Sustainable Recovery of CO2 by Using Visible-Light-Responsive Crystal Cuprous Oxide/Reduced Graphene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Crystal Cu2O Decorated with rGO
2.2. Characterizations
2.3. Photoreduction of CO2 Experiments
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- DeCicco, J.M. Methodological issues regarding biofuels and carbon uptake. Sustainability 2018, 10, 1581. [Google Scholar] [CrossRef]
- Sethupathi, S.; Zhang, M.; Rajapaksha, A.U.; Lee, S.R.; Nor, N.M.; Mohamed, A.R.; Al-Wabel, M.; Lee, S.S.; Ok, Y.S. Biochars as potential adsorbers of CH4, CO2 and H2S. Sustainability 2017, 9, 121. [Google Scholar] [CrossRef]
- Wieckol-Ryk, A.; Krzemien, A.; Smolinski, A.; Lasheras, F.S. Analysis of biomass blend co-firing for post combustion CO2 capture. Sustainability 2018, 10, 923. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Xu, Y.-J. Photocatalytic conversion of CO2 over graphene-based composites: Current status and future perspective. Nanoscale Horiz. 2016, 1, 185–200. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Sun, Y.W.; Jiang, X.; Zhang, A.F.; Song, C.S.; Guo, X.W. Pyrolyzing ZIF-8 to N-doped porous carbon facilitated by iron and potassium for CO2 hydrogenation to value-added hydrocarbons. J. CO2 Util. 2018, 25, 120–127. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, X.; Wang, X.; Wang, X. Progress in catalyst exploration for heterogeneous CO2 reduction and utilization: A critical review. J. Mater. Chem. A 2017, 5, 21625–21649. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Li, K.; Peng, B.; Peng, T. Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Rao, H.; Schmidt, L.C.; Bonin, J.; Robert, M. Visible-light-driven methane formation from CO2 with a molecular iron catalyst. Nature 2017, 548, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Perini, J.A.L.; Cardoso, J.C.; de Brito, J.F.; Zanoni, M.V.B. Contribution of thin films of ZrO2 on TiO2 nanotubes electrodes applied in the photoelectrocatalytic CO2 conversion. J. CO2 Util. 2018, 25, 254–263. [Google Scholar] [CrossRef]
- De Brito, J.F.; Hudari, F.F.; Zanoni, M.V.B. Photoelectrocatalytic performance of nanostructured p-n junction NtTiO2/NsCuO electrode in the selective conversion of CO2 to methanol at low bias potentials. J. CO2 Util. 2018, 24, 81–88. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. CdS/Graphene nanocomposite photocatalysts. Adv. Energy Mater. 2015, 5, 1500010. [Google Scholar] [CrossRef]
- Hou, J.; Cheng, H.; Takeda, O.; Zhu, H. Three-dimensional bimetal-graphene-semiconductor coaxial nanowire arrays to harness charge flow for the photochemical reduction of carbon dioxide. Angew. Chem. Int. Ed. 2015, 54, 8480–8484. [Google Scholar] [CrossRef] [PubMed]
- Penconi, M.; Rossi, F.; Ortica, F.; Elisei, F.; Gentili, P.L. Heterojunction p-n-p Cu2O/S-TiO2/CuO: Synthesis and application to photocatalytic conversion of CO2 to methane. J. CO2 Util. 2017, 20, 91–96. [Google Scholar]
- Pan, H.Q.; Steiniger, A.; Heagy, M.D.; Chowdhury, S. Efficient production of formic acid by simultaneous photoreduction of bicarbonate and oxidation of glycerol on gold-TiO2 composite under solar light. J. CO2 Util. 2017, 22, 117–123. [Google Scholar] [CrossRef]
- Zhang, D.; Maimaiti, H.; Awati, A.; Yisilamu, G.; Sun, F.; Wei, M. Synthesis and photocatalytic CO2 reduction performance of Cu2O/coal-based carbon nanoparticle composites. Chem. Phys. Lett. 2018, 700, 27–35. [Google Scholar]
- Abdullah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2 photocatalyst for CO2 photocatalytic reduction: An overview. J. CO2 Util. 2017, 22, 15–32. [Google Scholar] [CrossRef]
- Zubair, M.; Razzaq, A.; Grimes, C.A.; In, S.I. Cu2ZnSnS4 (CZTS)-ZnO: A noble metal-free hybrid Z-scheme photocatalyst for enhanced solar-spectrum photocatalytic conversion of CO2 to CH4. J. CO2 Util. 2017, 20, 301–311. [Google Scholar] [CrossRef]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Visible-light-activated oxygen-rich TiO2 as next generation photocatalyst: Importance of annealing temperature on the photoactivity toward reduction of carbon dioxide. Chem. Eng. J. 2016, 283, 1254–1263. [Google Scholar] [CrossRef]
- Akple, M.S.; Low, J.; Liu, S.W.; Cheng, B.; Yu, J.G.; Ho, W.K. Fabrication and enhanced CO2 reduction performance of N-self-doped TiO2 microsheet photocatalyst by bi-cocatalyst modification. J. CO2 Util. 2016, 16, 442–449. [Google Scholar] [CrossRef]
- Liu, S.-H.; Syu, H.-R. One-step fabrication of N-doped mesoporous TiO2 nanoparticles by self-assembly for photocatalytic water splitting under visible light. Appl. Energy 2012, 100, 148–154. [Google Scholar] [CrossRef]
- Liu, S.-H.; Syu, H.-R. High visible-light photocatalytic hydrogen evolution of C,N-codoped mesoporous TiO2 nanoparticles prepared via an ionic-liquid-template approach. Int. J. Hydrogen Energy 2013, 38, 13856–13865. [Google Scholar] [CrossRef]
- Yu, L.; Li, G.; Zhang, X.; Ba, X.; Shi, G.; Li, Y.; Wong, P.K.; Yu, J.C.; Yu, Y. Enhanced activity and stability of carbon-decorated cuprous oxide mesoporous nanorods for CO2 reduction in artificial photosynthesis. ACS Catal. 2016, 6, 6444–6454. [Google Scholar] [CrossRef]
- Siegfried, M.J.; Choi, K.S. Elucidating the effect of additives on the growth and stability of Cu2O surfaces via shape transformation of pre-grown crystals. J. Am. Chem. Soc. 2006, 128, 10356–10357. [Google Scholar] [CrossRef] [PubMed]
- An, X.Q.; Li, T.; Wen, B.; Tang, J.W.; Hu, Z.Y.; Liu, L.M.; Qu, J.H.; Huang, C.P.; Liu, H.J. New Insights into defect-Mediated heterostructures for photoelectrochemical water splitting. Adv. Energy Mater. 2016, 6, 1502268. [Google Scholar] [CrossRef]
- Wu, J.; Xu, K.; Liu, Q.Z.; Ji, Z.; Qu, C.H.; Zhang, H.; Guan, Y.; He, P.; Zhu, L.J. Controlling dominantly reactive (010) facets and impurity level by in-situ reduction of BiOIO3 for enhancing photocatalytic activity. Appl. Catal. B Environ. 2018, 232, 135–145. [Google Scholar] [CrossRef]
- He, W.J.; Sun, Y.J.; Jiang, G.M.; Huang, H.W.; Zhang, X.M.; Dong, F. Activation of amorphous Bi2WO6 with synchronous Bi metal and Bi2O3 coupling: Photocatalysis mechanism and reaction pathway. Appl. Catal. B Environ. 2018, 232, 340–347. [Google Scholar] [CrossRef]
- Tan, C.F.; Zing, A.; Chen, Z.H.; Liow, C.H.; Phan, H.T.; Tan, H.R.; Xu, Q.H.; Ho, G.W. Inverse stellation of CuAu-ZnO multimetallic-semiconductor nanostartube for plasmon-enhanced photocatalysis. ACS Nano 2018, 12, 4512–4520. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.S.; Wen, Z.H. Cu nanoparticles decorating rGO nanohybrids as electrocatalyst toward CO2 reduction. J. CO2 Util. 2017, 22, 231–237. [Google Scholar] [CrossRef]
- Ma, Y.J.; Zhu, X.Z.; Xu, S.S.; He, G.L.; Yao, L.; Hu, N.T.; Su, Y.J.; Feng, J.; Zhang, Y.F.; Yang, Z. Gold nanobipyramid@cuprous oxide jujube-like nanostructures for plasmon-enhanced photocatalytic performance. Appl. Catal. B Environ. 2018, 234, 26–36. [Google Scholar] [CrossRef]
- Kerour, A.; Boudjadar, S.; Bourzami, R.; Allouche, B. Eco-friendly synthesis of cuprous oxide (Cu2O) nanoparticles and improvement of their solar photocatalytic activities. J. Solid State Chem. 2018, 263, 79–83. [Google Scholar] [CrossRef]
- Kumar, S.; Parlett, C.M.A.; Isaacs, M.A.; Jowett, D.V.; Douthwaite, R.E.; Cockett, M.C.R.; Lee, A.F. Facile synthesis of hierarchical Cu2O nanocubes as visible light photocatalysts. Appl. Catal. B Environ. 2016, 189, 226–232. [Google Scholar] [CrossRef]
- Shang, Y.; Guo, L. Facet-controlled synthetic strategy of Cu2O-based crystals for catalysis and sensing. Adv. Sci. 2015, 2, 1500140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, Y.J.; Yang, Z.; Tang, X.H.; Li, X.L.; He, G.L.; Cheng, Y.Q.; Fang, Z.B.; He, R.; Zhang, Y.F. Analysis of synergistic effect between graphene and octahedral cuprous oxide in cuprous oxide-graphene composites and their photocatalytic application. J. Alloys Compd. 2017, 712, 704–713. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lu, J.-S. Facet-dependent cuprous oxide nanocrystals decorated with graphene as durable photocatalysts under visible light. Nanomaterials 2018, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Lu, J.-S.; Yang, S.-W. Highly visible-light-responsive Cu2O/rGO decorated with Fe3O4@SiO2 nanoparticles as a magnetically recyclable photocatalyst. Nanotechnology 2018, 29, 305606. [Google Scholar] [CrossRef] [PubMed]
- Handoko, A.D.; Tang, J. Controllable proton and CO2 photoreduction over Cu2O with various morphologies. Int. J. Hydrog. Energy 2013, 38, 13017–13022. [Google Scholar] [CrossRef]
- Ba, X.; Yan, L.L.; Huang, S.; Yu, J.G.; Xia, X.J.; Yu, Y. New way for CO2 reduction under visible light by a combination of a Cu electrode and semiconductor thin film: Cu2O conduction type and morphology effect. J. Phys. Chem. C 2014, 118, 24467–24478. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, P.; Sharma, O.P.; Jain, S.L.; Khatri, O.P. Reduced graphene oxide–CuO nanocomposites for photocatalytic conversion of CO2 into methanol under visible light irradiation. Appl. Catal. B Environ. 2016, 181, 352–362. [Google Scholar] [CrossRef]
- Schreier, M.; Gao, P.; Mayer, M.T.; Luo, J.; Moehl, T.; Nazeeruddin, M.K.; Tilley, S.D.; Grätzel, M. Efficient and selective carbon dioxide reduction on low cost protected Cu2O photocathodes using a molecular catalyst. Energy Environ. Sci. 2015, 8, 855–861. [Google Scholar] [CrossRef]
- Lee, C.; Shin, K.; Lee, Y.J.; Jung, C.; Lee, H.M. Effects of shell thickness on Ag-Cu2O core-shell nanoparticles with bumpy structures for enhancing photocatalytic activity and stability. Catal. Today 2018, 303, 313–319. [Google Scholar] [CrossRef]
- Niu, W.Z.; Moehl, T.; Cui, W.; Wick-Joliat, R.; Zhu, L.P.; Tilley, S.D. Extended light harvesting with dual Cu2O-based photocathodes for high efficiency water splitting. Adv. Energy Mater. 2018, 8, 1702323. [Google Scholar] [CrossRef]
- Periasamy, A.P.; Ravindranath, R.; Kumar, S.M.S.; Wu, W.P.; Jian, T.R.; Chang, H.T. Facet- and structure-dependent catalytic activity of cuprous oxide/polypyrrole particles towards the efficient reduction of carbon dioxide to methanol. Nanoscale 2018, 10, 11869–11880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, L.; Kang, S.; Yin, C.; Ma, Z.; Cui, L.; Wang, Y. CuO/Cu2O nanowire arrays grafted by reduced graphene oxide: Synthesis, characterization, and application in photocatalytic reduction of CO2. RSC Adv. 2017, 7, 43642–43647. [Google Scholar] [CrossRef]
- Liu, S.-H.; Wei, Y.-S.; Lu, J.-S. Visible-light-driven photodegradation of sulfamethoxazole and methylene blue by Cu2O/rGO photocatalysts. Chemosphere 2016, 154, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Cheng, B.; Yu, J. Graphene-based photocatalysts for solar-fuel generation. Angew. Chem. Int. Ed. 2015, 54, 11350–11366. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.C.; Ronconi, C.M. Self-assembled 3D mesoporous graphene oxides (MEGOs) as adsorbents and recyclable solids for CO2 and CH4 capture. J. CO2 Util. 2017, 20, 292–300. [Google Scholar] [CrossRef]
- Deerattrakul, V.; Dittanet, P.; Sawangphruk, M.; Kongkachuichay, P. CO2 hydrogenation to methanol using Cu-Zn catalyst supported on reduced graphene oxide nanosheets. J. CO2 Util. 2017, 16, 104–113. [Google Scholar] [CrossRef]
- An, X.; Li, K.; Tang, J. Cu2O/reduced graphene oxide composites for the photocatalytic conversion of CO2. ChemSusChem 2014, 7, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, X.; Zhao, Y.; Wu, W.; Chen, J.; Meng, H. Preparation and characterizations of Cu2O/reduced graphene oxide nanocomposites with high photo-catalytic performances. Powder Technol. 2014, 261, 42–48. [Google Scholar] [CrossRef]
- Liu, S.-H.; Yang, S.-W. Highly efficient cuprous oxide nanocrystals assisted with graphene for decolorization using visible light. Water Air Soil Pollut. 2018, 229, 67. [Google Scholar] [CrossRef]
- Huang, W.C.; Lyu, L.M.; Yang, Y.C.; Huang, M.H. Synthesis of Cu2O nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hao, C.; Jin, G.; Zhu, H.Y.; Guo, X.Y. Copper nanoparticles on graphene support: An efficient photocatalyst for coupling of nitroaromatics in visible light. Angew. Chem. Int. Ed. 2014, 53, 1973–1977. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Wang, Q.; Lu, A.; Zhang, L. Portable visible-light photocatalysts constructed from Cu2O nanoparticles and graphene oxide in cellulose matrix. J. Phys. Chem. C 2014, 118, 7202–7210. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, L.; Liu, L.; Wang, X.; Sun, J.; Wu, S.; Deng, Y.; Tang, C.; Gao, F.; Dong, L. Engineering the Cu2O–reduced graphene oxide interface to enhance photocatalytic degradation of organic pollutants under visible light. Appl. Catal. B Environ. 2016, 181, 495–503. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yang, Z.; Tang, X.; Ma, Y.; Li, M.; Hu, N.; Wei, H.; Zhang, Y. In situ preparation of cubic Cu2O-RGO nanocomposites for enhanced visible-light degradation of methyl orange. Nanotechnology 2016, 27, 265703. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, W.; Li, Z. One-pot self-assembly of Cu2O/RGO composite aerogel for aqueous photocatalysis. Appl. Surf. Sci. 2015, 358, 146–151. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, N.; Jiu, H.F.; Qi, G.S.; Huang, Y.J. ZnO-reduced graphene oxide nanocomposites as efficient photocatalysts for photocatalytic reduction of CO2. Ceram. Int. 2015, 41, 6256–6262. [Google Scholar] [CrossRef]
- Ma, G.X.; Jia, R.R.; Zhao, J.H.; Wang, Z.J.; Song, C.; Jia, S.P.; Zhu, Z.P. Nitrogen-doped hollow carbon nanoparticles with excellent oxygen reduction performances and their electrocatalytic kinetics. J. Phys. Chem. C 2011, 115, 25148–25154. [Google Scholar] [CrossRef]
- Xiang, C.X.; Kimball, G.M.; Grimm, R.L.; Brunschwig, B.S.; Atwater, H.A.; Lewis, N.S. 820 mV open-circuit voltages from Cu2O/CH3CN junctions. Energy Environ. Sci. 2011, 4, 1311–1318. [Google Scholar] [CrossRef]
- Yuan, J.L.; Wang, X.; Gu, C.H.; Sun, J.J.; Ding, W.M.; Wei, J.J.; Zuo, X.Y.; Hao, C.J. Photoelectrocatalytic reduction of carbon dioxide to methanol at cuprous oxide foam cathode. RSC Adv. 2017, 7, 24933–24939. [Google Scholar] [CrossRef]
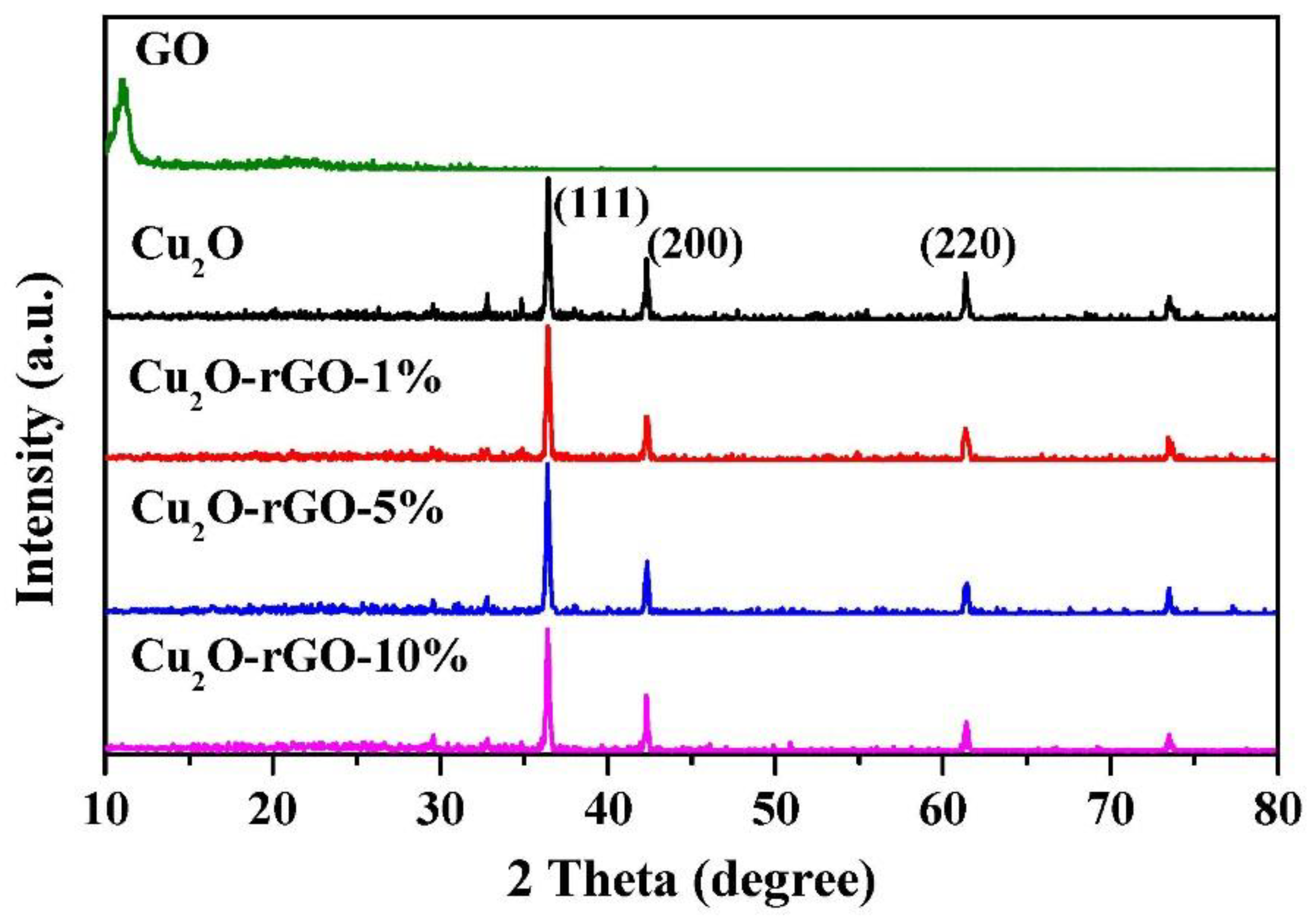



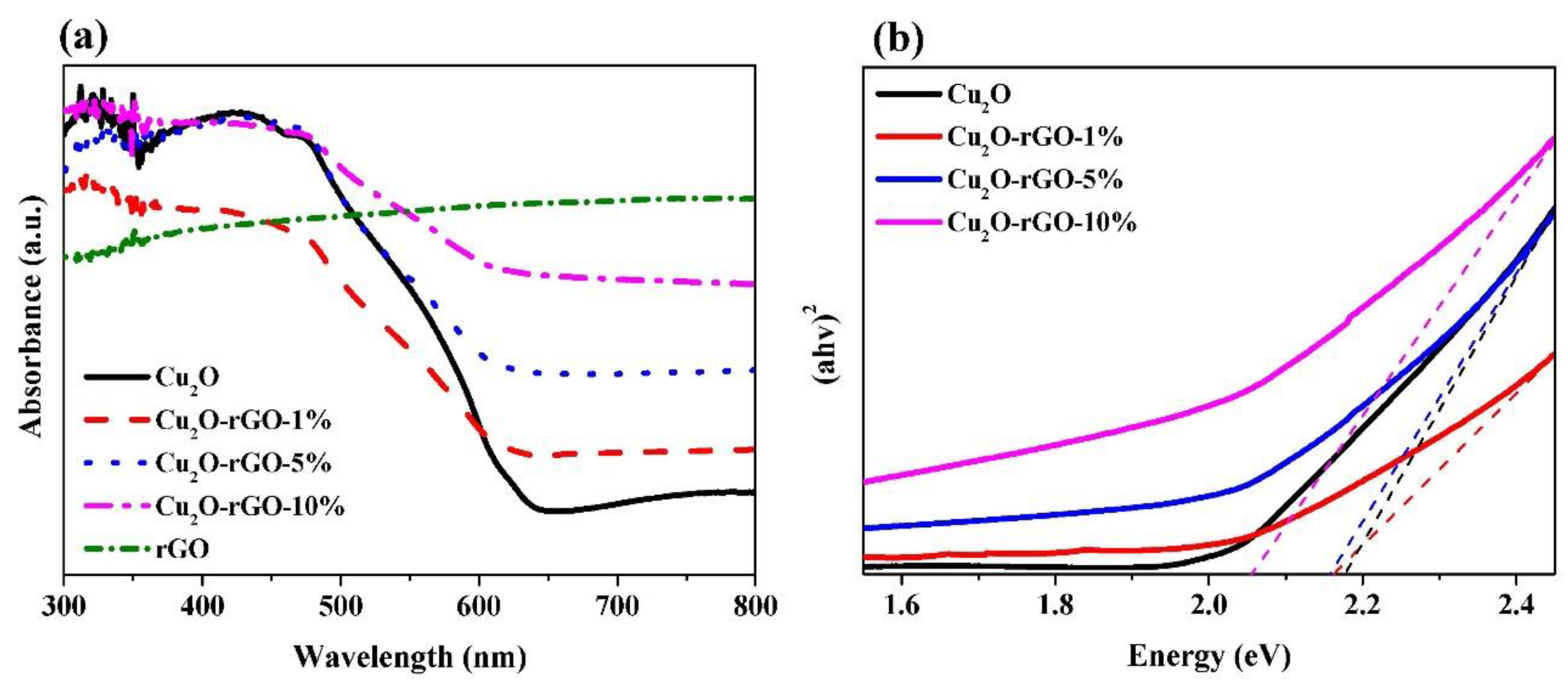
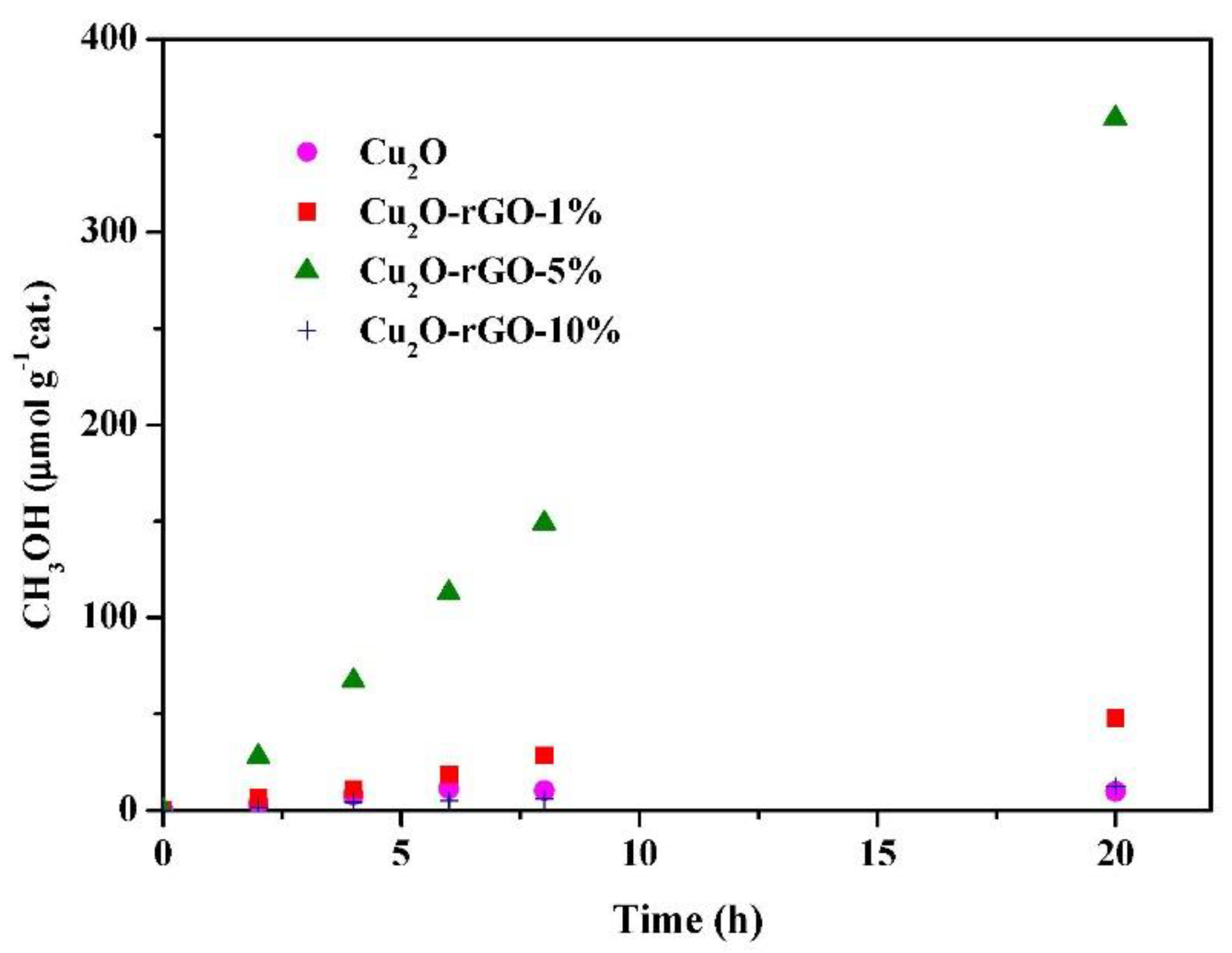

| Sample | Energy Gap (eV) | Threshold Wavelength (nm) |
|---|---|---|
| Cu2O | 2.18 | 570.11 |
| Cu2O-rGO-1% | 2.16 | 573.01 |
| Cu2O-rGO-5% | 2.16 | 574.87 |
| Cu2O-rGO-10% | 2.06 | 603.11 |
| Sample | Reaction Precursor | Visible Light Illumination (λ > 400 nm) | Methanol Yield (μmol g−1-cat) |
|---|---|---|---|
| None | CO2 | O | None |
| Cu2O | CO2 | O | 9.76 |
| Cu2O-rGO-1% | CO2 | O | 47.91 |
| Cu2O-rGO-5% | CO2 | O | 355.26 |
| Cu2O-rGO-5% | CO2 | X | None |
| Cu2O-rGO-5% | N2 | O | None |
| Cu2O-rGO-10% | CO2 | O | 12.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-H.; Lu, J.-S.; Chen, Y.-C. Sustainable Recovery of CO2 by Using Visible-Light-Responsive Crystal Cuprous Oxide/Reduced Graphene Oxide. Sustainability 2018, 10, 4145. https://doi.org/10.3390/su10114145
Liu S-H, Lu J-S, Chen Y-C. Sustainable Recovery of CO2 by Using Visible-Light-Responsive Crystal Cuprous Oxide/Reduced Graphene Oxide. Sustainability. 2018; 10(11):4145. https://doi.org/10.3390/su10114145
Chicago/Turabian StyleLiu, Shou-Heng, Jun-Sheng Lu, and Yi-Chiun Chen. 2018. "Sustainable Recovery of CO2 by Using Visible-Light-Responsive Crystal Cuprous Oxide/Reduced Graphene Oxide" Sustainability 10, no. 11: 4145. https://doi.org/10.3390/su10114145




