Self-Powered Bioelectrochemical Nutrient Recovery for Fertilizer Generation from Human Urine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrolysed Human Urine
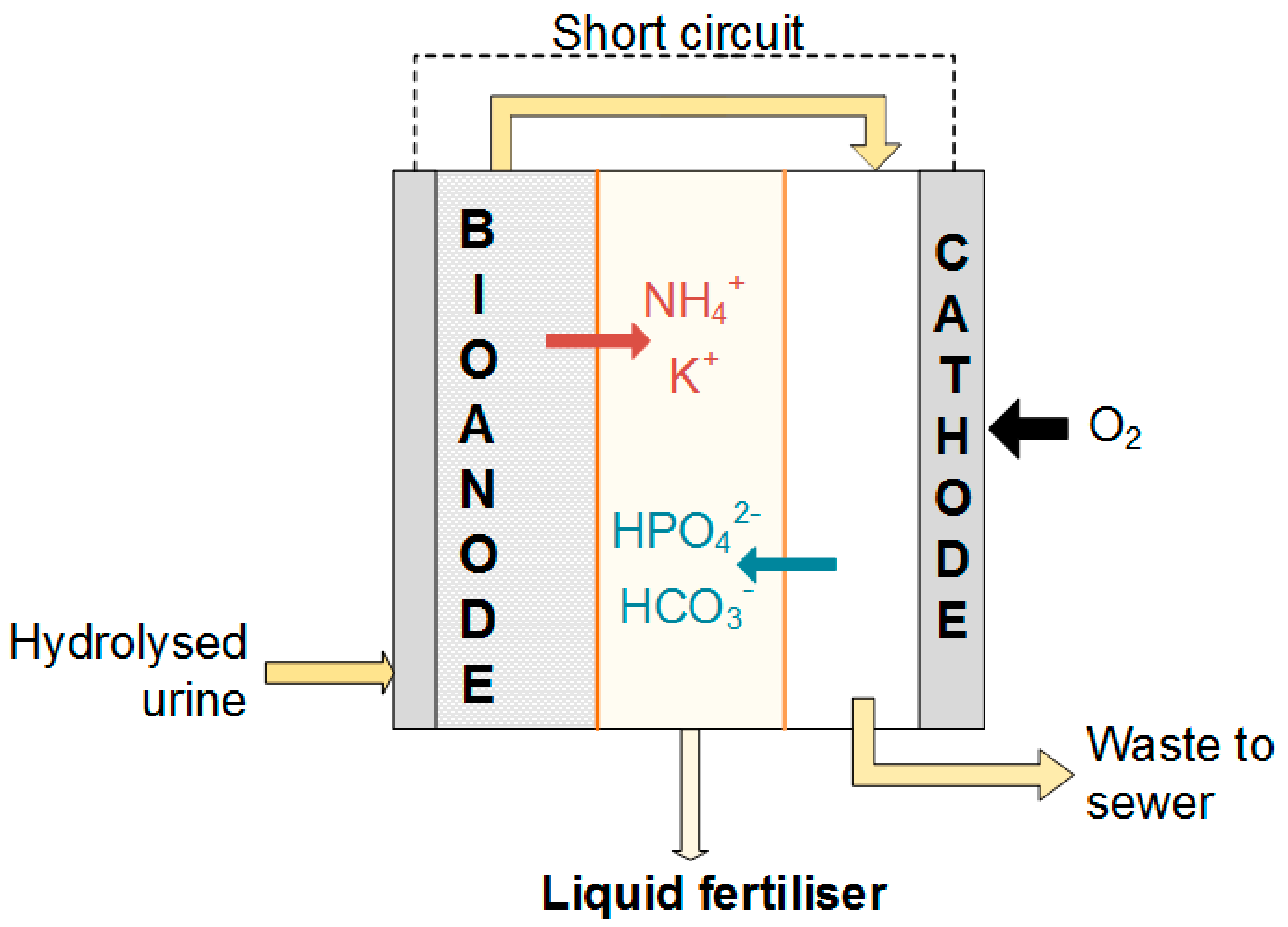
2.2. Electrochemical Cell Design and Operation
2.3. Air Cathode
2.4. Start-Up
2.5. Chemical Analysis
3. Results and Discussion
3.1. Cathode Catalytic Performance
3.2. Electricity Generation at Zero Power Input
3.3. Nutrient Recovery and Fertiliser Production
- (1)
- Normalized to total reactor volume
- (2)
- Normalized to membrane area (reactor cross-section)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cordell, D. Peak phosphorus and the role of P recovery in achieving food security. In Source Separation and Decentralisation for Wastewater Management; Larsen, T.A., Udert, K.M., Lienert, J., Eds.; IWA Publishing: London, UK, 2013; pp. 29–44. ISBN 9781843393481. [Google Scholar]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Kugler, K.; Ohs, B.; Scholz, M.; Wessling, M. Towards a carbon independent and CO2-free electrochemical membrane process for NH3 synthesis. Phys. Chem. Chem. Phys. 2014, 16, 6129–6138. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [Green Version]
- Cordell, D.; White, S. Peak phosphorus: Clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability 2011, 3, 2027–2049. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Ghilardi, P.; Boguniewicz-Zablocka, J. New paradigms in urban water management for conservation and sustainability. Water Pract. Technol. 2016, 11, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Capodaglio, A.G.; Callegari, A.; Cecconet, D.; Molognoni, D. Sustainability of decentralized wastewater treatment technologies. Water Pract. Technol. 2017, 12, 463–477. [Google Scholar] [CrossRef]
- Daneshgar, S.; Buttafava, A.; Callegari, A.; Capodaglio, A.G. Economic and energetic assessment of different phosphorus recovery options from aerobic sludge. J. Clean. Prod. 2019, 223, 729–738. [Google Scholar] [CrossRef]
- Johansson, M.; Jönsson, H.; Höglund, C.; Richert Stintzing, A.; Rodhe, L. Final Report for Source-Separated Human Urine: A Future Source of Fertilizer for Agriculture in the Stockholm Region; Stockholm Vatten, Stockholmshem & HSB National Federation: Stockholm, Sweden, 2002. [Google Scholar]
- Wilsenach, J.A.; Schuurbiers, C.A.H.; van Loosdrecht, M.C.M. Phosphate and potassium recovery from source separated urine through struvite precipitation. Water Res. 2007, 41, 458–466. [Google Scholar] [CrossRef]
- Hug, A.; Udert, K.M. Struvite precipitation from urine with electrochemical magnesium dosage. Water Res. 2013, 47, 289–299. [Google Scholar] [CrossRef]
- Etter, B.; Tilley, E.; Khadka, R.; Udert, K.M. Low-cost struvite production using source-separated urine in Nepal. Water Res. 2011, 45, 852–862. [Google Scholar] [CrossRef] [Green Version]
- Daneshgar, S.; Vanrolleghem, P.A.; Vaneeckhaute, C.; Buttafava, A.; Capodaglio, A.G. Optimization of P compounds recovery from aerobic sludge by chemical modeling and response surface methodology combination. Sci. Total Environ. 2019, 668, 668–677. [Google Scholar] [CrossRef]
- Kuntke, P.; Śmiech, K.M.; Bruning, H.; Zeeman, G.; Saakes, M.; Sleutels, T.H.J.A.; Hamelers, H.V.M.; Buisman, C.J.N. Ammonium recovery and energy production from urine by a microbial fuel cell. Water Res. 2012, 46, 2627–2636. [Google Scholar] [CrossRef]
- Christiaens, M.E.R.; Udert, K.M.; Arends, J.B.A.; Huysman, S.; Vanhaecke, L.; McAdam, E.; Rabaey, K. Membrane stripping enables effective electrochemical ammonia recovery from urine while retaining microorganisms and micropollutants. Water Res. 2018, 150, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Tarpeh, W.A.; Nelson, K.L. Electrochemical Stripping to Recover Nitrogen from Urine. Environ. Sci. Technol. 2018, 52, 1453–1460. [Google Scholar] [CrossRef]
- Siegrist, H.; Laureni, M.; Udert, K.M. (Eds.) Transfer into the gas phase: ammonia stripping. In Source Separation and Decentralisation for Wastewater Management; IWA Publishing: London, UK, 2013; pp. 337–350. [Google Scholar]
- Etter, B.; Hug, A.; Udert, K.M. Total Nutrient Recovery from Urine—Operation of a Pilot-Scale Nitrification Reactor. In Proceedings of the WEF/IWA International Conference on Nutrient Removal and Recovery 2013: Trends in Resource Recovery and Use, Vancouver, BC, Canada, 28–31 July 2013. [Google Scholar]
- Udert, K.M.; Buckley, C.A.; Wächter, M.; McArdell, C.S.; Kohn, T.; Strande, L.; Zöllig, H.; Fumasoli, A.; Oberson, A.; Etter, B. Technologies for the treatment of source-separated urine in the eThekwini Municipality. Water SA 2015, 41, 212–221. [Google Scholar] [CrossRef]
- Etter, B.; Udert, K.M.; Gounden, T. (Eds.) VUNA Final Report, Eawag; Swiss Federal Institute of Aquatic Science and Technology (EAWAG): Dübendorf, Switzerland, 2015. [Google Scholar]
- Pronk, W.; Zuleeg, S.; Lienert, J.; Escher, B.; Koller, M.; Berner, A.; Koch, G.; Boller, M. Pilot experiments with electrodialysis and ozonation for the production of a fertiliser from urine. Water Sci. Technol. 2007, 56, 219–227. [Google Scholar] [CrossRef]
- Pronk, W.; Biebow, M.; Boller, M. Electrodialysis for recovering salts from a urine solution containing micropollutants. Environ. Sci. Technol. 2006, 40, 2414–2420. [Google Scholar] [CrossRef]
- Fumasoli, A.; Etter, B.; Sterkele, B.; Morgenroth, E.; Udert, K.M. Operating a pilot-scale nitrification/distillation plant for complete nutrient recovery from urine. Water Sci. Technol. 2016, 73, 215–222. [Google Scholar] [CrossRef]
- Udert, K.M.; Wächter, M. Complete nutrient recovery from source-separated urine by nitrification and distillation. Water Res. 2012, 46, 453–464. [Google Scholar] [CrossRef]
- Pronk, W.; Palmquist, H.; Biebow, M.; Boller, M. Nanofiltration for the separation of pharmaceuticals from nutrients in source-separated urine. Water Res. 2006, 40, 1405–1412. [Google Scholar] [CrossRef]
- Ledezma, P.; Jermakka, J.; Keller, J.; Freguia, S. Recovering Nitrogen as a Solid without Chemical Dosing: Bio-Electroconcentration for Recovery of Nutrients from Urine. Environ. Sci. Technol. Lett. 2017, 4, 119–124. [Google Scholar] [CrossRef]
- Freguia, S.; Rabaey, K.; Yuan, Z.; Keller, J. Non-catalyzed cathodic oxygen reduction at graphite granules in microbial fuel cells. Electrochim. Acta 2007, 53, 598–603. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006. [Google Scholar] [CrossRef]
- Nabae, Y.; Sonoda, M.; Yamauchi, C.; Hosaka, Y.; Isoda, A.; Aoki, T. Highly durable Pt-free fuel cell catalysts prepared by multi-step pyrolysis of Fe phthalocyanine and phenolic resin. Catal. Sci. Technol. 2014, 4, 1400–1406. [Google Scholar] [CrossRef] [Green Version]
- Nabae, Y.; Kuang, Y.; Chokai, M.; Ichihara, T.; Isoda, A.; Hayakawa, T.; Aoki, T. High performance Pt-free cathode catalysts for polymer electrolyte membrane fuel cells prepared from widely available chemicals. J. Mater. Chem. A 2014, 2, 11561–11564. [Google Scholar] [CrossRef] [Green Version]
- Blázquez, E.; Gabriel, D.; Baeza, J.A.; Guisasola, A.; Freguia, S.; Ledezma, P. Recovery of elemental sulfur with a novel integrated bioelectrochemical system with an electrochemical cell. Sci. Total Environ. 2019, 677, 175–183. [Google Scholar] [CrossRef]
- Soukharev, V.; Mano, N.; Heller, A. A four-electron O2-electroreduction biocatalyst superior to platinum and a biofuel cell operating at 0.88 V. J. Am. Chem. Soc. 2004, 126, 8368–8369. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Arola, K.; Thompson Brewster, E.; Mehta, C.M.; Batstone, D.J. Nutrient recovery from wastewater through pilot scale electrodialysis. Water Res. 2018, 135, 57–65. [Google Scholar] [CrossRef]
- Cecconet, D.; Bolognesi, S.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Influence of reactor’s hydrodynamics on the performance of microbial fuel cells. J. Water Process Eng. 2018, 26, 281–288. [Google Scholar] [CrossRef]
- Monetti, J.; Ledezma, P.; Virdis, B.; Freguia, S. Nutrient Recovery by Bio-Electroconcentration is Limited by Wastewater Conductivity. ACS Omega 2019, 4, 2152–2159. [Google Scholar] [CrossRef]


| Reactor 1 | Reactor 2 | |
|---|---|---|
| Average current density | 3.1 | 2.7 |
| N-removal rate (g N m–3 d–1)(1) | 496 | 409 |
| N-removal rate (g N m–2 d–1)(2) | 30 | 25 |
| Energy input | 0 | 0 |
| N upconcentration factor | 1.5 | 1.4 |
| P upconcentration factor | 0.6 | 0.6 |
| K upconcentration factor | 1.7 | 1.6 |
| Na upconcentration factor | 1.4 | 1.4 |
| Product-to-feed ratio | 0.054 | 0.046 |
| Average | Standard Deviation (n = 6) | |
|---|---|---|
| pH | 8.1 | |
| Ionic conductivity, mS cm−1 | 77 | |
| NH4-N, mg L−1 | 11,600 | 600 |
| PO4-P, mg L−1 | 202 | 4 |
| K, mg L−1 | 3900 | 200 |
| Na, mg L−1 | 4000 | 200 |
| Ca, mg L−1 | 1 | 1 |
| Mg, mg L−1 | < 1 | |
| SO4-S, mg L−1 | 1020 | 70 |
| Acetate, mg L−1 | 19,000 | 600 |
| Cd, Cr, Cu, Pb, and Zn, mg L−1 | < 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freguia, S.; Logrieco, M.E.; Monetti, J.; Ledezma, P.; Virdis, B.; Tsujimura, S. Self-Powered Bioelectrochemical Nutrient Recovery for Fertilizer Generation from Human Urine. Sustainability 2019, 11, 5490. https://doi.org/10.3390/su11195490
Freguia S, Logrieco ME, Monetti J, Ledezma P, Virdis B, Tsujimura S. Self-Powered Bioelectrochemical Nutrient Recovery for Fertilizer Generation from Human Urine. Sustainability. 2019; 11(19):5490. https://doi.org/10.3390/su11195490
Chicago/Turabian StyleFreguia, Stefano, Maddalena E. Logrieco, Juliette Monetti, Pablo Ledezma, Bernardino Virdis, and Seiya Tsujimura. 2019. "Self-Powered Bioelectrochemical Nutrient Recovery for Fertilizer Generation from Human Urine" Sustainability 11, no. 19: 5490. https://doi.org/10.3390/su11195490





