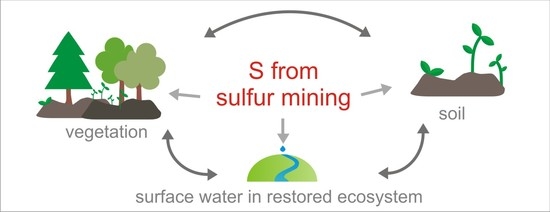
Comprehensive Study of Reclaimed Soil, Plant, and Water Chemistry Relationships in Highly S-Contaminated Post Sulfur Mine Site Jeziórko (Southern Poland)
Abstract
:1. Introduction
2. Materials and Methods
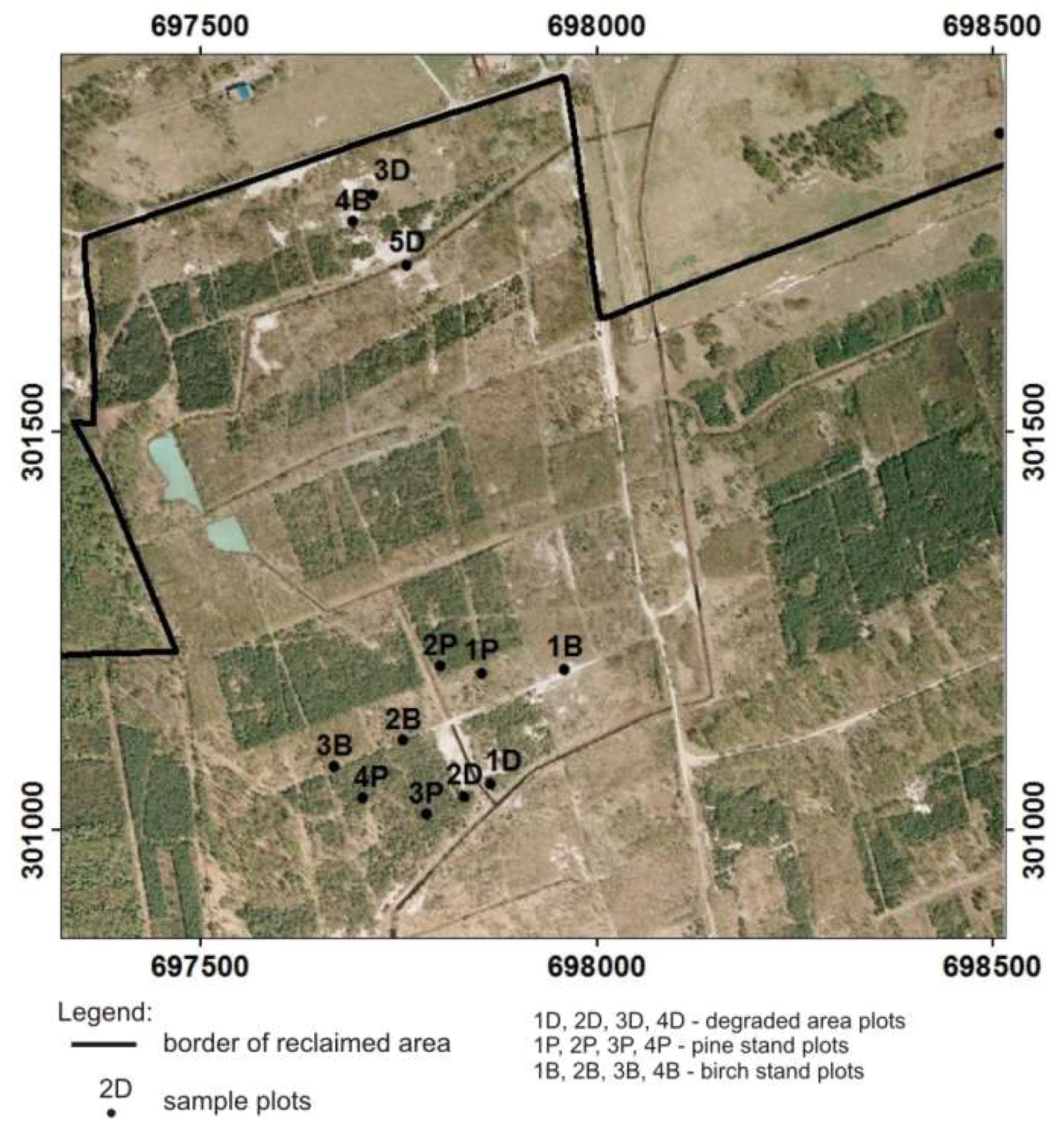
2.1. Study Site
2.2. Field and Laboratory Study
3. Results
3.1. Soil pH and Contamination Spatial Variability
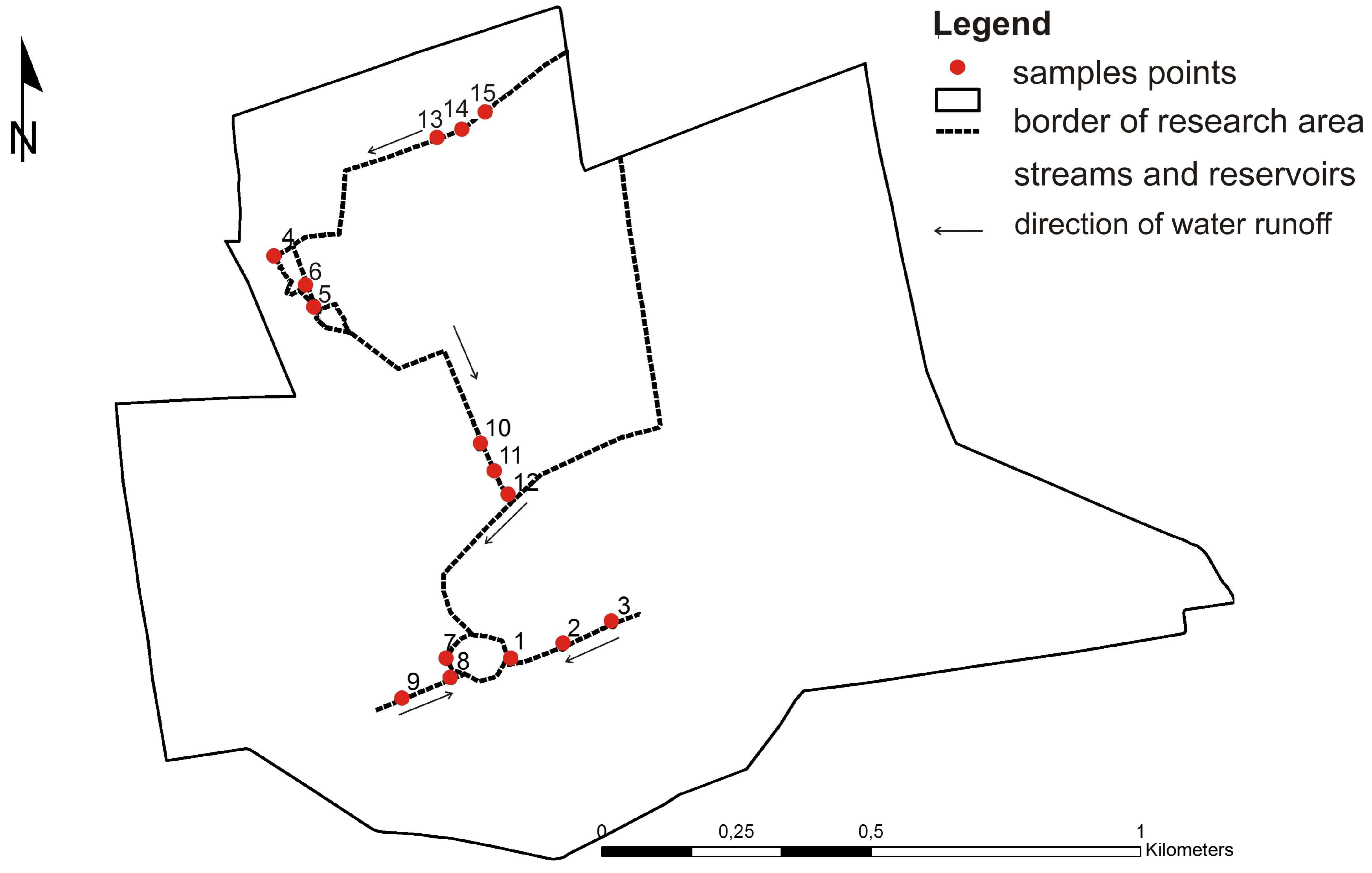
3.2. Surface Water Chemistry Variability
3.3. Soil and Vegetation Relations
3.4. Foliage Chemistry and Macronutrient Supply
3.5. Chemistry of Soil Solution in the Leaching Experiment under Controlled Conditions
4. Discussion
5. Summary and Conclusions
- Hotspots that are highly contaminated with sulfur still occur. The hotspots cover a relatively small area of about 2% of the monitored site (216.5 ha). However, they typically display a significant disturbance of soil and surface water chemistry, affecting the reaction of vegetation.
- The investigated surface waters mostly display relatively high pH (6.5 on average). Only in several points (three out 15), pH ranged from 2.6 to 3.1, indicating heavy acidification and AMD risk, so the area must be monitored.
- It was found that at sulfur concentration in soils of up to 3% after suitable neutralization with sludge lime (at a minimum dose of 500 Mg kg−1), the introduced birch and pine stands displayed good growth parameters and viability. The remaining ones occur in spots constituting about 2% of heavily contaminated sites, where neutralization was not done properly. In order to introduce vegetation and achieve a satisfactory yield, thorough neutralization treatment should be repeated.
- Wood small-reed proved extremely resistant to sulfur contamination and the relatively high salinity of soils, and it occurred even on extremely sulfated soils. Therefore, there is a perspective and an indication of the use of this species in the natural succession of similar sites as an alternative temporary biological stabilization.
- Soil detoxification will not be achieved without neutralization. Changes in the chemistry of soil solution after the application of organic matter were due to the process of litter mineralization and the leaching of nutrients. This effect was more pronounced in the case of birch litter application, where more intensive nitrogen and magnesium leaching took place, which is important in the perspective of soil-forming processes and plant nutrition.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USGS. 2014 Minerals Yearbook. Sulfur 9Advance Release; U.S. Department of the Interior: Washington, DC, USA; U.S. Geological Survey: Reston, VA, USA, 2014. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/sulfur/myb1-2014-sulfu.pdf (accessed on 6 February 2017).
- PGI. Polish Geological Institute National Research Institute. Available online: http://geoportal.pgi.gov.pl/surowce/chemiczne/siarka (accessed on 6 February 2017).
- Likus-Cieślik, J.; Pietrzykowski, M.; Śliwińska-Siuśta, M.; Krzaklewski, W.; Szostak, M. A preliminary assessment of soil sulfur contamination and vegetations in the vicinity of former boreholes on the afforested post-mine site Jeziórko. Geol. Geophys. Environ. 2015, 41, 371–380. [Google Scholar] [CrossRef]
- Likus-Cieślik, J.; Pietrzykowski, M.; Szostak, M.; Szulczewski, M. Spatial distribution and concentration of sulfur in relation to vegetation cover and soil properties on a reclaimed sulfur mine site (Southern Poland). Environ. Monit. Assess. 2017, 189, 87. [Google Scholar] [CrossRef] [PubMed]
- Ober, J.A. Materials Flow of Sulfur; Open-File Report 02–298; U.S. Geological Survey: Reston, VA, USA, 2002. Available online: https://pubs.usgs.gov/of/2002/of02-298/ (accessed on 7 February 2017).
- TSI. The Sulfur Institute. Available online: http://www.sulfurinstitute.org (accessed on 7 February 2017).
- Likus-Cieślik, J.; Pietrzykowski, M. Vegetation development and nutrients supply of trees in habitats with high sulfur concentration in reclaimed former sulfur mines Jeziórko (Southern Poland). Environ. Sci. Pollut. Res. Int. 2017, 24, 20556–20566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Likus-Cieślik, J.; Pietrzykowski, M. Ocena hydrochemiczna wód powierzchniowych na zrekultywowanym i zalesionym obszarze kopalni siarki “Jeziórko”. In Współczesne Problemy Ochrony Środowiska IV; Czop, M., Kajda-Szcześniak, M., Eds.; Katedra Technologii i Urządzeń Zagospodarowania Odpadów, Politechnika Śląska: Gliwice, Poland, 2017; pp. 41–47. ISBN 978-83-930232-6-4. (In Polish) [Google Scholar]
- Zhao, K.J.; McGrath, S.P.; Blake–Kalff, M.M.A.; Link, A.; Tucker, M. Crop responses to sulfur fertilization in Europe; International Fertiliser Society: York, UK, 2002; p. 28. ISBN 978-0853101406. [Google Scholar]
- Stern, D. Global sulfur emissions from 1850 to 2000. Chemosphere 2005, 58, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Katzur, J.; Haubold-Rosar, M. Amelioration and reforestation of sulfur ous mine soils in Lusatia (Eastern Germany). Water Air Soil Pollut. 1995, 91, 17–32. [Google Scholar] [CrossRef]
- Larssen, T.; Carmichael, G.R. Acid rain and acidification in China: The importance of base cation deposition. Environ. Pollut. 2000, 110, 89–102. [Google Scholar] [CrossRef]
- Liu, K.H.; Fang, Y.T.; Yu, F.M.; Liu, Q.; Li, R.F.; Peng, S.L. Soil Acidification in Response to Acid Deposition in Three Subtropical Forests of Subtropical China. Pedosphere 2010, 20, 399–408. [Google Scholar] [CrossRef]
- Esri. 2014. ArcMap-ArcGIS 10.2 (software system). Available online: https://www.esri.pl/ (accessed on 10 July 2018).
- Race, M.; Ferraro, A.; Fabbricino, M.; La Marca, A.; Panico, A.; Spasiano, D.; Tognacchini, A.; Pirozzi, F. Ethylenediamine-N,N′-Disuccinic Acid (EDDS)—Enhanced Flushing Optimization for Contaminated Agricultural Soil Remediation and Assessment of Prospective Cu and Zn Transport. Int. J. Environ. Res. Public Health 2018, 15, 543. [Google Scholar] [CrossRef] [PubMed]
- Pietrzykowski, M. Soil quality index as a tool for Scots pine (Pinus sylvestris) monoculture conversion planning on afforested, reclaimed mine land. J. For. Res. 2014, 25, 63–74. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Piotrowska, M.; Motowicka-Terelak, T.; Maliszewska-Kordybach, B.; Filipiak, K.; Krakowiak, A.; Pietruch, C. The Fundamentals of Assessment of Chemical Contamination of Soils. Heavy Metals, Sulfur and PAHs; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 1995; pp. 19–20. ISBN 9788386676354. (In Polish) [Google Scholar]
- Pietrzykowski, M.; Krzaklewski, W. Soil organic matter, C and N accumulation during natural succession and reclamation in an opencast sand quarry (southern Poland). Arch. Agron. Soil Sci. 2007, 53, 473–483. [Google Scholar] [CrossRef]
- Kopáček, J.; Fluksová, H.; Hejzlar, J.; Kaňa, J.; Porcal, P.; Turek, J. Changes in surface water chemistry caused by natural forest dieback in an unmanaged mountain catchment. Sci. Total Environ. 2017, 585–585, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Macioszczyk, A. Hydrogeochemistry; Wyd. Geol.: Warszawa, Poland, 1987; p. 475. ISBN 83-220-0298-X. (In Polish) [Google Scholar]
- StatSoft, Inc. 2014. STATISTICA (Data Analysis Software System), v. 12. Available online: www.statsoft.com (accessed on 10 July 2018).
- Woś, B.; Pietrzykowski, M. Simulation of Birch and Pine Litter Influence on Early Stage of Reclaimed Soil Formation Process under Controlled Conditions. J. Environ. Qual. 2015, 44, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Abrol, I.P.; Yadav, J.S.P.; Massoud, F.I. Salinity and plant growth. In Salt-Affected Soils and Their Management; FAO Soil Bulletin; FAO: Rome, Italy, 1988; Volume 39, Available online: http://www.fao.org/docrep/x5871e/x5871e04.htm#3.1.2 (accessed on 18 April 2018).
- RME. Regulation of the Ministry of Environment of 21 July 2016—Regulation Establishing Way of Classifying the State of Uniform Parts of Surface Waters and Environmental Quality Standards for Priority Substances (Rozporządzenie Ministra Środowiska z dn. 21 lipca 2016 r. w sprawie sposobu klasyfikacji stanu jednolitych części wód powierzchniowych oraz środowiskowych norm jakości dla substancji priorytetowych). 2016; Dz.U. 2016 poz. 1187. Available online: http://dziennikustaw.gov.pl/du/2016/1187 (accessed on 18 April 2018). (In Polish)
- Stefan, K.; Fürst, A.; Hacker, R.; Bartels, U. Forest Foliar Condition in Europe. Forest Foliar Coordinating Centre in Cooperating with the Australian Federal Forest Research Centre; EC-UN/ECE-FBV A; United Nations Economic Commision for Europe: Brussels, Geneva, 1997; Available online: http://bfw.ac.at/600/pdf/1451_01.pdf (accessed on 10 July 2018).
- Augusto, L.; Ranger, J.; Binkley, D.; Rothe, A. Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci. 2002, 59, 233–253. [Google Scholar] [CrossRef] [Green Version]
- Likus-Cieślik, J.; Pietrzykowski, M.; Chodak, M. Chemistry of sulfur-contaminated soil substrate from a former Frasch extraction method sulfur mine Leachate with various forms of litter in a controlled experiment. Water Air Soil Poll. 2018, 229, 71. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.J.; Cole, M.A. Cycles of Soil—Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients; John Wiley and Sons Inc.: New York, NY, USA, 1999; p. 448. ISBN 978-0-471-32071-5. [Google Scholar]
- Environmental Management Act (EMA). Contaminated Sites Regulation; Environmental Management Act (EMA): British Columbia, Canada, 1996; Available online: http://www.bclaws.ca (accessed on 20 December 2016).
- Cárcamo, H.A.; Parkinson, D.; Volney, J.W. Effects of sulfur contamination on macroinvertebrates in Canadian pine forests. Appl. Soil Ecol. 1998, 9, 459–464. [Google Scholar] [CrossRef]
- Benison, K.C.; Bowen, B.B. Extreme sulfur-cycling in acid brine lake environments of Western Australia. Chem. Geol. 2013, 351, 154–167. [Google Scholar] [CrossRef]
- Katzur, J.; Liebner, F. Effects of Superficial Tertiary Dump Substrates and Recultivation Variants on Acid Output, Salt Leaching and Development of Seepage Water Quality. In Acidic Mining Lakes (Environmental Science); Geller, W., Klapper, H., Salomons, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 251–265. [Google Scholar]
- Grande, J.A.; de la Torre, M.L.; Cerón, J.C.; Beltránc, R.; Gómez, T. Overall hydrochemical characterization of the Iberian Pyrite Belt. Main acid mine drainage-generating sources (Huelva, SW Spain). J. Hydrol. 2010, 390, 123–130. [Google Scholar] [CrossRef]
- Knoche, D.; Embacher, A.; Katzur, J. Element dynamics of oak ecosystems on acid-sulphurous mine soil in the Lusatian mining district (Eastern Germany). Lands Urban Plan 2000, 51, 113–122. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2011; p. 520. ISBN 978-1-4200-9368-1. [Google Scholar]
- Heinsdorf, D. Fertilization of Forest Crops on Lusatia Dumps (Düngung von Forstkulturen auf Lausitzer Kippen); Laubag: Eberswalde, Germany, 1999; p. 54. [Google Scholar]
- Baule, H.; Fricker, C. The Fertilizer Treatment of Forest Trees; PWRiL: Warsaw, Poland, 1973; p. 285. (In Polish) [Google Scholar]
- Pietrzykowski, M.; Woś, B.; Haus, M. Scots pine Needles macro-nutrient (N, P, K, Ca, Mg, and S) supply at different reclaimed mine soil substrates—As an indicator of the stability of developed forest ecosystems. Environ. Monit. Assess. 2013, 185, 7445–7457. [Google Scholar] [CrossRef] [PubMed]
- Holub, P.; Tůma, I.; Záhora, J.; Fiala, K. Different nutrient use strategies of expansive grasses Calamagrostis epigejos and Arrhenatherum elatius. Biologia 2012, 67, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Mudrák, O.; Frouz, J.; Velichová, V. Understory vegetation in reclaimed and unreclaimed post-mining forest stands. Ecol. Eng. 2010, 36, 783–790. [Google Scholar] [CrossRef]
- Menyailo, O.V.; Hungate, B.A.; Zech, W. Tree species mediated soil chemical changes in a Siberian artificial afforestation experiment. Plant Soil 2002, 242, 171–182. [Google Scholar] [CrossRef]
- Chodak, M.; Niklińska, M. The effect of different tree species on the chemical and microbial properties of reclaimed mine soils. Biol. Fertil. Soils 2010, 46, 555–566. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). The Use of Soil Amendments for Remediation, Revitalization, and Reuse; EPA (Environmental Protection Agency) National Service Center for Environmental Publications: Cincinnati, OH, USA, 2007. Available online: https://www.epa.gov/sites/production/files/2015-05/documents/the_use_of_soil_amendments_for_remediation_revitalization_and_reule.pdf (accessed on 6 January 2017).
- McTee, R.M.; Lekberg, Y.; Bullington, L.; Rummel, A.; Mummey, D.L.; Ramsey, P.W.; Hinman, N.W. Restoring ecological properties of acidic soils contaminated with elemental sulfur. Sci. Total Environ. 2017, 587–588, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| USDA | Sand | Silt | Clay | pHKCl | EC | NT | SOC | ST | |
|---|---|---|---|---|---|---|---|---|---|
| (%) | (µS cm−1) | (%) | (%) | (mg kg−1) | |||||
| Mean | S | 91 | 7 | 1 | 5.12 (3.30–7.59) | 92 (16–314) | 0.02 (0.00–0.08) | 0.57 (0.12–1.87) | 212 (10–1099) |
| SD | 1.58 | 101 | 0.02 | 0.47 | 322 | ||||
| Mean | LS | 77 | 19 | 4 | 4.43 (2.62–7.51) | 222 (15–1573) | 0.11 (0.00–0.41) | 2.59 (0.25–10.68) | 2185 (0–25,575) |
| SD | 1.79 | 425 | 0.14 | 3.10 | 6584 | ||||
| Mean | SL | 67 | 27 | 6 | 5.57 (2.75–7.49) | 412 (21–2080) | 0.08 (0.00–0.39) | 2.28 (0.69–8.15) | 5002 (10–40,805) |
| SD | 1.89 | 653 | 0.09 | 1.86 | 11,221 | ||||
| Mean | L | 47 | 43 | 10 | 6.46 (3.83–7.27) | 1332 (27–2000) | 0.03 (0.01–0.06) | 3.67 (0.95–7.58) | 15,388 (56–27,930) |
| SD | 1.47 | 833 | 0.02 | 2.43 | 11,985 | ||||
| Mean | SiL | 28 | 55 | 14 | 7.00 (6.79–7.13) | 1625 (1212–1940) | 0.02 (0.00–0.05) | 5.42 (2.76–6.76) | 35,731 (32,096–45,742) |
| SD | 0.16 | 308 | 0.02 | 1.88 | 6688 | ||||
| Season | ||||
|---|---|---|---|---|
| Summer | Autumn | Winter | Spring | |
| pH1 | 6.5 (3.1–7.7) | 6.6 (3.4–7.7) | 6.2 (2.7–7.2) | 6.7 (2.6–7.9) |
| µS cm−1 | ||||
| EC | 1819 (1660–2070) | 1823 (1720–2120) | 1566 (1083–2190) | 1980 (1118–3430) |
| mg L−1 | ||||
| Cl− | 76.83 (11.2–113.2) | 75.87 (9.5–122.4) | 67.42 (7.5–110.0) | 73.14 (7.2–123.7) |
| HCO3− | 8.63 (1.8–13.7) | 9.92 (2.4–15.6) | bdl | 78.33 (8.5–131.0) |
| NO3− | 1.00 (0.4–3.4) | 1.24 (0.4–2.7) | 1.86 (0.4–6.9) | bdl |
| SO42− | 1049.39 (829.5–1321.9) | 994.52 (593.8–1312.2) | 742.48 (472.0–988.1) | 954.11 (500.2–1502.9) |
| Na+ | 97.61 (22.5–134.9) | 96.82 (24.0–145.7) | 79.47 (13.7–122.9) | 93.87 (11.2–147.1) |
| Mg2+ | 21.44 (16.0–29.1) | 21.09 (14.4–30.8) | 15.83 (8.2–22.0) | 23.11 (14.3–30.8) |
| Ca2+ | 358.32 (276.3–405.8) | 343.00 (197.9–406.7) | 285.40 (174.5–390.6) | 342.75 (222.3–433.9) |
| Category | Soil Horizon | pHKCl | EC | ST | SOC | NT |
|---|---|---|---|---|---|---|
| µS cm−1 | Mg kg−1 | % | ||||
| B | 0–20 | 6.7 ± 1.3 | 707 ± 1132 | 17,609.83 ± 57,882.88 | 1.8 ± 1.32 | 0.05 ± 0.04 |
| 20–50 | 5 ± 1.2 | 421 ± 653 | 12,717.75 ± 36,221.24 | 0.6 ± 1.02 | 0.01 ± 0.02 | |
| 50–100 | 4.2 ± 1.8 | 564 ± 658 | 7891.63 ± 16,631.18 | 0.87 ± 0.7 | 0.03 ± 0.03 | |
| P | 0–20 | 6.0 ± 1.7 | 369 ± 480 | 1891.72 ± 5388.58 | 1.5 ± 0.83 | 0.06 ± 0.03 |
| 20–50 | 4.6 ± 1.1 | 347 ± 469 | 978.8 ± 3284.43 | 0.76 ± 0.76 | 0.03 ± 0.03 | |
| 50–100 | 5.2 ± 1.1 | 296 ± 262 | 274.87 ± 223 | 0.63 ± 0.44 | 0.02 ± 0.01 | |
| D | 0–20 | 3.9 ± 1.8 | 1840 ± 1220 | 24,569.68 ± 37,249.22 | 1.39 ± 1.15 | 0.03 ± 0.02 |
| 20–50 | 3.3 ± 1.4 | 1860 ± 1410 | 23,775.14 ± 43,725.62 | 1.11 ± 0.89 | 0.02 ± 0.02 | |
| 50–100 | 2.7 ± 0.9 | 1612 ± 967 | 89,326.42 ± 107,323.19 | 1.06 ± 0.79 | 0.03 ± 0.03 | |
| Category | N | DBH | Ht | V | IUFRO | Number of Vascular Plant Species | Cover Abundance of Vegetation | ||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | |||||||
| (pcs ha−1) 1 | (cm) | (m) | (m3 ar−1) | (%) | (pcs plot−1) | (%) | |||
| D | 612 (238–1090) | 7.7 (5.0–10.04) | 5.5 (3.61–6.88) | 0.06 (0.02–0.11) | 7 | 40 | 53 | 10 (1–16) | 26 (1–55) |
| P | 1457 (1051–2133) | 10.6 (9.17–11.53) | 10 (9.54–10.63) | 0.93 (0.73–1.09) | 25 | 63 | 12 | 14 (8–21) | 35 (3–75) |
| B | 1558 (637–2038) | 7.8 (5.67–8.89) | 8.3 (6.1–11.35) | 0.50 (6.4–11.35) | 9 | 70 | 21 | 14 (12–19) | 74 (60–85) |
| Foliage Species 1,2 | |||||
|---|---|---|---|---|---|
| B | P | ||||
| Element | Pc | Pc+1 | |||
| N | (%) | mean (median) | 1.71 a (1.68) | 1.12 b (1.07) | 1.11 b (1.10) |
| range | 1.58–1.89 | 1.05–1.30 | 1.05–1.81 | ||
| P | (mg kg−1) | mean (median) | 2320.4 a (2309.3) | 1348.1 b (1358.3) | 1045.4 b (1049.6) |
| range | 1695.8–2969.3 | 1221.3–1454.3 | 970.6–1111.8 | ||
| Ca | (%) | mean (median) | 1.35 a (1.30) | 0.33 b (0.32) | 0.75 c (0.78) |
| range | 1.15–1.63 | 0.25–0.44 | 0.52–0.93 | ||
| Mg | (mg kg−1) | mean (median) | 1525.7 a (1494.9) | 705.2 b (705.2) | 715.0 b (710.6) |
| range | 1039.9–2073.1 | 645.0–765.5 | 687.5–751.5 | ||
| K | (%) | mean (median) | 0.80 a (0.72) | 0.50 b (0.49) | 0.50 b (0.49) |
| range | 0.68–1.07 | 0.46–0.56 | 0.48–0.53 | ||
| Na | (mg kg−1) | mean (median) | 34.9 a (37.1) | 10.3 b (11.0) | 10.0 b (8.5) |
| range | 17.46–47.88 | bdl.—19.1 | 1.3–21.74 | ||
| S | (mg kg−1) | mean (median) | 1954 a (1987) | 1272 b (1287) | 1221 b (1208) |
| range | 1218–2626 | 1054–1459 | 955–1513 | ||
| Category 1 | ||||
|---|---|---|---|---|
| P | B | D | ||
| % | ||||
| N | mean (median) | 0.93 a (0.97) | 0.75 ab (0.73) | 0.62 b (0.67) |
| Range | 0.71–1.09 | 0.56–0.99 | 0.40–0.72 | |
| K | mean (median) | 0.95 a (0.87) | 0.72 a (0.76) | 0.57 a (0.57) |
| Range | 0.82–1.25 | 0.37–0.98 | 0.23–0.91 | |
| Ca | mean (median) | 0.34 a (0.33) | 0.27 a (0.31) | 0.22 a (0.24) |
| Range | 0.20–0.50 | 0.15–0.33 | 0.09–0.28 | |
| mg kg−1 | ||||
| S | mean (median) | 2249.1 a (2262.8) | 1717.3 a (1689.1) | 1778.5 a (2042.4) |
| Range | 2087.8–2383.0 | 1406.6–2084.4 | 790.6–2238.5 | |
| P | mean (median) | 1351.2 a (1381.1) | 1132.5 a (1182.3) | 904 a (739.3) |
| Range | 1235.3–1407.3 | 749.2–1416.3 | 519.3–1618.3 | |
| Mg | mean (median) | 706.1 a (644.1) | 485.5 ab (506.1) | 363.9 b (390.6) |
| Range | 603.4–932.5 | 296.8–633.1 | 175.1–499.5 | |
| Na | mean (median) | 25.0 a (15.8) | 22.2 a (22.4) | 11.3 a (5.9) |
| Range | 13.6–54.7 | 13.3–30.4 | 4.2–29.3 | |
| N:P ratio | mean (median) | 6.9 | 6.7 | 7.7 |
| Properties | LS 1 | LS-c | LS-B | LS-P | HS | HS-c | HS-B | HS-P | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 2.1 a | ± | 0.0‡ | 2.6 bc | ± | 0.0 | 2.5 bd | ± | 0.1 | 2.9 e | ± | 0.3 | 2.5 bc | ± | 0.0 | 2.6 c | ± | 0.0 | 2.3 d | ± | 0.0 | 2.5 bc | ± | 0.1 | |
| EC | (mS cm−1) | 1.87 a | ± | 0.057 | 0.39 b | ± | 0.032 | 0.59 c | ± | 0.11 | 0.23 d | ± | 0.23 | 2.59 e | ± | 0.053 | 2.53 e | ± | 0.075 | 2.98 f | ± | 0.123 | 2.58 e | ± | 0.047 |
| St | (mg kg−1) | 5090 a | ± | 483 | 6447 a | ± | 397 | 5947 a | ± | 1319 | 6178 a | ± | 1870 | 42,521 b | ± | 2670 | 35,634 c | ± | 9568 | 33,247 c | ± | 4033 | 34,157 c | ± | 4291 |
| SOC | (g kg−1) | 3.22 a | ± | 0.09 | 2.89 a | ± | 0.32 | 2.97 a | ± | 0.16 | 3.09 a | ± | 0.20 | 7.78 b | ± | 0.13 | 7.23 b | ± | 0.34 | 7.80 b | ± | 1.14 | 7.20 b | ± | 0.44 |
| Nt | 0.03 a | ± | 0.03 | 0.02 a | ± | 0.01 | 0.03 a | ± | 0.01 | 0.02 a | ± | 0.00 | 0.16 b | ± | 0.02 | 0.19 bc | ± | 0.04 | 0.22 c | ± | 0.04 | 0.18 b | ± | 0.01 | |
| Ca | 7.06 a | ± | 0.50 | 0.07 b | ± | 0.05 | 0.06 b | ± | 0.00 | 0.38 b | ± | 0.36 | 11.68 c | ± | 1.56 | 4.21 de | ± | 2.25 | 2.95 d | ± | 0.73 | 5.99 de | ± | 2.23 | |
| Mg | 0.09 ac | ± | 0.01 | 0.04 b | ± | 0.01 | 0.04 b | ± | 0.00 | 0.05 b | ± | 0.00 | 0.11 a | ± | 0.00 | 0.08 c | ± | 0.04 | 0.11 a | ± | 0.01 | 0.11 a | ± | 0.01 | |
| Al | 0.97 ab | ± | 0.09 | 0.37 c | ± | 0.25 | 0.43 c | ± | 0.03 | 0.49 c | 0.04 | 1.51 e | ± | 0.00 | 0.77 ad | ± | 0.44 | 1.02 ab | ± | 0.18 | 1.25 be | ± | 0.17 | ||
| RESP | (μg CO2/g/24 h) | n.d. 3 | 2.49 a | ± | 0.48 | 2.75 a | ± | 1.38 | 1.91 a | ± | 0.71 | n.d.2 | 2.40 a | ± | 0.57 | 0.99 a | ± | 0.48 | 2.24 a | ± | 0.59 | ||||
| LS-c | LS-B | LS-P | HS-c | HS-B | HS-P | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | s 1 | 1.8 b 3 | ± | 0.04 | 1.8 b | ± | 0.02 | 1.8 b | ± | 0.10 | 2.2 b | ± | 0.03 | 2.2 | ± | 0.10 | 2.3 b | ± | 0.10 |
| f 2 | 2.5 a | ± | 0.08 | 2.8 a | ± | 0.12 | 3.8 a | ± | 0.26 | 2.6 a | ± | 0.18 | 2.2 | ± | 0.22 | 2.9 a | ± | 0.26 | |
| mS cm−1 | |||||||||||||||||||
| EC | s | 10.80 a | ± | 1.38 | 11.48 a | ± | 0.42 | 10.91 a | ± | 3.07 | 4.85 a | ± | 0.29 | 5.52 | ± | 0.77 | 4.51 a | ± | 0.49 |
| f | 1.12 b | ± | 0.32 | 0.71 b | ± | 0.23 | 0.10 b | ± | 0.03 | 2.74 b | ± | 0.34 | 4.75 | ± | 1.00 | 2.40 b | ± | 0.36 | |
| mg L−1 | |||||||||||||||||||
| St | s | 1570.8 a | ± | 156.5 | 1585.9 a | ± | 100.1 | 1583.3 a | ± | 428.6 | 814.3 a | ± | 31.4 | 841.4 | ± | 73.6 | 741.0 a | ± | 74.4 |
| f | 71.0 b | ± | 20.3 | 53.7 b | ± | 17.2 | 9.6 b | ± | 4.6 | 625.7 b | ± | 36.6 | 725.4 | ± | 63.6 | 588.0 b | ± | 24.0 | |
| DOC | s | 82.84 a | ± | 8.95 | 92.97 a | ± | 9.38 | 117.13 a | ± | 18.80 | 101.41 a | ± | 9.54 | 127.00 a | ± | 33.11 | 110.37 a | ± | 15.38 |
| f | 5.08 b | ± | 0.33 | 20.47 b | ± | 3.17 | 37.20 b | ± | 10.72 | 9.78 b | ± | 1.65 | 32.00 b | ± | 4.03 | 29.56 b | ± | 2.48 | |
| Nt | s | 14.65 a | ± | 1.97 | 18.05 a | ± | 1.02 | 16.42 a | ± | 4.11 | 5.72 a | ± | 0.31 | 7.68 a | ± | 1.55 | 6.54 a | ± | 0.69 |
| f | 0.53 b | ± | 0.04 | 1.46 b | ± | 0.17 | 1.17 b | ± | 0.33 | 0.43 b | ± | 0.02 | 2.58 b | ± | 0.67 | 0.82 b | ± | 0.05 | |
| C | s | 474.41 a | ± | 39.07 | 445.30 a | ± | 26.10 | 442.78 a | ± | 33.14 | 550.23 a | ± | 17.79 | 552.79 a | ± | 14.18 | 553.38 | ± | 50.04 |
| f | 5.84 b | ± | 1.28 | 13.66 b | ± | 4.08 | 5.20 b | ± | 6.11 | 467.27 b | ± | 24.03 | 494.78 b | ± | 21.99 | 495.56 | ± | 17.26 | |
| Mg | s | 2.21 a | ± | 1.73 | 7.85 a | ± | 1.48 | 8.01 a | ± | 3.14 | 0.01 c | ± | 0.00 | 1.62 ad | ± | 0.96 | 0.85 d | ± | 1.70 |
| f | 0.01 b | ± | 0.00 | 0.68 b | ± | 0.12 | 0.07 b | ± | 0.14 | 0.01 c | ± | 0.03 | 2.24 a | ± | 0.49 | 0.48 d | ± | 0.09 | |
| Al | s | 73.24 a | ± | 12.42 | 61.17 a | ± | 6.95 | 60.68 a | ± | 25.23 | 42.95 a | ± | 6.32 | 32.42 a | ± | 10.98 | 21.66 a | ± | 9.22 |
| f | 0.22 b | ± | 0.16 | 0.05 b | ± | 0.04 | 0.06 b | ± | 0.04 | 0.74 b | ± | 0.41 | 1.77 b | ± | 0.47 | 0.21 b | ± | 0.14 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrzykowski, M.; Likus-Cieślik, J. Comprehensive Study of Reclaimed Soil, Plant, and Water Chemistry Relationships in Highly S-Contaminated Post Sulfur Mine Site Jeziórko (Southern Poland). Sustainability 2018, 10, 2442. https://doi.org/10.3390/su10072442
Pietrzykowski M, Likus-Cieślik J. Comprehensive Study of Reclaimed Soil, Plant, and Water Chemistry Relationships in Highly S-Contaminated Post Sulfur Mine Site Jeziórko (Southern Poland). Sustainability. 2018; 10(7):2442. https://doi.org/10.3390/su10072442
Chicago/Turabian StylePietrzykowski, Marcin, and Justyna Likus-Cieślik. 2018. "Comprehensive Study of Reclaimed Soil, Plant, and Water Chemistry Relationships in Highly S-Contaminated Post Sulfur Mine Site Jeziórko (Southern Poland)" Sustainability 10, no. 7: 2442. https://doi.org/10.3390/su10072442