Progress and Bottlenecks in the Early Domestication of the Perennial Oilseed Silphium integrifolium, a Sunflower Substitute
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Locations
2.1.1. Kansas
2.1.2. Patagonia
2.2. Plant Material
2.2.1. Botany
2.2.2. Partially Domesticated Silflower Populations
2.2.3. Wild Populations for Common Garden Experiments
2.3. Silphim Biology and Ecology Methods
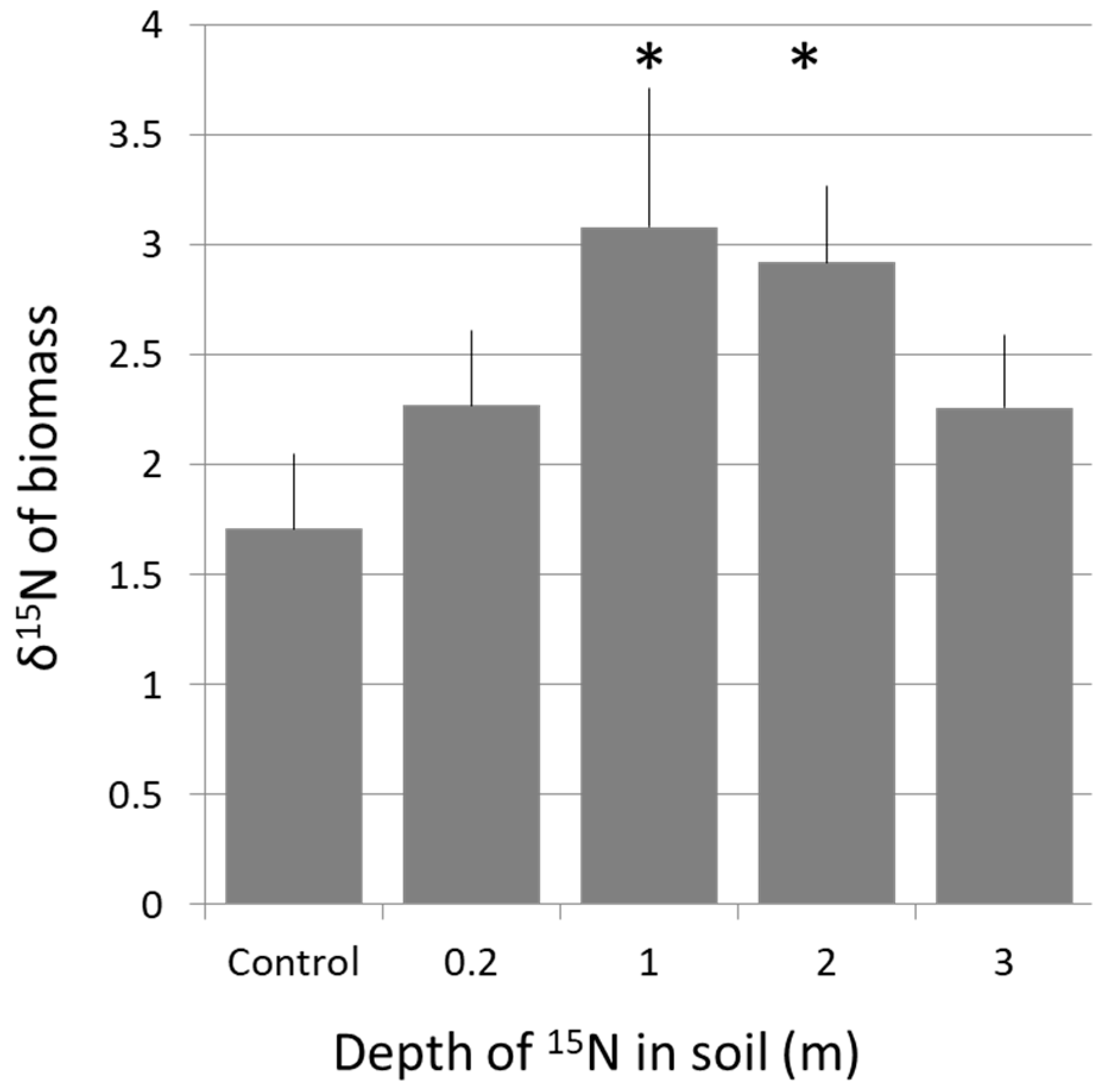
2.3.1. 15N Enrichment with Soil Depth
2.3.2. Leaf Water Potential
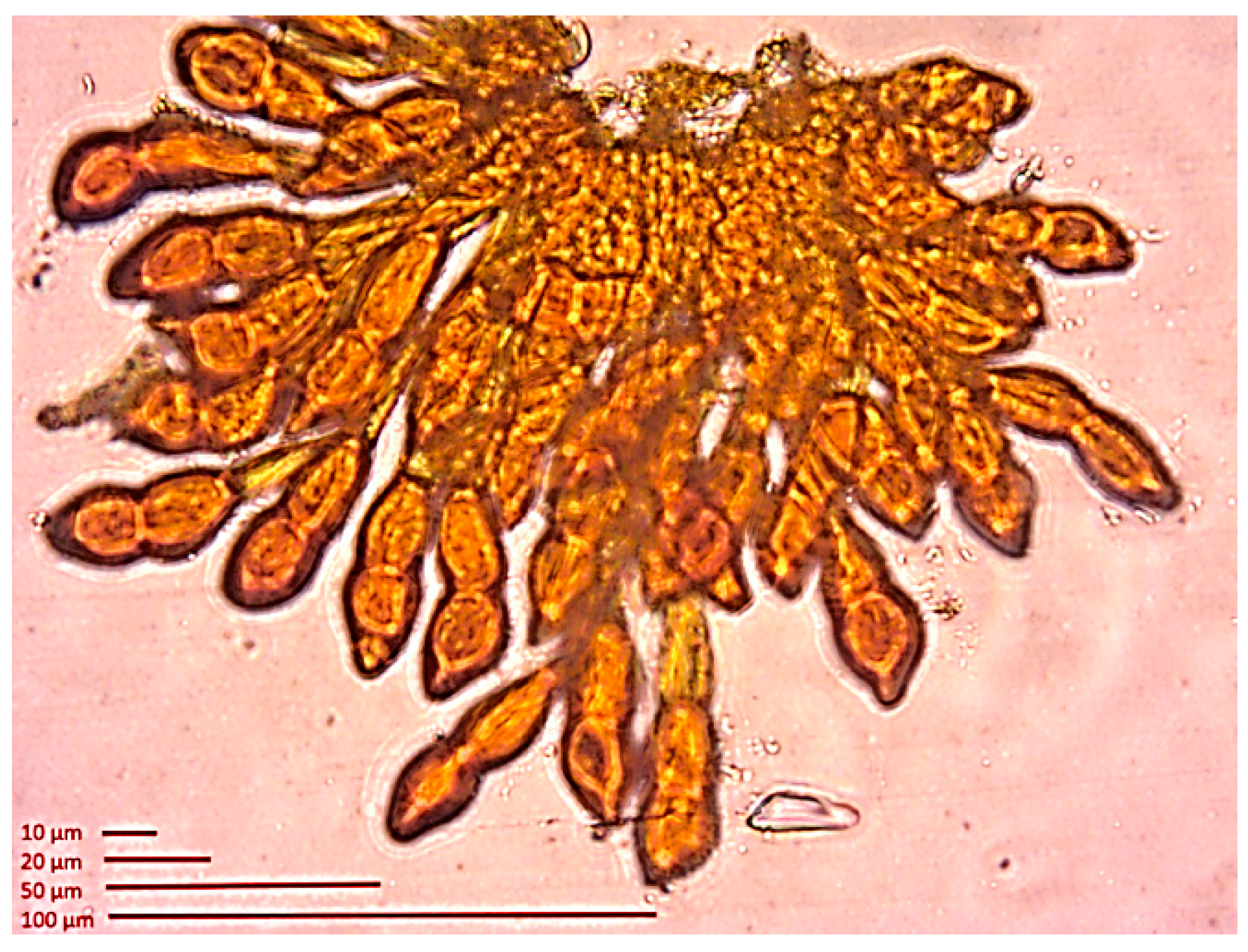
2.3.3. Fungal Pathogens
2.3.4. Insect Damage
2.4. Agronomic Practice Development
2.4.1. Propagation
2.4.2. N Fertilization Experiment
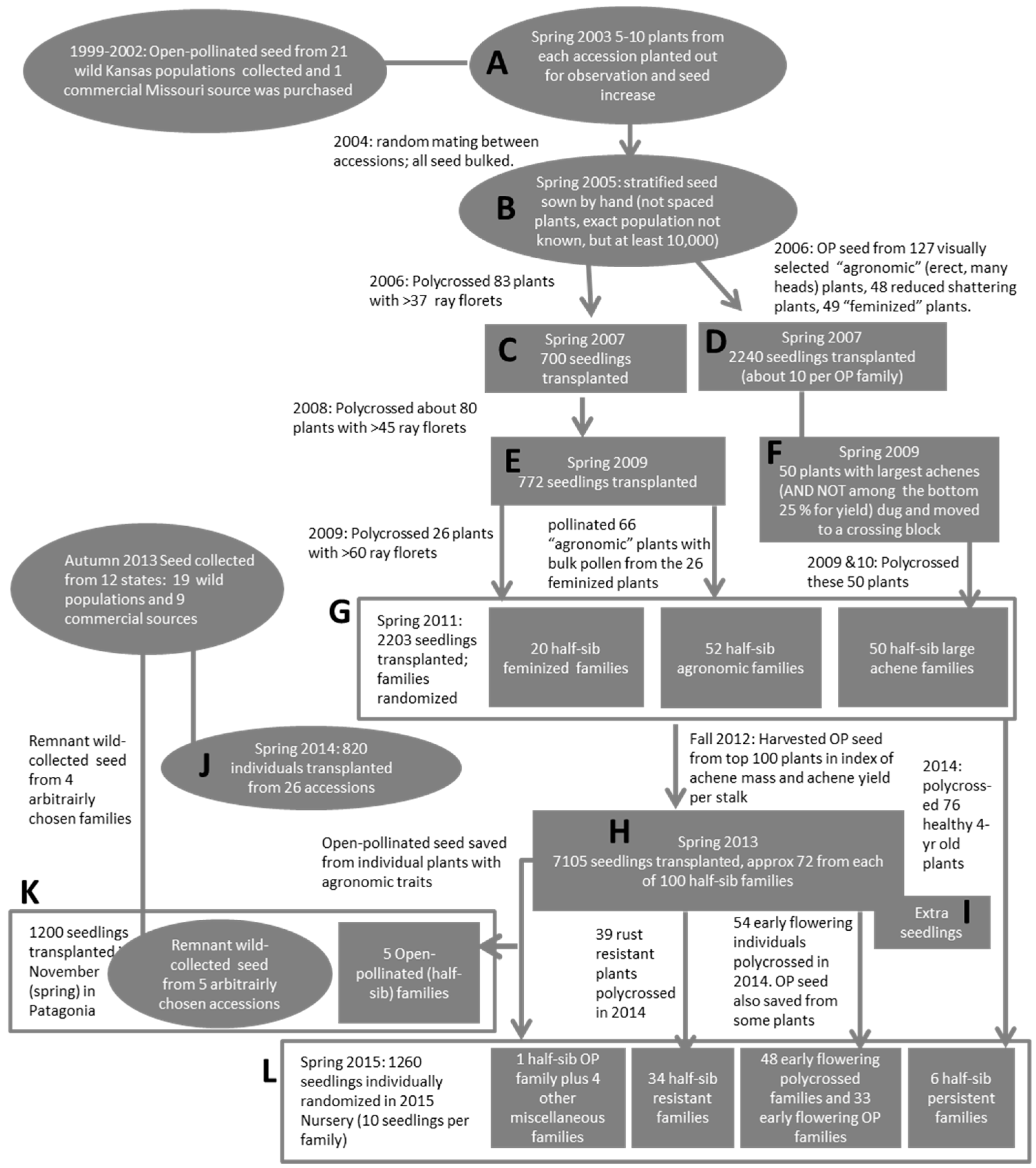
2.5. Breeding Methods and Breeding Progress Evaluation
2.5.1. Comparison of Wild and Selected Accessions of Silphium integrifolium Growing in Patagonia Argentina
2.5.2. General Crossing Method (Kansas)
2.5.3. Harvesting, Seed Cleaning (Kansas)
3. Results
3.1. Progress in Characterizing the Biology and Ecology of Silflower
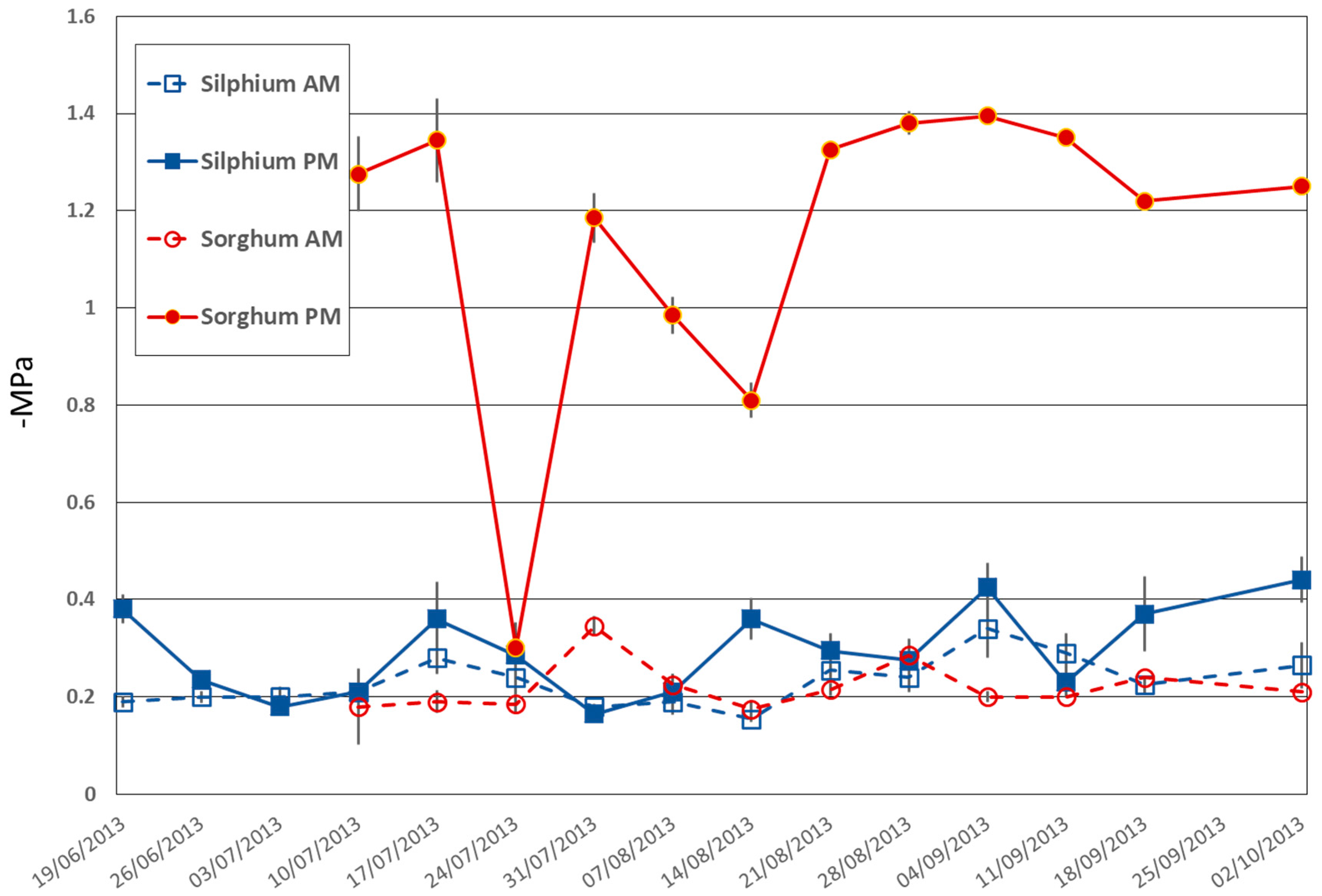
3.1.1. Evidence of Distinctive Water Use Strategy
Leaf Water Potential
15N Uptake at Depth
3.1.2. Pathogens and Herbivores
Diseases
Insects
3.2. Progress in Crop Management
3.2.1. Propagation
3.2.2. Fertilization
3.3. Progress in Domestication
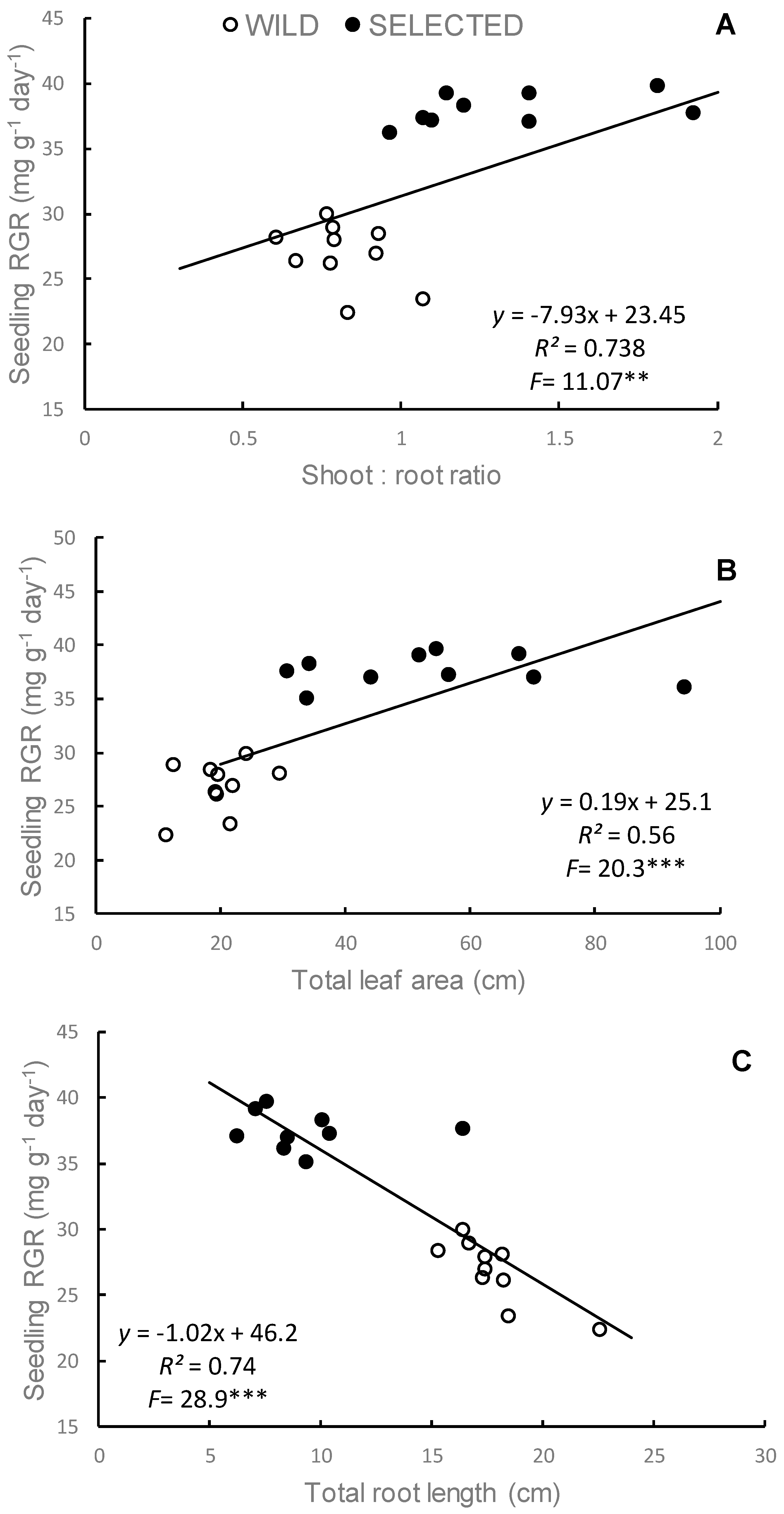
3.3.1. Unintentional Selection for Seedling Vigor
3.3.2. Yield Components
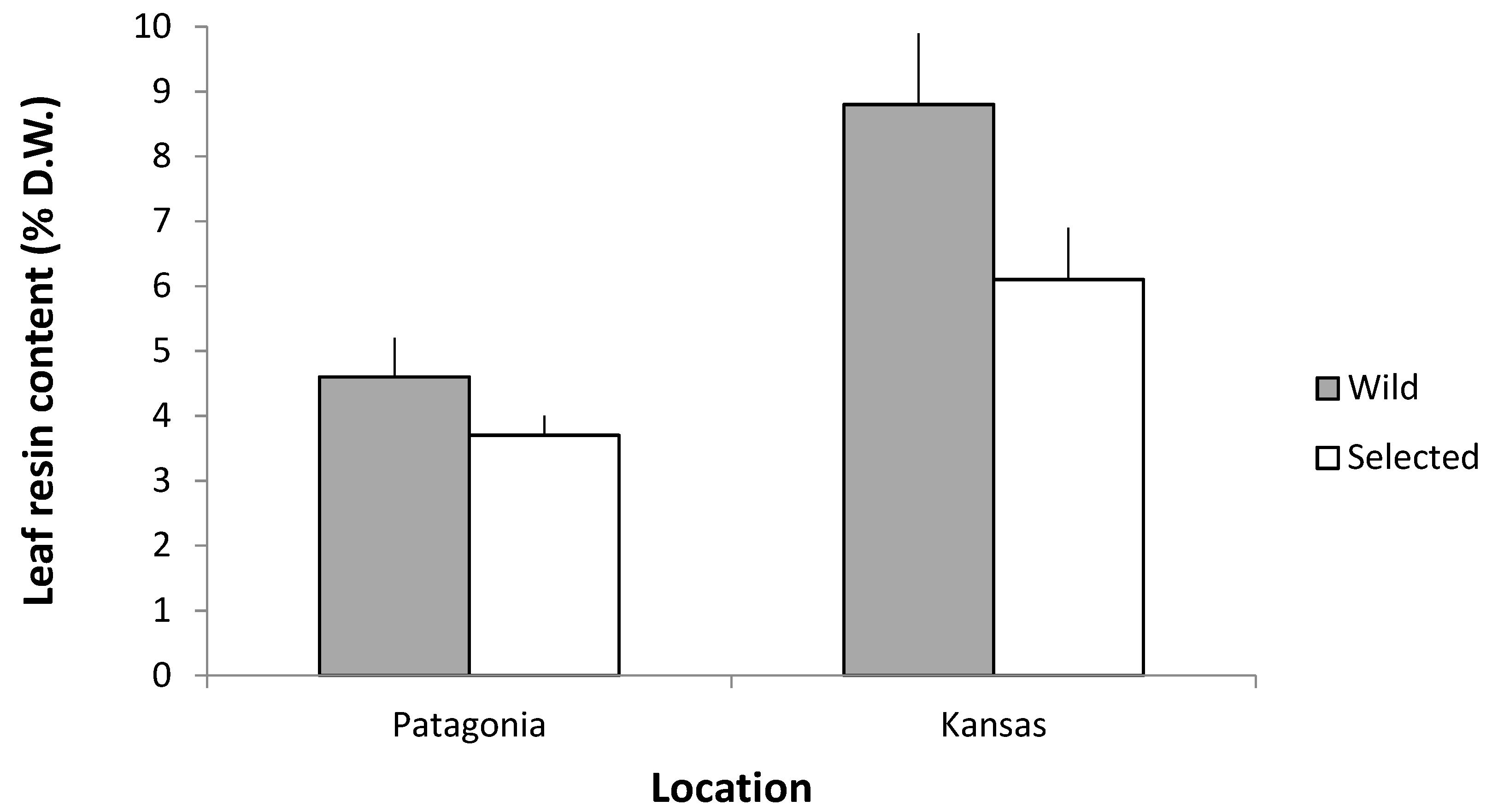
3.3.3. Resin Content
3.4. Heritability of Traits
4. Discussion
4.1. Crop Biology and Ecology
4.2. Crop Management
4.3. Crop Domestication
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Glover, J.D.; Reganold, J.P.; Bell, L.W.; Borevitz, J.; Brummer, E.C.; Buckler, E.S.; Cox, C.M.; Cox, T.S.; Crews, T.E.; Culman, S.W.; et al. Increased food and ecosystem security via perennial grains. Science 2010, 328, 1638–1639. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.; Glover, J.; Tassel, D. Van Prospects for developing perennial grain crops. Bioscience 2006, 56, 649–659. [Google Scholar] [CrossRef]
- Crews, T.E.; Blesh, J.; Culman, S.W.; Hayes, R.C.; Jensen, E.S.; Mack, M.C.; Peoples, M.B.; Schipanski, M.E. Going where no grains have gone before: From early to mid-succession. Agric. Ecosyst. Environ. 2016, 223, 223–238. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Adler, P.R. Perennial forages as second generation bioenergy crops. Int. J. Mol. Sci. 2008, 9, 768–788. [Google Scholar] [CrossRef] [PubMed]
- Cadisch, G.; Row, E.; van Noordwijk, M. Nurient harvesting—The tree-root safety net. Agrofor. Forum 1997, 8, 31–33. [Google Scholar]
- Bhat, R.; Karim, A.A. Exploring the Nutritional Potential of Wild and Underutilized Legumes. Compr. Rev. Food Sci. Food Saf. 2009, 8, 305–331. [Google Scholar] [CrossRef]
- Lefroy, E.C.; Flugge, F.; Avery, A.; Hume, I. Potential of current perennial plant-based farming systems to deliver salinity management outcomes and improve prospects for native biodiversity: A review. Aust. J. Exp. Agric. 2005, 45, 1357–1367. [Google Scholar] [CrossRef]
- Singh, A.; Krause, P.; Panda, S.N.; Flugel, W.-A. Rising water table: A threat to sustainable agriculture in an irrigated semi-arid region of Haryana, India. Agric. Water Manag. 2010, 97, 1443–1451. [Google Scholar] [CrossRef]
- Van Tassel, D.L.; Albrecht, K.A.; Bever, J.D.; Boe, A.A.; Brandvain, Y.; Crews, T.E.; Gansberger, M.; Gerstberger, P.; González-Paleo, L.; Hulke, B.S.; et al. Accelerating silphium domestication: An opportunity to develop new crop ideotypes and breeding strategies informed by multiple disciplines. Crop Sci. 2017, 57, 1274–1284. [Google Scholar] [CrossRef]
- Weaver, J.E.; Stoddart, L.A.; Noll, W. Response of the Prairie to the Great Drought of 1934. Ecology 1935, 16, 612–629. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Natural Resources Conservation Service. The PLANTS Database. Available online: http://plants.usda.gov (accessed on 17 September 2016).
- Cox, S.; Nabukalu, P.; Paterson, A.; Kong, W.; Nakasagga, S. Development of Perennial Grain Sorghum. Sustainability 2018, 10, 172. [Google Scholar] [CrossRef]
- Vail, J.; Kulakow, P.; Benson, L. Illinois Bundleflower: Prospects For A Perennial Seed Crop. In Proceedings of the Twelfth North American Prairie Conference, Cedar Rapids, IA, USA, 4–6 August 1980; pp. 31–32. [Google Scholar]
- Jackson, W. Nature as the measure for agriculture. In Ecology, Economics, Ethics: The Broken Circle; Borkmann, F.H., Kellert, S.R., Eds.; Yale University Press: New York, NY, USA; London, UK, 1991; pp. 43–58. [Google Scholar]
- Jackson, W. New Roots for Agriculture; University of Nebraska Press: Lincoln, NE, USA, 1985. [Google Scholar]
- Van Tassel, D.L. (The Land Institute, Salina, KS, USA). Personal communication, 2017.
- Boyer, J.S. Measuring the Water Status of Plants and Soils; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef]
- R_Core_Team R: A Language and Environment for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 1 December 2017).
- Strobl, C.; Malley, J.; Tutz, G. An introduction to recursive partitioning: Rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol. Methods 2009, 14, 323–348. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Curtis, J. Germination studies of Wisconsin prairie plants. Am. Midll. Nat. 1950, 43, 186–194. [Google Scholar] [CrossRef]
- Hunt, R.; Causton, D.R.; Shipley, B.; Askew, A.P. A modern tool for classical plant growth analysis. Ann. Bot. 2002, 90, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, H.L.; Thorup-Kristensen, K. Root Growth and Nitrate Uptake of Three Different Catch Crops in Deep Soil Layers. Soil Sci. Soc. Am. J. 2004, 68, 529–537. [Google Scholar] [CrossRef]
- Prasifka, J.R.; Mallinger, R.E.; Hulke, B.S.; Larson, S.R.; Van Tassel, D. Plant–Herbivore and Plant–Pollinator Interactions of the Developing Perennial Oilseed Crop, Silphium integrifolium. Environ. Entomol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Horst, R. Westcott’s Plant Disease Handbook; Springer: New York, NY, USA, 2008. [Google Scholar]
- Wagner, A.; Jamiolkowska, A. First Report of Alternaria alternata Causing Stem Blight of Compass Plant (Silphium laciniatum ) in Poland. Plant Dis. 2004, 88, 1045. [Google Scholar] [CrossRef]
- Siddiqui, M.; Brown, J. Effects of simulated rust epidemics on the growth and yield of sunflower. Aust. J. Agric. Res. 1977, 28, 389. [Google Scholar] [CrossRef]
- Markell, S.; Gulya, T.; McKay, K.; Hutter, M.; Hollingsworth, C.; Ulstad, V.; Koch, R.; Knudsvig, A. Widespread Occurrence of the Aecial Stage of Sunflower Rust Caused by Puccinia helianthi in North Dakota and Minnesota in 2008. Plant Dis. 2009, 93, 668. [Google Scholar] [CrossRef]
- Arthur, J.C. Manual of the Rusts in United States and Canada; Purdue Research Foundation: Lafayette, IN, USA, 1934. [Google Scholar]
- Parmelee, J.A. The Autoecious species of Puccinia on Heliantheae in North America. Can. J. Bot. 1967, 45, 2267–2327. [Google Scholar] [CrossRef]
- Hambleton, S. (Agriculture and Agri-Food Canada, Ottawa, ON, Canada). Personal communication, 2017.
- Prasifka, J.R. (United States Department of Agriculture, Fargo, ND, USA). Personal communication, 2017.
- Sartwell, C.; Daterman, G.E.; Koerber, T.W.; Stevens, R.E.; Sower, L.L. Distribution and Hosts of Eucosma sonomana in the Western United States as Determined by Trapping with Synthetic Sex Attractants. Ann. Entomol. Soc. Am. 1980, 73, 254–256. [Google Scholar] [CrossRef]
- Rogers, C.E.; Thompson, T.E.; Jones, O.R. Eucosma womonana Kearfott (Lepidoptera: Olethreutidae): A New Pest of Sunflower in the Southern Plains. J. Kansas Entomol. Soc. 1979, 52, 373–376. [Google Scholar]
- Lacey, L.; Georgis, R. Entomophagenic nematodes for control of insect pests above and below gruound with comments on vommercial production. J. Nematol. 2012, 44, 218–225. [Google Scholar] [PubMed]
- Fiedler, A.K.; Landis, D.A.; Wratten, S.D. Maximizing ecosystem services from conservation biological control: The role of habitat management. Biol. Control 2008, 45, 254–271. [Google Scholar] [CrossRef]
- Wassner, D.; Ravetta, D. Vegetative propagation of Grindelia chiloensis (Asteraceae). Ind. Crops Prod. 2000. [Google Scholar] [CrossRef]
- Vilela, A.E. (MEF-CONICET, Trelew, Patagonia Argentina). Personal communication, 2016.
- Schiffner, S.; Sheaffer, C. (University of Minnesota, Minneapolis, MN, USA). Personal communication, 2017.
- Özer, H.; Polat, T.; Öztürk, E. Response of irrigated sunflower (Helianthus annuus L.) hybrids to nitrogen fertilization: Growth, yield and yield components. Plant Soil Environ. 2006, 50, 205–211. [Google Scholar] [CrossRef]
- Tomar, H.P.S.; Dadhwal, K.S.; Singh, H.P. Effect of irrigation, N, and P on yield and yield attributes of spring sunflower (Helianthus annuus L.). Trop. Agric. 1999, 76, 228–231. [Google Scholar]
- Hocking, P.J.; Steer, B.T. Effects of timing and supply of nitrogen on nitrogen remobilization from vegetative organs and redistribution to developing seeds of sunflower. Plant Soil 1995. [Google Scholar] [CrossRef]
- González-Paleo, L. (MEF-CONICET, Trelew, Patagonia Argentina). Personal communication, 2017.
- Singh, U.P.; Singh, Y.; Kumar, V.; Ladha, J.K. Evaluation and promotion of resource conserving tillage and crop establishment techniques in rice-wheat system in Eastern India. In Integrated Crop and Resource Management in the Rice-Wheat System of South Asia; International Rice Research Institute: Los Baños, Philippines, 2009; pp. 151–176. [Google Scholar]
- Zhang, Z.-H.; Yu, S.-B.; Yu, T.; Huang, Z.; Zhu, Y.-G. Mapping quantitative trait loci (QTLs) for seedling-vigor using recombinant inbred lines of rice (Oryza sativa L.). Field Crops Res. 2005. [Google Scholar] [CrossRef]
- Asch, F.; Sow, A.; Dingkuhn, M. Reserve mobilization, dry matter partitioning and specific leaf area in seedlings of African rice cultivars differing in early vigor. Field Crops Res. 1999, 62, 191–202. [Google Scholar] [CrossRef]
- Caton, B.P.; Cope, A.E.; Mortimer, M. Growth traits of diverse rice cultivars under severe competition: Implications for screening for competitiveness. Field Crops Res. 2003, 83, 157–172. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- López-Castañeda, C.; Richards, R.A.; Farquhar, G.D.; Williamson, R.E. Seed and seedling characteristics contributing to variation in early vigor among temperate cereals. Crop Sci. 1996, 36, 1257–1266. [Google Scholar] [CrossRef]
- Rebolledo, M.C.; Luquet, D.; Courtois, B.; Henry, A.; Soulié, J.-C.; Rouan, L.; Dingkuhn, M. Can early vigour occur in combination with drought tolerance and efficient water use in rice genotypes? Funct. Plant Biol. 2013, 40, 582–594. [Google Scholar] [CrossRef]
- González-Paleo, L.; Ravetta, D.A. Indirect changes associated with a selection program for increased seed-yield in wild species of Lesquerella (Brassicaceae): Are we developing a phenotype opposite to the expected ideotype? Ind. Crops Prod. 2011, 34, 1372–1380. [Google Scholar] [CrossRef]
- McKey, D.; Elias, M. Ecological approaches to crop domestication. In Biodiversity in Agriculture: Domestication, Evolution and Sustainability; Gepts, P., Famula, T.R., Bettinger, R.L., Brush, S.B., Damania, A.B., Eds.; Cambridge University Press: Cambridge, MA, USA, 2012; pp. 377–406. [Google Scholar]
- Langenheim, J.H. Plant Resins: Chemistry, Evolution, Ecology and Ethnobotany; Timber Press: Portland, OR, USA; Cambridge, MA, USA, 2003. [Google Scholar]

| Plant Regulator | Water | Potting Mix | Soil |
|---|---|---|---|
| Control | 0% | 46.6 ± 6.6% | 33.4 ± 6.6% |
| IBA 0.1% | 0% | 13.4 ± 6.6% | 26.6 ± 6.6% |
| IBA 0.8% | 0% | 86.6 ± 6.6% | 53.4 ± 13.5% |
| TRAITS | F-Test | Treatment | |
|---|---|---|---|
| Control | Fertilized | ||
| Plant height (m) | 7.54 ** | 1.02 ± 0.08 | 1.44 ± 0.03 |
| Total biomass (g) | 31.28 *** | 153.19 ± 12.2 | 276.25 ± 18.26 |
| Number of stalks | 2.15 ns | 4.4 ± 0.6 | 5.8 ± 0.7 |
| Stalk diameter (cm) | 43.5 *** | 0.95 ± 0.03 | 1.48 ± 0.07 |
| Total leaf area (cm2) | 8.87 ** | 69.93 ± 5.38 | 100.14 ± 8.60 |
| [N] green leaves (mg∙g−1) | 13.3 ** | 10.22 ± 0.39 | 25. 68 ± 1.68 |
| A (µmoles CO2 cm−2∙s−1) | 5.8 * | 24.7 ± 1.03 | 28.60 ± 0.93 |
| [N] crown (mg∙g−1) | 10.1 ** | 14.98 ± 0.93 | 28. 2 ± 2.13 |
| Leaf senescence (%) | 8.39 * | 18.16 ± 4.18 | 10.30 ± 3.10 |
| Number of lateral roots | 11.26 *** | 30.8 ± 2.86 | 19.50 ± 1.80 |
| Seed yield (g) | 8.64 ** | 31.13 ± 3.32 | 41.44 ± 4.41 |
| Harvest index (%) | 13.5 * | 23 ± 2 | 14 ± 3 |
| Number of heads per plant | 23.83 *** | 30.40 ± 3.14 | 51.20 ± 2.88 |
| Number of seeds per head | 0.35 ns | 40.15 ± 3.26 | 42.75 ± 2.97 |
| Seed weight (g) | 0.64 ns | 0.023 ± 0.0015 | 0.021 ± 0.0012 |
| Trait | Selected | Wild | T-test |
|---|---|---|---|
| Total Biomass (g) | 1099 ± 82.24 | 827.77 ± 62.90 | 2.49 * |
| Stalk Biomass (g∙DW) | 583.54 ± 50.54 | 402.23 ± 32.28 | 3.07 *** |
| Stalks: number | 9.88 ± 0.66 | 11.32 ± 0.82 | –1.38 ns |
| Plant height (cm) | 216.38 ± 7.02 | 181.07 ± 7.33 | 3.43 ** |
| Reproductive biomass (g∙DW) | 200.85 ± 20.03 | 136.66 ± 11.45 | 2.36 * |
| Heads (num. per plant) | 168.76 ± 18.74 | 307.00 ± 52.78 | –2.51 * |
| Individual seed weight (g∙DW) | 0.03 ± 0.0001 | 0.02 ± 0.0001 | 3.88 *** |
| Harvest Index | 0.21 ± 0.01 | 0.15 ± 0.02 | 2.98 *** |
| Yield per plant (g∙DW) | 194.77 ± 18.43 | 108.24 ± 12.21 | 4.05 *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, A.; González-Paleo, L.; Turner, K.; Peterson, K.; Ravetta, D.; Crews, T.E.; Van Tassel, D. Progress and Bottlenecks in the Early Domestication of the Perennial Oilseed Silphium integrifolium, a Sunflower Substitute. Sustainability 2018, 10, 638. https://doi.org/10.3390/su10030638
Vilela A, González-Paleo L, Turner K, Peterson K, Ravetta D, Crews TE, Van Tassel D. Progress and Bottlenecks in the Early Domestication of the Perennial Oilseed Silphium integrifolium, a Sunflower Substitute. Sustainability. 2018; 10(3):638. https://doi.org/10.3390/su10030638
Chicago/Turabian StyleVilela, Alejandra, Luciana González-Paleo, Kathryn Turner, Kelsey Peterson, Damián Ravetta, Timothy E. Crews, and David Van Tassel. 2018. "Progress and Bottlenecks in the Early Domestication of the Perennial Oilseed Silphium integrifolium, a Sunflower Substitute" Sustainability 10, no. 3: 638. https://doi.org/10.3390/su10030638
APA StyleVilela, A., González-Paleo, L., Turner, K., Peterson, K., Ravetta, D., Crews, T. E., & Van Tassel, D. (2018). Progress and Bottlenecks in the Early Domestication of the Perennial Oilseed Silphium integrifolium, a Sunflower Substitute. Sustainability, 10(3), 638. https://doi.org/10.3390/su10030638





