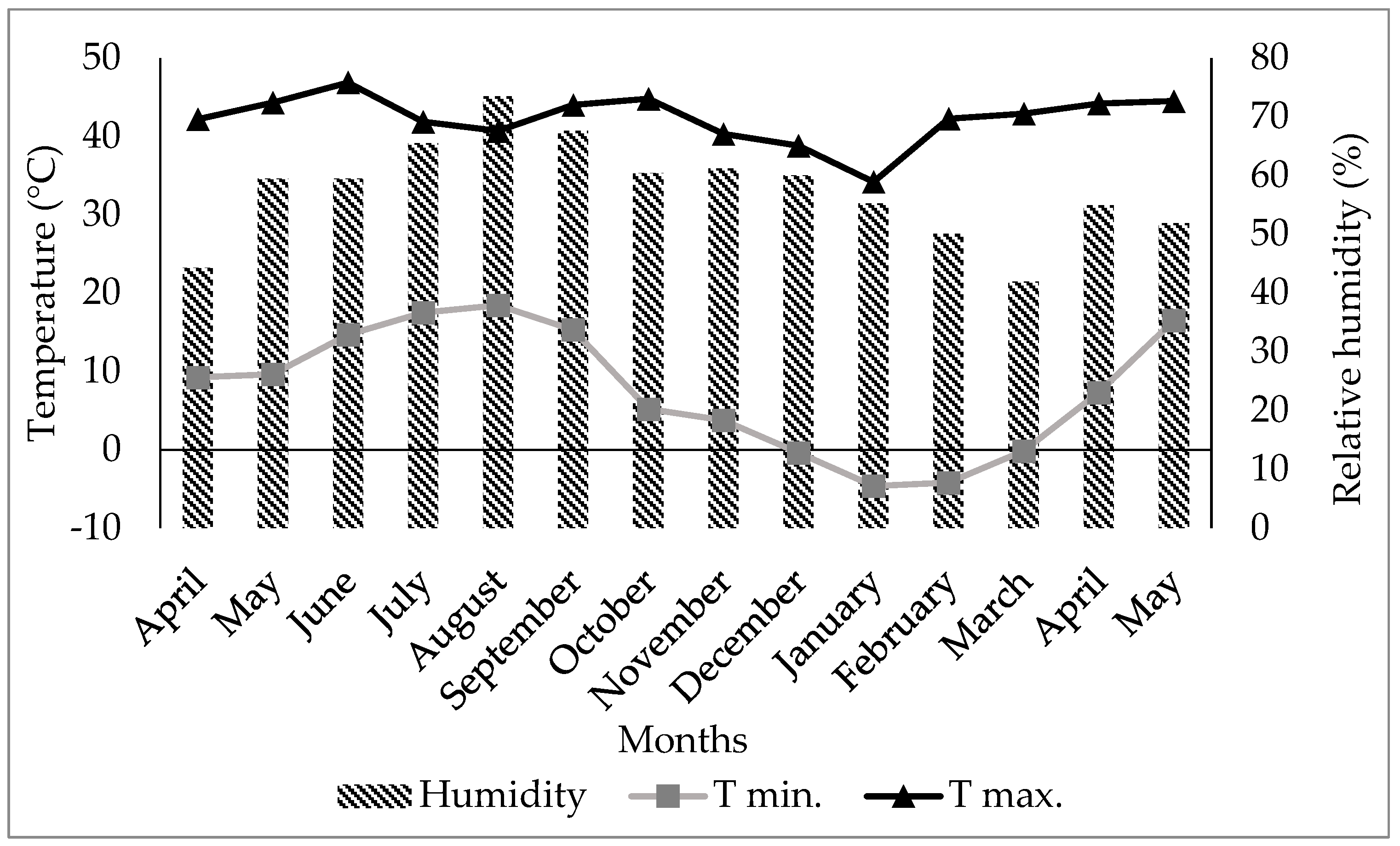
3.1. Temperature and Relative Humidity
3.2. Phenols
3.3. Flavonoids
3.4. Tannins
3.5. Saponins
3.6. Radical Scavenging Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stott, P. How climate change affects extreme weather events. Science 2016, 352, 1517–1518. [Google Scholar] [CrossRef] [PubMed]
- UN (United Nations). The World Population Reached 8000 Million Inhabitants. 2022. Available online: https://www.un.org/en/dayof8billion (accessed on 15 May 2022).
- Mozammel, H.A.; Mondal, S.S.A. Sustainable livestock production and food security. In Emerging Issues in Climate Smart Livestock Production; Mondal, S., Singh, R.L., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 71–90. [Google Scholar]
- OECD. Water Use in Agriculture. 2018. Available online: https://www.oecd.org/environment/water-use-in-agriculture.htm (accessed on 17 October 2022).
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water scarcity in agriculture: An overview of causes, impacts, and approaches for reducing the risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef] [PubMed]
- Elouafi, I.; Shahid, M.; Begmuratov, A.; Hirich, A. The Contribution of Alternative Crops to Food Security in Marginal Environments. In Emerging Research in Alternative Crops; Hirich, A., Choukr-Allah, R., Ragab, R., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 1–23. Available online: http://www.springer.com/series/5921 (accessed on 17 June 2022).
- Kumar, A.; Nayak, A.K.; Das, B.S.; Panigrahi, N.; Dasgupta, P.; Mohanty, S.; Kumar, U.; Panneerselvam, P.; Pathak, H. Effects of water deficit stress on agronomic and physiological responses of rice and greenhouse gas emission from rice soil under elevated atmospheric CO2. Sci. Total Environ. 2019, 650, 2032–2050. [Google Scholar] [CrossRef] [PubMed]
- Pedroza, S.A. Cambio Climático, Impacto Ambiental y Seguridad Alimentaria: Un Reto Permanente. El Siglo de Torreón. 2016. Available online: https://www.elsiglodetorreon.com.mx/noticia/2016/cambio-climatico-impacto-ambiental-y-seguridad-alimentaria-un-reto-permanente.html (accessed on 17 January 2017).
- Miñon, D.; Gallegos, J.; Barbosa, R. Producción de Forraje de Especies y Cultivares de Leguminosas en Valles Regados Norpatagónicos. In INTA—Información Técnica; EEA Valle Inferior-Convenio Provincia de Río Negro-INT. 2013, Volume 53, p. 74. Available online: http://repositorio.inta.gob.ar/handle/20.500.12123/4022 (accessed on 25 November 2022).
- Álvarez-Vázquez, P.; García de los Santos, G.; Guerrero-Rodríguez, J.D.; Mendoza-Pedroza, S.; Ortega-Cerrila, M.E.; Hernández-Garay, A. Comportamiento productivo de Lotus corniculatus L. dependiente de la estrategia de cosecha. Agrociencia 2018, 52, 1081–1093. [Google Scholar]
- Castillo-Cabrera, C.; Mendoza-Pedroza, S.I.; Escalante-Estrada, J.A.S.; García-de los Santos, G.; Pro-Martínez, A.; González-Cerón, F. Análisis de crecimiento de trébol pata de pájaro a diferente densidad de siembra. Rev. Mex. Cienc. Agrícolas 2023, 14, 265–276. [Google Scholar] [CrossRef]
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Nabi, S.Z.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant drought stress tolerance: Understanding its physiological, biochemical and molecular mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 1912–1925. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011, 6, 1720–1731. [Google Scholar]
- Tasiu, I. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Casler, M.D.; Undersander, D.J. Identification of Temperate Pasture Grasses and Legumes. In Horse Pasture Management; Sharpe, P., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 11–35. [Google Scholar]
- Wong-Paz, J.E.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Carrillo-Inungaray, M.L.; López, L.I.; Nevárez-Morillón, G.V.; Aguilar, C.N. Total phenolic content, in vitro antioxidant activity and chemical composition of plant extracts from semiarid Mexican region. Asian Pac. J. Trop. Med. 2015, 8, 104–111. [Google Scholar] [CrossRef]
- Carvajal, C.C. Especies Reactivas del Oxígeno: Formación, Función y Estrés Oxidativo. Med. Leg. Costa Rica 2019, 36, 91–100. Available online: https://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S1409-00152019000100091&lang=es (accessed on 12 April 2022).
- Baali, N.; Mezrag, A.; Bouheroum, M.; Benayache, F.; Benayache, S.; Souad, A. Anti-inflammatory and antioxidant effects of Lotus corniculatus on paracetamol-induced hepatitis in rats. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2019, 19, 128–139. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Bensegem, F. Reactive oxygen species signaling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Medina, G.G.G.; Díaz, P.J.; López, H.J.A.; Ruíz, C.y.M.; Marín, S. Estadísticas Climatológicas Básicas del Estado de Durango. (Periodo 1961–2003); Libro Técnico № 1; Campo Experimental Valle del Guadiana; CIRNOC-INIFAP: Durango, México, 2005; p. 224. [Google Scholar]
- Richard, L.A. Porous Plate Apparatus for Measuring Moisture Retention and Transmission by Soil. Soil Sci. 1948, 66, 105–110. Available online: https://journals.lww.com/soilsci/Citation/1948/08000/POROUS_PLATE_APPARATUS_FOR_MEASURING_MOISTURE.3.aspx (accessed on 14 July 2022). [CrossRef]
- Chávez-Barrantes, N.; Gutiérrez-Soto, M.V. Respuestas al estrés por calor en los cultivos. Tolerancia y tratamiento agronómico. Agron. Mesoam. 2017, 28, 255–271. [Google Scholar] [CrossRef]
- Barbazán, M.M.; Ferrando, M.; Zamalvide, J. Diagnóstico Nutricional de Lotus corniculatus L. en Suelos de Uruguay. Inf. Agronómicas 2008, 39, 6–13. Available online: https://fertilizar.org.ar/wp-content/uploads/2008/09/2.pdf (accessed on 17 May 2022).
- Melgarejo, L.; Romero, M.; Hernández, S.; Barrera, J.; Solarte, M.; Suárez, D.; Pérez, L.; Rojas, A.; Cruz, M.; Moreno, L.; et al. Experimentos en Fisiología Vegetal; Universidad Nacional de Colombia: Bogotá, Colombia, 2010. [Google Scholar]
- Singleton, V.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Donkor, S.; Larbie, C.; Komlaga, G.; Emikpe, B.O. Phytochemical, antimicrobial, and antioxidant profiles of Duranta erecta L. Parts. Biochem. Res. Int. 2019, 2019, 8731595. [Google Scholar] [CrossRef]
- Maksimovíc, Z.; Maleňcíc, D.; Kovǎcevíc, N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 2005, 96, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, B.; Cruz, D.L.; Alvarado, J.A.; Mollinedo, P. Cuantificación de saponinas en muestras de Cañihua chenopodium pallidicaule aellen. Rev. Boliv. De Química 2013, 30, 131–136. [Google Scholar]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolics phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Radzikowska, D.; Ivanišová, E.; Szwengiel, A.; Kačániová, M.; Sawinska, Z. Influence of abiotic stress factors on the antioxidant properties and polyphenols profile composition of Green Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2020, 21, 397. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.; Carter, E.B.; Hauck, B.; Lanot, A.; Allison, G. Responses of Lotus corniculatus to environmental change 3: The sensitivity of phenolic accumulation to growth temperature and light intensity and effects on tissue digestibility. Planta 2021, 253, 35. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhou, S.; Liu, J.; Feng, Q.; Wei, R.; Yu, H.; Wang, B.; Zhang, Y.; Bai, X. Widely targeted metabolomics provides new insights into the flavonoid metabolism in ‘Kyoho’ grapes under a two-crop-a-year cultivation system. Horticulturae 2023, 9, 154. [Google Scholar] [CrossRef]
- Gebrehiwot, L.; Beuselinck, P.R.; Roberts, C.A. Seasonal variations in condensed tannin concentration of three Lotus species. Agron. J. 2002, 94, 1059–1065. [Google Scholar] [CrossRef]
- Vignolio, O.; Fernández, O. Bioecología de Lotus glaber Mill. (Fabaceae) en la Pampa Deprimida (provincia de Buenos Aires, Argentina). Rev. Argent. Prod. Anim. 2014, 26, 113–130. Available online: www.semanticscholar.org (accessed on 27 November 2022).
- Acuña, H.; Concha, A.; Figueroa, M. Condensed tannin concentrations of three Lotus species grown in different environments. Chil. J. Agric. Res. 2008, 68, 31–41. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011, 10, 471–491. [Google Scholar] [CrossRef]
- Santacoloma, V.L.E. Fitometabolitos secundarios que inciden en el valor nutricional de Lotus corniculatus como forraje para rumiantes. Rev. Investig. Agrar. Y Ambient. 2014, 5, 131. [Google Scholar] [CrossRef]
- Albergaria, E.T.; Oliveira, A.F.M.; Albuquerque, U.P. The effect of water deficit stress on the composition of phenolic compounds in medicinal plants. S. Afr. J. Bot. 2020, 131, 12–17. [Google Scholar] [CrossRef]


| Ecotype Identification/Variety * | Country of Origin | Growth Habit |
|---|---|---|
| 255301 | France | Semi-erect |
| 255305 | Italy | Semi-erect |
| 202700 | Uruguay | Erect |
| 226792 | Canada | Semi-erect |
| Estanzuela Ganador | Uruguay | Erect |
| Ecotypes/Variety | SWC | Total Phenols Concentration (mg GAE/gFW) | |||
|---|---|---|---|---|---|
| Summer 2021 | Autumn 2021 | Winter 2021–2022 | Spring 2022 | ||
| 255301 | 100% FC (NWD) | 38.793 ± 4.5 a | 20.134 ± 3.4 a | 61.325 ± 4.5 b | 25.359 ± 3.2 a |
| 89% FC (WD) | 47.301 ± 0.5 a | 33.275 ± 2.6 a | 86.622 ± 3.8 a | 31.116 ± 1.3 a | |
| 255305 | 100% FC (NWD) | 50.670 ± 1.5 a | 27.374 ± 5.3 a | 73.453 ± 1.8 ab | 29.323 ± 0.7 a |
| 89% FC (WD) | 45.181 ± 7.7 a | 26.850 ± 1.9 a | 81.437 ± 5.7 ab | 30.906 ± 1.4 a | |
| 202700 | 100% FC (NWD) | 41.521 ± 8.8 a | 20.848 ± 3.4 a | 62.495 ± 5.2 b | 23.894 ± 3.5 a |
| 89% FC (WD) | 40.451 ± 1.4 a | 29.363 ± 2.8 a | 76.156 ± 6.2 ab | 30.715 ± 1.0 a | |
| 226792 | 100% FC (NWD) | 38.975 ± 1.9 a | 28.591 ± 1.2 a | 61.450 ± 0.7 b | 25.035 ± 0.2 a |
| 89% FC (WD) | 39.017 ± 3.4 a | 31.117 ± 4.9 a | 87.248 ± 1.4 a | 36.392 ± 3.0 a | |
| Estanzuela Ganador | 100% FC (NWD) | 49.593 ± 5.4 a | 30.404 ± 3.8 a | 66.796 ± 2.0 ab | 25.353 ± 3.8 a |
| 89% FC (WD) | 38.962 ± 1.9 a | 27.781 ± 1.8 a | 73.926 ± 6.7 ab | 33.561 ± 3.0 a | |
| Ecotypes/Variety | SWC | Total Flavonoid Concentration (mg QE/gFW) | |||
|---|---|---|---|---|---|
| Summer 2021 | Autumn 2021 | Winter 2021–2022 | Spring 2022 | ||
| 255301 | 100% FC (NWD) | 16.592 ± 1.4 a | 18.440 ± 1.6 a | 34.557 ± 6.8 cd | 12.898 ± 1.6 a |
| 89% FC (WD) | 19.084 ± 2.1 a | 17.478 ± 1.1 a | 57.965 ± 0.4 ab | 20.454 ± 1.0 a | |
| 255305 | 100% FC (NWD) | 19.919 ± 1.9 a | 19.452 ± 1.4 a | 42.826 ± 0.9 bcd | 16.385 ± 0.2 a |
| 89% FC (WD) | 17.084 ± 2.5 a | 19.253 ± 1.3 a | 51.887 ± 3.8 abc | 18.975 ± 2.2 a | |
| 202700 | 100% FC (NWD) | 20.731 ± 0.5 a | 17.512 ± 2.8 a | 43.294 ± 5.3 bcd | 13.975 ± 1.2 a |
| 89% FC (WD) | 16.220 ± 0.5 a | 16.517 ± 1.2 a | 56.008 ± 3.1 ab | 18.703 ± 0.6 a | |
| 226792 | 100% FC (NWD) | 16.594 ± 0.5 a | 15.385 ± 0.8 a | 27.187 ± 1.4 d | 13.393 ± 1.7 a |
| 89% FC (WD) | 16.415 ± 1.6 a | 16.345 ± 2.6 a | 53.210 ± 0.4 ab | 19.485 ± 0.3 a | |
| Estanzuela Ganador | 100% FC (NWD) | 19.016 ± 2.3 a | 15.070 ± 1.5 a | 48.435 ± 3.7 abc | 13.076 ± 1.8 a |
| 89% FC (WD) | 16.377 ± 0.2 a | 18.839 ± 0.7 a | 63.291 ± 4.3 a | 19.668 ± 1.8 a | |
| Ecotypes/Variety | SWC | Total Tannins Concentration (mg GAE/gFW) | |||
|---|---|---|---|---|---|
| Summer 2021 | Autumn 2021 | Winter 2021–2022 | Spring 2022 | ||
| 255301 | 100% FC (NWD) | 34.331 ± 3.5 a | 34.543 ± 3.6 a | 48.345 ± 1.3 bc | 20.656 ± 3.2 b |
| 89% FC (WD) | 36.300 ± 2.2 a | 43.844 ± 3.0 a | 71.710 ± 3.4 a | 32.353 ± 1.0 a | |
| 255305 | 100% FC (NWD) | 41.277 ± 3.7 a | 37.889 ± 5.6 a | 59.382 ± 3.3 abc | 23.502 ± 0.7 ab |
| 89% FC (WD) | 38.756 ± 2.6 a | 40.720 ± 2.9 a | 65.365 ± 4.7 a | 27.314 ± 4.1 ab | |
| 202700 | 100% FC (NWD) | 30.542 ± 5.0 a | 33.179 ± 2.7 a | 49.949 ± 3.0 bc | 22.043 ± 2.7 ab |
| 89% FC (WD) | 32.745 ± 2.3 a | 37.277 ± 1.7 a | 62.004 ± 2.9 ab | 26.880 ± 2.0 ab | |
| 226792 | 100% FC (NWD) | 36.121 ± 0.7 a | 38.794 ± 0.8 a | 44.550 ± 1.6 c | 23.777 ± 0.7 ab |
| 89% FC (WD) | 30.868 ± 1.8 a | 37.666 ± 3.8 a | 60.701 ± 3.0 ab | 28.751 ± 1.8 ab | |
| Estanzuela Ganador | 100% FC (NWD) | 41.653 ± 0.5 a | 35.806 ± 3.4 a | 49.798 ± 3.0 bc | 20.471 ± 1.7 b |
| 89% FC (WD) | 26.447 ± 0.1 a | 41.340 ± 0.8 a | 60.463 ± 1.9 ab | 32.006 ± 2.0 a | |
| Ecotypes/Variety | SWC | Total Saponins Concentration (mg Saponin/gFW) | |||
|---|---|---|---|---|---|
| Summer 2021 | Autumn 2021 | Winter 2021–2022 | Spring 2022 | ||
| 255301 | 100% FC (NWD) | 180.460 ± 14.2 ab | 161.762 ± 8.9 a | 92.652 ± 9.4 b | 31.211 ± 3.9 a |
| 89% FC (WD) | 254.869 ± 14.3 a | 186.385 ± 1.1 a | 145.342 ± 7.0 a | 45.301 ± 7.7 a | |
| 255305 | 100% FC (NWD) | 222.720 ± 11.6 ab | 138.888 ± 2.8 a | 131.771 ± 3.6 ab | 34.169 ± 9.5 a |
| 89% FC (WD) | 247.373 ± 14.6 ab | 186.005 ± 2.2 a | 107.550 ± 7.6 ab | 38.773 ± 8.1 a | |
| 202700 | 100% FC (NWD) | 176.378 ± 16.5 ab | 146.048 ± 1.9 a | 140.397 ± 14.3 ab | 30.338 ± 6.3 a |
| 89% FC (WD) | 187.382 ± 8.0 ab | 171.172 ± 6.9 a | 128.749 ± 5.8 ab | 42.577 ± 8.4 a | |
| 226792 | 100% FC (NWD) | 142.177 ± 18.6 b | 174.395 ± 2.1 a | 122.021 ± 13.2 ab | 34.887 ± 7.8 a |
| 89% FC (WD) | 138.890 ± 17.4 b | 143.549 ± 9.9 a | 120.065 ± 3.9 a | 42.595 ± 1.1 a | |
| Estanzuela Ganador | 100% FC (NWD) | 195.905 ± 3.9 ab | 169.869 ± 3.8 a | 106.424 ± 7.4 ab | 28.424 ± 2.8 a |
| 89% FC (WD) | 183.737 ± 6.9 ab | 195.811 ± 3.2 a | 136.781 ± 3.4 ab | 35.935 ± 2.0 a | |
| Ecotypes/Variety | SWC | Radical Scavenging Activity (mg of TEAC/gFW) | |||
|---|---|---|---|---|---|
| Summer 2021 | Autumn 2021 | Winter 2021–2022 | Spring 2022 | ||
| 255301 | 100% FC (NWD) | 42.528 ± 9.4 cd | 51.819 ± 5.9 abc | 133.138 ± 12.0 bcd | 47.110 ± 4.8 a |
| 89% FC (WD) | 78.613 ± 3.6 a | 114.253 ± 10.9 a | 244.910 ± 10.6 a | 70.941 ± 6.7 a | |
| 255305 | 100% FC (NWD) | 73.382 ± 3.9 ab | 105.165 ± 10.7 a | 209.139 ± 11.2 abc | 75.290 ± 10.3 a |
| 89% FC (WD) | 79.895 ± 3.2 a | 87.647 ± 12.8 ab | 221.492 ± 16.3 ab | 64.810 ± 3.1 a | |
| 202700 | 100% FC (NWD) | 60.7661 ± 2.2 bc | 85.630 ± 8.2 ab | 165.731 ± 8.9 a–d | 58.623 ± 5.8 a |
| 89% FC (WD) | 66.822 ± 2.7 abc | 23.665 ± 1.4 bc | 187.358 ± 14.1 a–d | 72.837 ± 2.6 a | |
| 226792 | 100% FC (NWD) | 27.992 ± 7.5 d | 24.782 ± 1.1 bc | 141.540 ± 17.0 bcd | 47.339 ± 2.6 a |
| 89% FC (WD) | 47.137 ± 5.5 bcd | 80.657 ± 4.1 abc | 179.409 ± 8.9 a | 85.027 ± 1.4 a | |
| Estanzuela Ganador | 100% FC (NWD) | 43.686 ± 8.9 cd | 62.400 ± 6.6 abc | 93.630 ± 6.1 d | 62.555 ± 7.2 a |
| 89% FC (WD) | 49.230 ± 3.1 bcd | 15.478 ± 1.6 c | 114.749 ± 9.4 cd | 67.850 ± 10.9 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Espíndola, L.A.; Pedroza-Sandoval, A.; de los Santos, G.G.; Trejo-Calzada, R.; Álvarez-Vázquez, P.; del Rosario Jacobo-Salcedo, M. Secondary Metabolites and Their Antioxidant Activity Enhance the Tolerance to Water Deficit on Clover Lotus corniculatus L. through Different Seasonal Times. Int. J. Plant Biol. 2024, 15, 175-186. https://doi.org/10.3390/ijpb15010014
González-Espíndola LA, Pedroza-Sandoval A, de los Santos GG, Trejo-Calzada R, Álvarez-Vázquez P, del Rosario Jacobo-Salcedo M. Secondary Metabolites and Their Antioxidant Activity Enhance the Tolerance to Water Deficit on Clover Lotus corniculatus L. through Different Seasonal Times. International Journal of Plant Biology. 2024; 15(1):175-186. https://doi.org/10.3390/ijpb15010014
Chicago/Turabian StyleGonzález-Espíndola, Luis Angel, Aurelio Pedroza-Sandoval, Gabino García de los Santos, Ricardo Trejo-Calzada, Perpetuo Álvarez-Vázquez, and Maria del Rosario Jacobo-Salcedo. 2024. "Secondary Metabolites and Their Antioxidant Activity Enhance the Tolerance to Water Deficit on Clover Lotus corniculatus L. through Different Seasonal Times" International Journal of Plant Biology 15, no. 1: 175-186. https://doi.org/10.3390/ijpb15010014
APA StyleGonzález-Espíndola, L. A., Pedroza-Sandoval, A., de los Santos, G. G., Trejo-Calzada, R., Álvarez-Vázquez, P., & del Rosario Jacobo-Salcedo, M. (2024). Secondary Metabolites and Their Antioxidant Activity Enhance the Tolerance to Water Deficit on Clover Lotus corniculatus L. through Different Seasonal Times. International Journal of Plant Biology, 15(1), 175-186. https://doi.org/10.3390/ijpb15010014







