Change of the Extractability of Cadmium Added to Different Soils: Aging Effect and Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Samples and Treatments
2.2. Determination of EDTA-Extractable Cd
2.3. Considerations for the Model to Predict EDTA-Extractable Cd
2.3.1. Processes of Precipitation/Nucleation
2.3.2. Processes of Mesopore/Micropore Diffusion
2.3.3. Processes of Occlusion within Organic Matter
3. Results and Discussion
3.1. Change of EDTA-Extractable Cd in Different Soils with Time
3.2. Semi-Mechanistic Models for EDTA-Cd
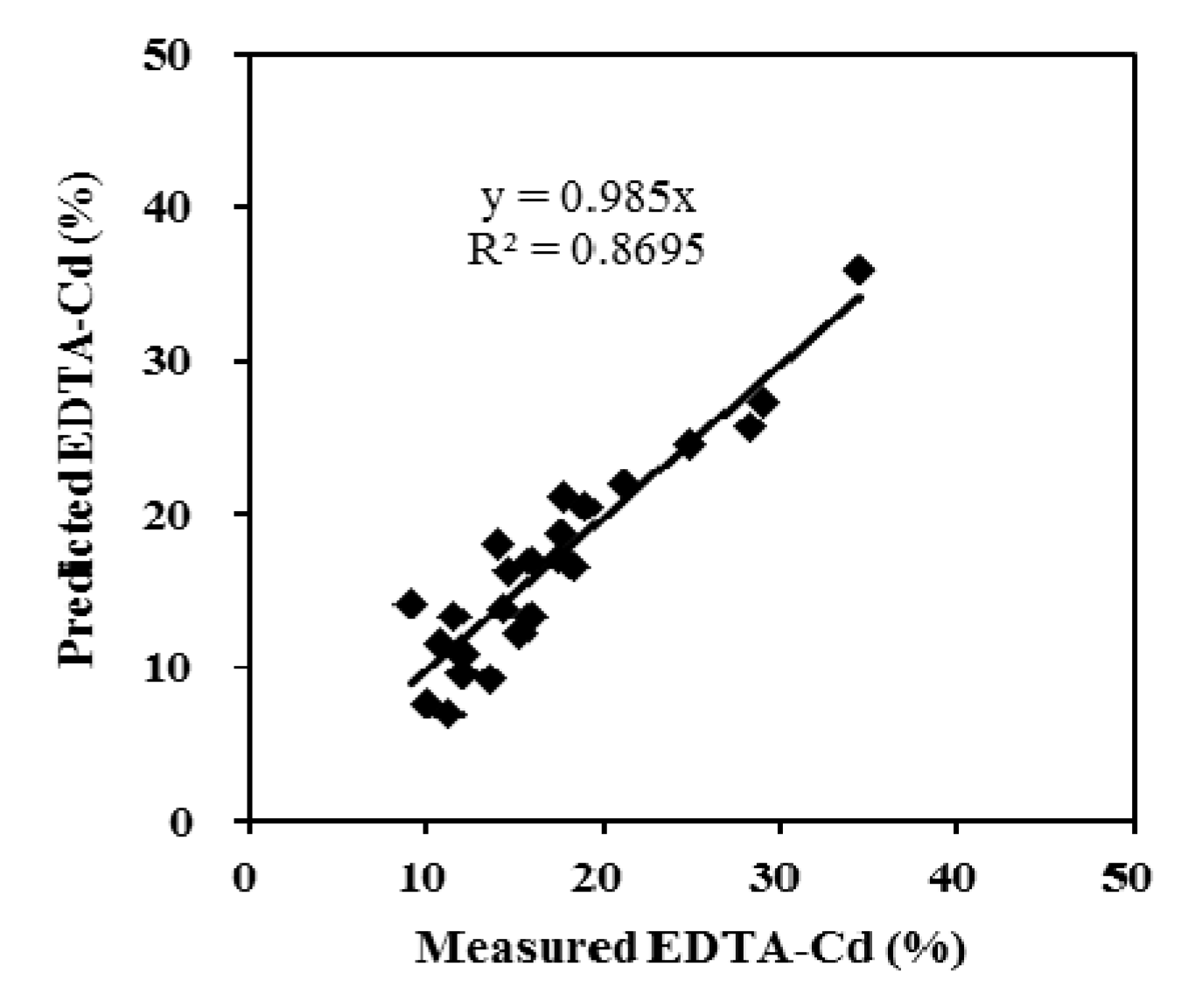
3.3. Model Validation
3.4. Practical Implications
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bermond, A.; Varrault, G.; Sappin-Didier, V.; Mench, M. A kinetic approach to predict soil trace metal bioavailability: Preliminary results. Plant Soil 2005, 275, 21–29. [Google Scholar] [CrossRef]
- Bermond, A.; Ghestem, J.P.; Yousfi, I. Kinetic approach to the chemical speciation of trace metals in soils. Analyst 1998, 123, 785–789. [Google Scholar] [CrossRef]
- Gutzman, D.W.; Langford, C.H. Kinetic study of the speciation of copper (II) bound to a hydrous ferric oxide. Environ. Sci. Technol. 1993, 27, 1388–1393. [Google Scholar] [CrossRef]
- Lo, I.M.; Yang, X. EDTA extraction of heavy metals from different soil fractions and synthetic soils. Water Air Soil Pollut. 1999, 109, 219–236. [Google Scholar] [CrossRef]
- Young, S.D.; Tye, A.; Carstensen, A.; Resende, L.; Crout, N. Methods for determining labile cadmium and zinc in soil. Eur. J. Soil Sci. 2000, 51, 129–136. [Google Scholar] [CrossRef]
- Labanowski, J.; Monna, F.; Bermond, A.; Cambier, P.; Fernandez, C.; Lamy, I.; Van Oort, F. Kinetic extractions to assess mobilization of Zn, Pb, Cu, and Cd in a metal-contaminated soil: EDTA vs. citrate. Environ. Pollut. 2008, 152, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, D.; Bermond, A.; Santos, E.; Carapuça, H.; Duarte, A. Heavy metal mobility assessment in sediments based on a kinetic approach of the EDTA extraction: Search for optimal experimental conditions. Anal. Chim. Acta 2002, 459, 245–256. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Lopez-Valdivia, L.M.; Novillo, J.; Obrador, A.; Rico, M.I. Comparison of EDTA and sequential extraction tests for phytoavailability prediction of manganese and zinc in agricultural alkaline soils. Geoderma 2006, 132, 450–463. [Google Scholar] [CrossRef]
- Manouchehri, N.; Besancon, S.; Bermond, A. Major and trace metal extraction from soil by EDTA: Equilibrium and kinetic studies. Anal. Chim. Acta 2006, 559, 105–112. [Google Scholar] [CrossRef]
- Paya-Perez, A.; Sala, J.; Mousty, F. Comparison of ICP-AES and ICP-MS for the analysis of trace elements in soil extracts. Int. J. Environ. Sci. Technol. 1993, 51, 223–230. [Google Scholar] [CrossRef]
- Crout, N.M.; Tye, A.M.; Zhang, H.; McGrath, S.P.; Young, S.D. Kinetics of metal fixation in soils: Measurement and modeling by isotopic dilution. Environ. Toxicol. Chem. 2006, 25, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Uren, N. Transformations of heavy metals added to soil-application of a new sequential extraction procedure. Geoderma 1998, 84, 157–168. [Google Scholar] [CrossRef]
- Mann, S.; Ritchie, G. Changes in the forms of cadmium with time in some western Australian soils. Soil Res. 1994, 32, 241–250. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, Y.; Cui, Y.; Duan, J.; Tang, L. The effect of ageing on the bioaccessibility and fractionation of cadmium in some typical soils of China. Environ. Int. 2006, 32, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ma, C.; Ma, Y.; Zhang, S.; Lv, J.T.; Cui, M. New insights into the sorption mechanism of cadmium on red mud. Environ. Pollut. 2011, 159, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.J. Ageing of metals in soils changes bioavailability. Face Sheet Environ. Risk Assess. 2001, 4, 1–6. [Google Scholar]
- Aringhieri, R.; Carrai, P.; Petruzzelli, G. Kinetics of Cu2+ and Cd2+ adsorption by an Italian soil. Soil Sci. 1985, 139, 197–204. [Google Scholar] [CrossRef]
- Barrow, N.J. Reactions with Variable-Charge Soils; Martinus Nijhoff Publishers: Boston, MA, USA, 1987. [Google Scholar]
- Sigel, A.; Sigel, H.; Sigel, R.K. Cadmium: From Toxicity to Essentiality; Springer: Berlin, Germany, 2013. [Google Scholar]
- Bruemmer, G.; Gerth, J.; Tiller, K. Reaction kinetics of the adsorption and desorption of nickel, zinc and cadmium by goethite. I. Adsorption and diffusion of metals. J. Soil Sci. 1988, 39, 37–52. [Google Scholar] [CrossRef]
- Cavallaro, N.; McBride, M. Copper and cadmium adsorption characteristics of selected acid and calcareous soils. Soil Sci. Soc. Am. J. 1978, 42, 550–556. [Google Scholar] [CrossRef]
- Elzinga, E.; Reeder, R. X-ray absorption spectroscopy study of Cu2+ and Zn2+ adsorption complexes at the calcite surface: Implications for site-specific metal incorporation preferences during calcite crystal growth. Geochim. Cosmochim. Acta 2002, 66, 3943–3954. [Google Scholar] [CrossRef]
- Ma, Y.; Lombi, E.; Nolan, A.L.; McLaughlin, M.J. Short-term natural attenuation of copper in soils: Effects of time, temperature, and soil characteristics. Environ. Toxicol. Chem. 2006, 25, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Schosseler, P.; Wehrli, B.; Schweiger, A. Uptake of Cu2+ by the calcium carbonates vaterite and calcite as studied by continuous wave (CW) and pulse electron paramagnetic resonance. Geochim. Cosmochim. Acta 1999, 63, 1955–1967. [Google Scholar] [CrossRef]
- Wendling, L.A.; Ma, Y.; Kirby, J.K.; McLaughlin, M.J. A predictive model of the effects of aging on cobalt fate and behavior in soil. Environ. Sci. Technol. 2008, 43, 135–141. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Streck, T.; Richter, J. Heavy metal displacement in a sandy soil at the field scale: I. Measurements and parameterization of sorption. J. Environ. Qual. 1997, 26, 49–56. [Google Scholar] [CrossRef]
- Proffit, S.; Marin, B.; Cances, B.; Ponthieu, M.; Sayen, S.; Guillon, E. Using synthetic models to simulate aging of Cu contamination in soils. Environ. Sci. Pollut. Res. 2015, 22, 7641–7652. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lombi, E.; Oliver, I.; Nolan, A.; McLaughlin, M.J. Long-term aging of copper added to soils. Environ. Sci. Technol. 2006, 40, 6310–6317. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lombi, E.; McLaughlin, M.J.; Oliver, I.W.; Nolan, A.L.; Oorts, K.; Smolders, E. Aging of nickel added to soils as predicted by soil pH and time. Chemosphere 2013, 92, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Brouwere, K.D.; Smolders, E.; Merckx, R. Soil properties affecting solid-liquid distribution of As (V) in soils. Eur. J. Soil Sci. 2004, 55, 165–173. [Google Scholar] [CrossRef]
- Smolders, E.; Oorts, K.; Van Sprang, P.; Schoeters, I.; Janssen, C.R.; McGrath, S.P.; McLaughlin, M.J. Toxicity of trace metals in soil as affected by soil type and aging after contamination: Using calibrated bioavailability models to set ecological soil standards. Environ. Toxicol. Chem. 2009, 28, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.J.; Lofts, S.; Warne, M.S.J.; Amorim, M.J.; Fairbrother, A.; Lanno, R.; Hendershot, W.; Schlekat, C.E.; Ma, Y.; Paton, G.I. Derivation of ecologically based soil standards for trace elements. In Soil Quality Standards for Trace Elements: Derivation, Implementation and Interpretation; Merrington, G., Schoeters, I., Eds.; CRC Press: Pensacola, FL, USA, 2010; pp. 7–80. [Google Scholar]
- Liu, B.; Sun, C.; Chen, S.; Zhang, X.; Song, W.; Li, N. Dynamic characteristics and ageing factors of Cd added to paddy soils with various properties. China Environ. Sci. 2015, 35, 2137–2145. [Google Scholar]
- Rayment, G.; Higginson, F.R. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press Pty Ltd.: Melbourne, Australia, 1992. [Google Scholar]
- Matejovic, I. Determination of carbon and nitrogen in samples of various soils by the dry combustion. Commun. Soil Sci. Plant 1997, 28, 1499–1511. [Google Scholar] [CrossRef]
- Sherrod, L.; Dunn, G.; Peterson, G.; Kolberg, R. Inorganic carbon analysis by modified pressure-calcimeter method. Soil Sci. Soc. Am. J. 2002, 66, 299–305. [Google Scholar] [CrossRef]
- Mehra, O.; Jackson, M. Iron Oxide Removal from Soils and Clays by a Dithionite-Citrate System Buffered with Sodium Bicarbonate. In Proceedings of the National Conference on Clays and Clays Minerals, Washington, DC, USA, 20–23 October 1958; pp. 317–327. [Google Scholar]
- McKenzie, N.; Coughlan, K.; Cresswell, H. Soil Physical Measurement and Interpretation for Land Evaluation; CSIRO Publishing: Clayton, Australia, 2002. [Google Scholar]
- Smith, C.J.; Hopmans, P.; Cook, F.J. Accumulation of Cr, Pb, Cu, Ni, Zn and Cd in soil following irrigation with treated urban effluent in Australia. Environ. Pollut. 1996, 94, 317–323. [Google Scholar] [CrossRef]
- Barrow, N.; Brümmer, G.; Fischer, L. Rate of desorption of eight heavy metals from goethite and its implications for understanding the pathways for penetration. Eur. J. Soil Sci. 2012, 63, 389–398. [Google Scholar] [CrossRef]
- Donner, E.; McLaughlin, M.J.; Hodson, M.E.; Heemsbergen, D.; Warne, M.S.J.; Nortcliff, S.; Broos, K. Ageing of zinc in highly-weathered iron-rich soils. Plant Soil 2012, 361, 83–95. [Google Scholar] [CrossRef]
- Guo, G.; Yuan, T.; Wang, W.; Li, D.; Wang, J. Effect of aging on bioavailability of copper on the fluvo aquic soil. Int. J. Environ. Sci. Technol. 2011, 8, 715–722. [Google Scholar] [CrossRef]
- Ford, R.G.; Sparks, D.L. The nature of Zn precipitates formed in the presence of pyrophyllite. Environ. Sci. Technol. 2000, 34, 2479–2483. [Google Scholar] [CrossRef]
- Christensen, T.H. Cadmium soil sorption at low concentrations: Ⅱ. Reversibility, effect of changes in solute composition, and effect of soil aging. Water Air Soil Pollut. 1984, 21, 115–125. [Google Scholar] [CrossRef]
- Smolders, E.; Brans, K.; Földi, A.; Merckx, R. Cadmium fixation in soils measured by isotpoic dilution. Soil Sci. Soc. Am. J. 1999, 63, 78–85. [Google Scholar] [CrossRef]
- Lee, J.D. A New Concise Inorganic Chemistry, 3rd ed.; Van Nostrand Reinhold Co. Ltd.: Berkshire, UK, 1977. [Google Scholar]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 1979. [Google Scholar]
- Perrin, D.D. Dissociation constants of inorganic acids and bases in aqueous solution. Pure Appl. Chem. 1969, 20, 133–236. [Google Scholar] [CrossRef]
- Almås, Å.; Singh, B.; Salbu, B. Mobility of cadmium-109 and zinc-65 in soil influenced by equilibration time, temperature, and organic matter. J. Environ. Qual. 1999, 28, 1742–1750. [Google Scholar] [CrossRef]
- Kirkham, M. Cadmium in plants on polluted soils: Effects of soil factors, hyperaccumulation, and amendments. Geoderma 2006, 137, 19–32. [Google Scholar] [CrossRef]
- Krishnamurti, G.S.; Naidu, R. Solid-solution equilibria of cadmium in soils. Geoderma 2003, 113, 17–30. [Google Scholar] [CrossRef]
- Christensen, T.H. Cadmium soil sorption at low concentrations: I. Effect of time, cadmium load, pH, and calcium. Water Air Soil Pollut. 1984, 21, 105–114. [Google Scholar] [CrossRef]
- Fairbrother, A.; Wenstel, R.; Sappington, K.; Wood, W. Framework for metals risk assessment. Ecotoxicol. Environ. Saf. 2007, 68, 145–227. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Bolan, N.S.; Kookana, R.S.; Tiller, K. Ionic-strength and pH effects on the sorption of cadmium and the surface charge of soils. Eur. J. Soil Sci. 1994, 45, 419–429. [Google Scholar] [CrossRef]
- Liang, Z.; Ding, Q.; Wei, D.; Li, J.; Chen, S.; Ma, Y. Major controlling factors and predictions for cadmium transfer from the soil into spinach plants. Ecotoxicol. Environ. Saf. 2013, 93, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Owsianiak, M.; Holm, P.E.; Fantke, P.; Christiansen, K.S.; Borggaard, O.K.; Hauschild, M.Z. Assessing comparative terrestrial ecotoxicity of Cd, Co, Cu, Ni, Pb, and Zn: The influence of aging and emission source. Environ. Pollut. 2015, 206, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Owsianiak, M.; Rosenbaum, R.K.; Huijbregts, M.A.J.; Hauschild, M.Z. Addressing geographic variability in the comparative toxicity potential of copper and nickel in soils. Environ. Sci. Technol. 2013, 47, 3241–3250. [Google Scholar] [CrossRef] [PubMed]

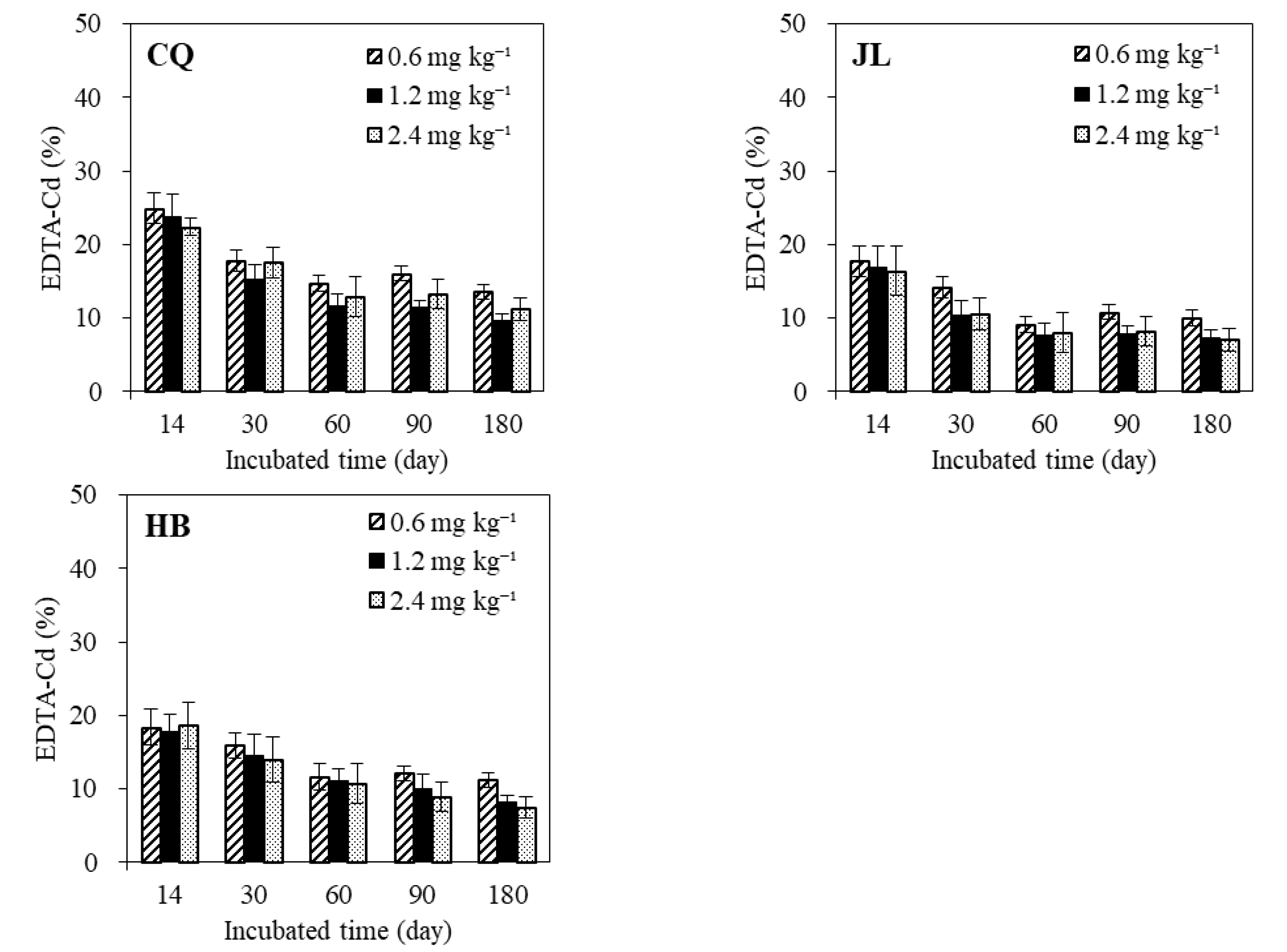




| Soil Code | Soil Location | Soil Type | pH (H2O) | OC (%) | CEC (cmol kg−1) | EC (μS cm−1) | FeOX (g kg−1) | Cd (mg kg−1) | Clay (<0.002 mm, %) |
|---|---|---|---|---|---|---|---|---|---|
| HN | Hunan | Red soil | 4.54 | 0.87 | 7.47 | 74.2 | 3.76 | 0.121 | 46.1 |
| ZJ | Zhejiang | Paddy soil | 6.72 | 2.46 | 12.82 | 203.4 | 2.14 | 0.119 | 38.9 |
| CQ | Chongqing | Purple soil | 7.02 | 0.99 | 22.31 | 71.3 | 3.1 | 0.207 | 27.3 |
| JL | Jilin | Black soil | 7.31 | 2.17 | 28.81 | 147.1 | 1.26 | 0.142 | 44.6 |
| HB | Hebei | Alluvial soil | 7.81 | 0.63 | 6.36 | 5.7 | 0.98 | 0.172 | 19.1 |
| Cd Concentration (mg kg−1) | B | C | pK0 | F | G | H | R2 | RMSE |
|---|---|---|---|---|---|---|---|---|
| 0.6 | 0.05 | 8.25 | 7.04 | 0.10 | 0.35 | 8.70 | 0.83 | 0.02 |
| 1.2 | 0.07 | 6.31 | 6.81 | 0.10 | 1.03 | 3.94 | 0.87 | 0.01 |
| 2.4 | 0.05 | 6.67 | 7.26 | 0.17 | 0.20 | 13.38 | 0.92 | 0.01 |
| 0.6, 1.2 and 2.4 | 0.05 | 7.18 | 7.03 | 0.12 | 0.49 | 7.88 | 0.84 | 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zeng, S.; Chen, S.; Ma, Y. Change of the Extractability of Cadmium Added to Different Soils: Aging Effect and Modeling. Sustainability 2018, 10, 885. https://doi.org/10.3390/su10030885
Zhang X, Zeng S, Chen S, Ma Y. Change of the Extractability of Cadmium Added to Different Soils: Aging Effect and Modeling. Sustainability. 2018; 10(3):885. https://doi.org/10.3390/su10030885
Chicago/Turabian StyleZhang, Xi, Saiqi Zeng, Shibao Chen, and Yibing Ma. 2018. "Change of the Extractability of Cadmium Added to Different Soils: Aging Effect and Modeling" Sustainability 10, no. 3: 885. https://doi.org/10.3390/su10030885
APA StyleZhang, X., Zeng, S., Chen, S., & Ma, Y. (2018). Change of the Extractability of Cadmium Added to Different Soils: Aging Effect and Modeling. Sustainability, 10(3), 885. https://doi.org/10.3390/su10030885





