Reduced Carbon Dioxide Sink and Methane Source under Extreme Drought Condition in an Alpine Peatland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Experimental Observation
2.2.1. Sampling Land Design
2.2.2. Greenhouse Gas Fluxes and Environmental Factors
2.2.3. Data Processing and Analysis
2.2.4. Statistical Analysis
3. Results
3.1. Hydrothermal Conditions
3.2. The Diurnal Patterns of Carbon Fluxes
3.3. The Effects of Extreme Drought on CO2 Fluxes
3.4. The Effects of Extreme Drought on CH4 Fluxes
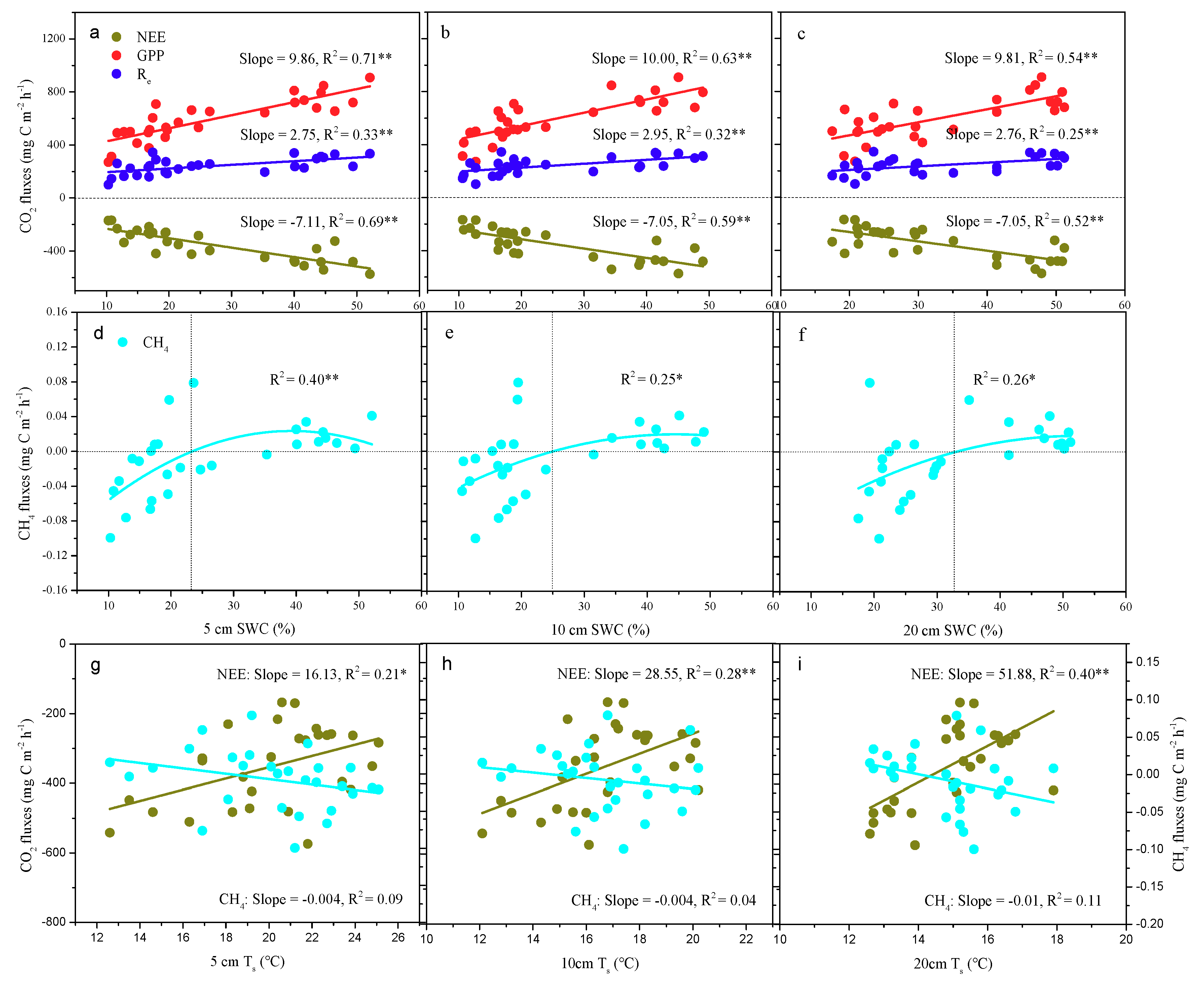
3.5. The Relationship between Carbon Fluxes and the Soil Microenvironment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Kreyling, J.; Wenigmann, M.; Beierkuhnlein, C.; Jentsch, A. Effects of extreme weather events on plant productivity and tissue die-back are modified by community composition. Ecosystems 2008, 11, 752–763. [Google Scholar] [CrossRef]
- Glaser, B.; Jentsch, A.; Kreyling, J.; Beierkuhnlein, C. Soil-moisture change caused by experimental extreme summer drought is similar to natural inter-annual variation in a loamy sand in Central Europe. J. Plant. Nutr. Soil Sci. 2013, 176, 27–34. [Google Scholar] [CrossRef]
- Graw, V.; Ghazaryan, G.; Dall, K.; Delgado Gómez, A.; Abdel-Hamid, A.; Jordaan, A.; Piroska, R.; Post, J.; Szarzynski, J.; Walz, Y.; et al. Drought Dynamics and Vegetation Productivity in Different Land Management Systems of Eastern Cape; South Africa—A Remote Sensing Perspective. Sustainability 2017, 9, 1728. [Google Scholar] [CrossRef]
- Thakur, M.P.; Reich, P.B.; Hobbie, S.E. Reduced feeding activity of soil detritivores under warmer and drier conditions. Nat. Clim. Chang. 2018, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Knox, R.G.; Levine, N.M.; Alves, L.F.; Bonal, D.; Camargo, P.B.; Fitzjarrald, D.R.; Hayek, M.N.; Restrepo-Coupe, N.; Saleska, S.R.; et al. Ecosystem heterogeneity and diversity mitigate Amazon forest resilience to frequent extreme droughts. New Phytol. 2018, 219, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Melinda, D.; Smith, A.K.K.; Scott, L.; Collins, A. Framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 2009, 90, 3279–3289. [Google Scholar]
- Um, M.J.; Kim, M.M.; Kim, Y.; Park, D. Drought Assessment with the Community Land Model for 1951–2010 in East Asia. Sustainability 2018, 10, 2100. [Google Scholar] [CrossRef]
- Reichstein, M. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D. The ecological role of climate extremes: Current understanding and future prospects. J. Ecol. 2011, 99, 651–655. [Google Scholar] [CrossRef]
- Jiang, Z. Extreme climate events in China: IPCC-AR4 model evaluation and projection. Clim. Chang. 2012, 110, 385–401. [Google Scholar] [CrossRef]
- Du, L.; Mikle, N.; Zou, Z. Global patterns of extreme drought-induced loss in land primary production: Identifying ecological extremes from rain-use efficiency. Sci. Total Environ. 2018, 628–629, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, A.; Kreyling, J.; Beierkuhnlein, C. A new generation of climate-change experiments: Events, not trends. Front. Ecol. Environ. 2007, 5, 365–374. [Google Scholar] [CrossRef]
- Turunen, J.; Tomppo, E.; Tolonen, K.; Reinikainen, A. Estimating carbon accumulation rates of undrained mires in Finland–application to boreal and subarctic regions. Holocene 2002, 12, 69–80. [Google Scholar] [CrossRef]
- Fleischer, E.; Khashimov, I.; Hölzel, N.; Klemm, O. Carbon exchange fluxes over peatlands in Western Siberia: Possible feedback between land-use change and climate change. Sci. Total Environ. 2016, 545, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.M.; Hao, Y.B.; Cui, X.Y.; Chen, H.; Huang, S.X.; Du, Y.G.; Li, W.; Kardol, P.; Xiao, X.M.; Cui, L.J. Variability and changes in climate, phenology, and gross primary production of an Alpine wetland ecosystem. Remote Sens. 2016, 8, 391. [Google Scholar] [CrossRef]
- Cui, L.J.; Kang, X.M.; Li, W.; Hao, Y.B.; Zhang, Y.; Wang, J.Z.; Yan, L.; Zhang, X.D.; Zhang, M.Y.; Zhou, J.P.; et al. Rewetting decreases carbon emissions from the Zoige alpine peatland on the Tibetan Plateau. Sustainability 2017, 9, 948. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, G.; Jia, Q.Y. Annual cycle of CO2 exchange over a reed (Phragmites australis) wetland in Northeast China. Aquat. Bot. 2009, 91, 91–98. [Google Scholar] [CrossRef]
- Hao, Y.B.; Cui, X.Y.; Wang, Y.F.; Mei, X.R.; Kang, X.M.; Wu, N.; Luo, P.; Zhu, D. Predominance of precipitation and temperature controls on ecosystem CO2 exchange in Zoige alpine wetlands of Southwest China. Wetlands 2011, 31, 413–422. [Google Scholar] [CrossRef]
- Polsenaere, P.; Lamaud, E.; Lafon, V. Spatial and temporal CO2 exchanges measured by eddy covariance over a temperate intertidal flat and their relationships to net ecosystem production. Biogeosciences 2012, 9, 249–268. [Google Scholar] [CrossRef]
- Sanaullah, M.; Chabbi, A.; Rumpel, C.; Kuzyakov, Y. Carbon allocation in grassland communities under drought stress followed by 14C pulse labeling. Soil Boil. Biochem. 2012, 55, 132–139. [Google Scholar] [CrossRef]
- Jentsch, A. Climate extremes initiate ecosystem-regulating functions while maintaining productivity. J. Ecol. 2011, 99, 689–702. [Google Scholar] [CrossRef] [Green Version]
- Royer, P.D.; Cobb, N.S.; Clifford, M.J.; Huang, C.Y.; Breshears, D.D.; Adams, H.D.; Villegas, J.C. Extreme climatic event-triggered overstorey vegetation loss increases understorey solar input regionally: Primary and secondary ecological implications. J. Ecol. 2011, 99, 714–723. [Google Scholar] [CrossRef]
- Kolb, T.; Dore, S.; Montes-Helu, M. Extreme late-summer drought causes neutral annual carbon balance in southwestern ponderosa pine forests and grasslands. Environ. Res. Lett. 2013, 8, 015015. [Google Scholar] [CrossRef] [Green Version]
- Sotta, E.D.; Veldkamp, E.; Schwendenmann, L.; Guimarães, B.R.; Paixão, R.K.; Ruivo, M.D.L.P.; Lola Da Costa, A.C.; Meir, P. Effects of an induced drought on soil carbon dioxide (CO2) efflux and soil CO2 production in an Eastern Amazonian rainforest, Brazil. Glob. Chang. Biol. 2007, 13, 2218–2229. [Google Scholar] [CrossRef]
- Cox, P.M.; Betts, R.A.; Jones, C.D.; Spall, S.A.; Totterdell, I.J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 2000, 408, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.D.; Macalady, A.K.; Breshears, D.D.; Allen, C.D.; Stephenson, N.L.; Saleska, S.R.; Huxman, T.E. Climate-induced tree mortality: Earth system consequences. Eos Trans. Am. Geophys. Union 2010, 91, 153. [Google Scholar] [CrossRef]
- Hartley, I.P.; Armstrong, A.F.; Murthy, R. The dependence of respiration on photosynthetic substrate supply and temperature: Integrating leaf, soil and ecosystem measurements. Glob. Chang. Biol. 2006, 12, 1954–1968. [Google Scholar] [CrossRef]
- Mueller, R.C. Differential tree mortality in response to severe drought: Evidence for long-term vegetation shifts. J. Ecol. 2005, 93, 1085–1093. [Google Scholar] [CrossRef]
- Olefeldt, D.; Euskirchen, E.S.; Harden, J. A decade of boreal rich fen greenhouse gas fluxes in response to natural and experimental water table variability. Glob. Chang. Biol. 2017, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, G.; Peng, C.H.; Zhang, Y.; Zhu, D.; Zhu, Q.A.; Hu, J.; Wang, M.; Zhan, W.; Zhu, E.X.; et al. The carbon stock of alpine peatlands on the QinghaieTibetan Plateau during the Holocene and their future fate. Quat. Sci. Rev. 2014, 95, 151–158. [Google Scholar] [CrossRef]
- Zheng, D.; Li, B.Y. Progress in studies on geographical environments of the Qinghai-Xizang plateau. Sci. Geo Sin. 1999, 19, 295–302. [Google Scholar]
- Kang, X.M.; Wang, Y.F.; Chen, H.; Tian, J.Q.; Cui, X.Y.; Rui, Y.C.; Zhong, L.; Kardol, P.; Hao, Y.B.; Xiao, X.M. Modeling carbon fluxes using multi-temporal MODIS imagery and CO2 eddy flux tower data in Zoige Alpine Wetland. Wetlands 2014, 34, 603–618. [Google Scholar] [CrossRef]
- Wang, M.; Yang, G.; Gao, Y.H.; Chen, H.; Wu, N.; Peng, C.H.; Zhu, Q.A.; Zhu, D.; Wu, J.H.; He, Y.X.; et al. Higher recent peat C accumulation than that during the Holocene on the Zoige Plateau. Quat. Sci. Rev. 2015, 114, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Corrigan, E.; Nieuwenhuis, M. Using Goal-Programming to Model the Effect of Stakeholder Determined Policy and Industry Changes on the Future Management of and Ecosystem Services Provision by Ireland’s Western Peatland Forests. Sustainability 2017, 9, 11. [Google Scholar] [CrossRef]
- Tian, Y.B.; Xiong, M.B.; Xiong, X.S.; Song, G.Y. The organic carbon distribution and flow in wetland soil-plant system in ruoergai plateau. Acta Phytoecol. Sin. 2003, 27, 490–495. [Google Scholar]
- Chen, H.; Wu, N.; Gao, Y.H.; Wang, Y.F.; Luo, P.; Tian, J.Q. Spatial variations on methane emissions from Zoige alpine wetlands of Southwest China. Sci. Total Environ. 2009, 407, 1097–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, M.; Zhu, C.; Song, Y.; Ma, C.; Yang, Z. Paleoenvironment change and its impact on carbon and nitrogen accumulation in the Zoige wetland, northeastern Qinghai-Tibetan Plateau over the past 14,000 years. Geochem. Geophys. Geosyst. 2017, 18, 1775–1792. [Google Scholar] [CrossRef]
- China Meteorological Data Network. Available online: http://data.cma.cn/site/index.html (accessed on 10 November 2018).
- Mastepanov, M.; Sigsgaard, C.; Dlugokencky, E.J.; Houweling, S.; Ström, L.; Tamstorf, M.P.; Christensen, T.R. Large tundra methane burst during onset of freezing. Nature 2008, 456, 628–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, B.E.; Falge, E.; Gu, L.; Baldocchi, D.D.; Bakwin, P.; Berbigier, P.; Davis, K. Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agr. For. Meteorol. 2002, 113, 97–120. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.N. Low-Rank Scale-Invariant Tensor Product Smooths for Generalized Additive Mixed Models. Biometrics 2006, 62, 1025–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2006. [Google Scholar]
- van Straaten, O. Spatial and temporal effects of drought on soil CO2 efflux in a cacao agroforestry system in Sulawesi. Biogeosciences 2010, 7, 1223–1235. [Google Scholar] [CrossRef]
- Li, L.F.; Fan, W.Y.; Kang, X.M.; Wang, Y.F.; Cui, X.Y.; Xu, C.Y.; Griffin, K.L.; Hao, Y.B. Responses of greenhouse gas fluxes to climate extremes in a semiarid grassland. Atmos. Environ. 2016, 142, 32–42. [Google Scholar] [CrossRef]
- Shi, Z.; Thomey, M.L.; Mowll, W. Differential effects of extreme drought on production and respiration: Synthesis and modeling analysis. Biogeosciences 2014, 11, 621–633. [Google Scholar] [CrossRef] [Green Version]
- Van der Molen, M.K. Drought and ecosystem carbon cycling. Agric. For. Meteorol. 2011, 151, 765–773. [Google Scholar] [CrossRef]
- Lefi, E.; Medrano, H.; Cifre, J. Water uptake dynamics, photosynthesis and water use efficiency in field-grown Medicago arborea and Medicago citrina under prolonged Mediterranean drought conditions. Ann. Appl. Biol. 2004, 144, 299–307. [Google Scholar] [CrossRef]
- Flanagan, L.B. Phenology of plant production in the Northwestern Great Plains: Relationships with carbon isotope discrimination, net ecosystem productivity and ecosystem respiration. Phenol. Ecosyst. Processes 2009, 10, 169–185. [Google Scholar]
- Jia, X.; Zha, T.; Gong, J. Carbon and water exchange over a temperate semi-arid shrubland during three years of contrasting precipitation and soil moisture patterns. Agric. For. Meteorol. 2016, 228–229, 120–129. [Google Scholar] [CrossRef]
- Pitchford, J.L.; Wu, C.; Lin, L.S. Climate Change Effects on Hydrology and Ecology of Wetlands in the Mid-Atlantic Highlands. Wetlands 2011, 32, 21–33. [Google Scholar] [CrossRef]
- Austin, A.T.; Yahdjian, L.; Stark, J.M.; Belnap, J.; Porporato, A.; Norton, U.; Ravetta, D.A.; Schaeffer, S.M. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 2004, 141, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhu, J.J.; Liu, H.M.; Pan, Q.M. Effects of extreme rainfall and drought events on grassland ecosystems. Acta Phytoecol. Sin. 2014, 38, 1008–1018. [Google Scholar]
- Wieder, R.K.; Vitt, D.H.; Benscoter, B.W. Peatlands and the Boreal Forest. Boreal Peatland Ecosystems. Boreal Peatl. Ecosyst. 2006, 188, 1–8. [Google Scholar]
- Tiemeyer, B.; Albiac Borraz, E.; Augustin, J.; Bechtold, M.; Beetz, S.; Beyer, C.; Drösler, M.; Ebli, M.; Eickenscheidt, T.; Fiedler, S. High emissions of greenhouse gases from grasslands on peat and other organic soils. Glob. Chang. Biol. 2016, 22, 4134–4149. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.J.; Mooij, W.M.; Bragazza, L. Plant functional types define magnitude of drought response in peatland CO2 exchange. Ecology 2014, 95, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, F.A.; Morgan, J.A.; Follett, R.F. Climate change reduces the net sink of CH4 and N2O in a semiarid grassland. Glob. Chang. Biol. 2013, 19, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Blankinship, J.C.; Brown, J.R.; Dijkstra, P. Effects of interactive global changes on methane uptake in an annual grassland. J. Geophy. Res. Biogeosci. 2010, 115, G02008. [Google Scholar] [CrossRef]
- Tian, J.Q.; Zhu, Y.B.; Kang, X.M. Effects of drought on the archaeal community in soil of the Zoige wetlands of the Qinghai–Tibetan plateau. Eur. J. Soil Biol. 2012, 52, 84–90. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, X.; Yan, L.; Cui, L.; Zhang, X.; Hao, Y.; Wu, H.; Zhang, Y.; Li, W.; Zhang, K.; Yan, Z.; et al. Reduced Carbon Dioxide Sink and Methane Source under Extreme Drought Condition in an Alpine Peatland. Sustainability 2018, 10, 4285. https://doi.org/10.3390/su10114285
Kang X, Yan L, Cui L, Zhang X, Hao Y, Wu H, Zhang Y, Li W, Zhang K, Yan Z, et al. Reduced Carbon Dioxide Sink and Methane Source under Extreme Drought Condition in an Alpine Peatland. Sustainability. 2018; 10(11):4285. https://doi.org/10.3390/su10114285
Chicago/Turabian StyleKang, Xiaoming, Liang Yan, Lijuan Cui, Xiaodong Zhang, Yanbin Hao, Haidong Wu, Yuan Zhang, Wei Li, Kerou Zhang, Zhongqing Yan, and et al. 2018. "Reduced Carbon Dioxide Sink and Methane Source under Extreme Drought Condition in an Alpine Peatland" Sustainability 10, no. 11: 4285. https://doi.org/10.3390/su10114285




