Abstract
Public access to high quality green environments has become a key issue for city managers and a matter of environmental justice. Urban pressures on ecosystem remnants may act to favor the expansion of some invasive species in cities. Whilst the negative impacts of invasive species on ecosystem function is well documented, little is known about how invasive species influence the use of green space by people. Here, we examined one of the few remnants of urban riparian forests in Europe, the Vistula river valley in Warsaw, which has recently become an attractive recreation site. Despite their high ecological value, the poplar and willow forests have been increasingly taken over by the invasive tree species Acer negundo. We examined the status of the invasion process and the relationship between recreational ecosystem services and the characteristics of the tree stands—tree species, tree density, and age and NDVI values. We found the willow forest to be more susceptible to invasion by A. negundo than the poplar forest, which was revealed in significantly higher share of the maple individuals and their greater volume per unit area. Ash-leaved maples also prevailed in numbers in younger stands (<10 years) than in older ones. The presence of A. negundo affected biodiversity, resulting in decreased undergrowth density and biodiversity expressed in number of species. The use intensity by the public, assessed on the basis of soil compaction of existing informal tracks, as revealed by GLM analysis, was related to volume of invasive maple and distance from the main track. This study highlights the need to integrate invasive species management into green infrastructure planning and management.
1. Introduction
The increasing urban population of cities worldwide combined with anxiety over the life quality of residents has resulted in an increasing interest in the benefits to be derived from city green areas [1,2,3,4,5,6,7]. Many cities were established on the banks of rivers, and frequently the remnants of former riparian forests constitute an important part of the green systems of the city [8,9]. Despite remaining under strong anthropogenic pressure, including land-use change, pollution, lack of cyclic flooding, human trampling, or the introduction of non-indigenous organisms [10], riparian ecosystems generate multiple important services for the city residents [11,12]. Natural riparian forests are recognized for their positive contribution to nutrient removal, carbon capture, air purification, pollination, noise buffering, and water cleaning [13,14,15,16,17]. The few examples of riparian forests which were preserved in cities play an additional important role by contributing recreation opportunities to citizens and allowing them to interact with nature. The role of recreation in city green spaces in improving citizen health and well-being has been widely recognized and is regarded as one of the most important ecosystem services urban green spaces offer [18,19,20,21,22,23,24]. More and more studies also reveal an increasing demand from city residents for “less ordered” green areas providing the possibility to experience nature more directly than the traditional urban green spaces, such as parks, can offer [25,26].
Physical disturbances to nature in cities, such as pollution, drought, or drainage, lead to irreversible changes in the existing ecosystems. Preserving high biodiversity in urban environments faces major difficulties, and river valleys are additionally highly susceptible to other negative changes, including a high risk of biological invasions [27]. Invasive plant species (IAS) not only severely alter the biodiversity of the areas they colonize, but can also alter the ecosystem services provisioning by those areas [28]. The negative effects of various IAS on ecosystem services provisioning were recognized for multiple services [29]. The impact of different IAS on the provision of ecosystem services is considered negative, listing these more important effects—they hinder land use, thus limiting food production [30], replace natural ecosystems as barriers for many human pathogens to their easier dissemination [31,32], attract native pollinator species, thus decreasing their activity on native plant species [33], colonize water banks, and are ineffective in coastal stabilization [34,35], and reduce recreational and tourist benefits [29,30]. IAS species also provide certain benefits [29], but the economic balance seems to be unfavorable for these species.
Yet little is known about the impact of IAS on recreational ecosystem services (RES), as one of the cultural ecosystem services, understood as “the contributions to recreation opportunities”. Even less is known about ecosystem services in riparian forests due to their scarcity and the difficulties in recreational potential assessment, and the socio-cultural context of the human perception of invasive species is also very poorly understood [36]. Nature preservation in cities is strongly driven by social approval, and a participatory approach to spatial urban planning has become good practice [37]. Consequently, it is crucial to recognize if IAS in urban areas, riparian forests in particular, affect the perception of urban green areas and result in a decline of ecosystem service provisioning [29]. Invasive tree species are common in urban forests, and are likely to remain so due to low effectiveness of removal actions [29], yet little is known concerning their perception as a component of natural vegetation remnants or regenerating forms. Invasive tree species can affect ecosystem services provisioning [38,39,40,41,42], but also change their visual appearance, which can affect how people use the space [42,43]. Invasive plant species may likely be perceived negatively by the public and be associated with their environmental impact, but still a significant share of society might have no negative attitude towards the presence of invasive species [44]. Many plants, now declared as invasive species, were deliberately introduced to urban recreational areas due to their aesthetic values, and despite any ecosystem services lost services, such as use in medicine or biofuels, may occur [29]. Effectively, the public may perceive IAS similarly to native trees, and IAS management consequently receives low priority [45,46].
Ash-leaved maple (Acer negundo), a native of North America, was commonly planted in Europe from the 17th century [47,48]. Due to high tolerance to heat and water stress, it has become one of the most invasive plant species occurring in riparian forests in Europe. Due to intensive seed production and easy dispersal by wind and water, it has spread throughout Poland, and other parts of Europe, being most successful in urbanized regions along rivers [49,50]. In the 20th century, the tree became one of the most valued in urban plantings [51], and in the same period there was information about its escape from cultivation [52]. Species penetration takes place in many regions of Europe on a massive scale in disturbed urban habitats and along river valleys [53], hence it is expected that A. negundo will become a permanent component of the riparian forest [54]. In this study, we investigated the process of invasion in riparian forests by A. negundo and evaluate how it affects the structure of the riparian forests preserved in Vistula river in Warsaw and the effect of A. negundo on recreational ecosystem services of this area. We address the following questions: (1) What are the forest stand characteristics of riparian forest invaded to various extent by A. negundo—habitat type, tree density, and age; (2) What is the effect of A. negundo on the biodiversity and Normalized Difference Vegetation Index (NDVI), as indirect measure of biomass and quality of the riparian forest stands; (3) How are the recreational ecosystem services, expressed in intensity of penetration by visitors, linked to the presence of A. negundo and the forest characteristics.
2. Materials and Methods
2.1. Study Area
The investigation was conducted in Warsaw, Poland’s capital city, which covers an area of 517 square kilometers and is inhabited by over 1.76 million citizens (GUS, 2018). The city is characterized by high greenness, with the green infrastructure including agricultural areas accounting for nearly 50% of the city’s area, along with over 14% forested lands [55]. The urban green space of Warsaw consists of 201 parks and forests, and 12 nature reserves. The unique element of Warsaw’s green system is the strip of natural riparian forest which developed along the Vistula river (Figure 1) in between the embankments. The case of Warsaw is a unique example of a natural riparian forest formed within a narrow strip between the river and the embankments, which is also located in the strict city center. Today, the area is covered by the 50-year-old riparian forests of poplar Populetum albae and willow Salicion albae—code 91E0 (Interpretation Manual—EUR28 2013), accompanied by the oxbow lakes, represented by the Potamion and Nymphaeion plant communities (code 3150-2), and muddy banks with Chenopodion rubri p.p. Bidention p.p. vegetation (code 3270), all being Natura 2000 habitat types, temporarily appearing between the groynes. Despite the high ecological value, revealed in number of plant species, the area is subjected to various pressures such as increased visitor pressure, resulting in vegetation mechanical damage and soil compaction. These negative changes can be revealed in the presence of the invasive tree species such as A. negundo, which has been noted in substantial numbers in the forest stand, both in the canopy and undergrowth [56].
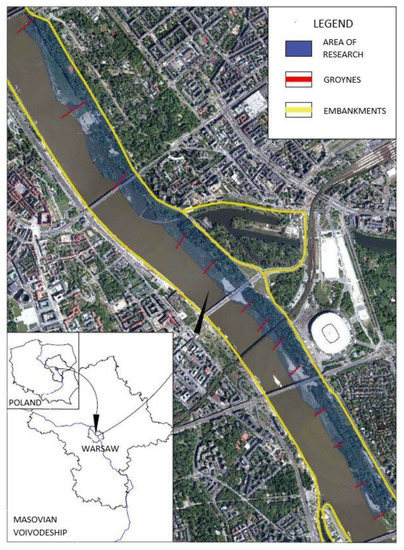
Figure 1.
Location of the investigated area.
Since the 1970s, the riparian forests of the Vistula received little attention from the city’s managers, and remained mostly unmanaged as a result of cyclic floods and associated lack of installed permanent infrastructure (Figure 2). In 2007, the Municipality of Warsaw made the area accessible to the public by creating a “nature track” along the river in the forest. The track was an approximately 20 km section of a gravel route parallel to the river course designated for walking and cycling, which remains periodically flooded along with the temporary infrastructure. Since that time, the number of visitors has grown substantially. In the year 2007, the area was not used for recreation and during the vegetation inventory performed at that time we observed only a few visitors daily (unpublished data). The counts of pedestrians and cyclists in 2013 indicated 25,000 visitors per year [56]. In years 2014–2018, the yearly number of cyclists recorded has grown to over 140,000 (source: http://rowery.um.warszawa.pl). The track along the natural shore of the river, accompanied by cultural and educational events, remains one of the most popular places in Warsaw [56], offering the citizens a wide range of ecosystem services and the possibility to experience nature in the middle of a major city (Figure 2). Apart from the main formal trail along the river, growing interest from citizens in nature-seeking has resulted in increased activity of the visitors off the main track and led to multiple off-trail routes throughout the forests (Figure 3). The selected site represents a well-preserved riparian forest, but at the same time it is subject to visitor pressure, when compared to other sections of the forests along the river (due to the presence of recreational infrastructure). The number of visitors yearly is comparable to the visits made to the biggest public parks in Warsaw [56].

Figure 2.
Main formal route along the riparian forest (formal trail) (a), informal trails in poplar riparian forest Populetum albae (b), willow riparian forest with Populus x canescens, (c) and willow riparian forest heavily invaded by A. negundo (d).
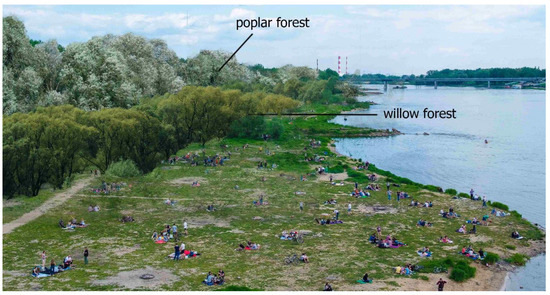
Figure 3.
Photograph presenting the investigated riparian habitats—typical willow and poplar forests used for recreation in Warsaw.
2.2. Canopy Tree Inventory
In the years 2015–2017, a detailed tree canopy inventory was performed in the 20 km riparian forest strip. We identified all tree individuals and recorded the species, the area was divided into homogenous forest patches in terms of tree age, species, and habitat. A detailed vector map of the forest stand patches was created based on field maps and the orthophotomap from 2017, as in Figure 4a. The patches varied from 0.01 to 1.63 ha. Within each distinguished patch, every single tree trunk of breast height diameter larger than 4 cm was recorded. All tree species were identified, including the hybrids between Populus sp. and Salix sp. We also estimated the age of the forest stand patches on the basis of rectified RGB orthophotomaps from years (1945, 1975, 1977, 1982, 1987, 1994, 2001, 2005, 2008, 2010, 2011, and 2012 retrieved from the Warsaw Municipality Office).
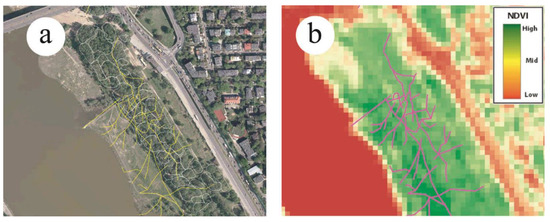
Figure 4.
Example of the results of tree stand mapping—white lines represent distinguished homogenous tree patches (a), results of the informal tracks inventory, (b) and NDVI values calculated from SENTINEL.
The forest stand patches were categorized based on the dominant tree species in the canopy and vegetation composition. We singled out two habitat types—poplar and willow riparian forests, and further we analyzed them separately, due to the differences in habitat characteristics. The willow forest patches were generally located closer to the river and were of approximately 50 m width, the undergrowth was dominated by the species characteristic of the Salicetum albo-fragilis type, the content of sand in soil was >50%, and cyclic flooding took place every 2–10 years [57]. The poplar forest stand patches were located further from the river and stretched further to the embankment. The habitat was less frequently flooded, and the vegetation composition was characteristic for the Populetum albae type [58].
2.3. The Effect of A. negundo on Biodiversity
The distribution dynamics and effects of invasive A. negundo presence on the riparian forest structure was investigated in detail. Vegetation was recorded in 83 representative 20 × 20 m plots in homogenous areas to a various extent invaded by A. negundo. In each plot the percentage of A. negundo, number of species in the canopy and undergrowth, and percentage cover of each of the layers—trees, shrubs, and undergrowth cover were recorded.
Vegetation quality assessment was performed by calculating average Normalized Difference Vegetation Index (NDVI) for each of the previously identified forest patches. We used satellite imagery from Sentinel-2 multispectral images. NDVI is frequently used to quantitatively assess urban vegetation, and values vary from −1 to 1, where maximum values correspond to the highest coverage of vegetation. The index has been successfully used to assess canopy structure and quality [59]. We used a cloudless scene obtained during the vegetation season (6 August 2017) to calculate NDVI using a software addendum (Quality Assurance Tools) (van Leeuwen, TBRS, Tucson, Arizona) applied to ENVI software. Normalized difference vegetation index was calculated using red and NIR reflectance as: NDVI = [(NIR − Red)/(NIR + Red)] [60].
2.4. Off Trail Activity as an Indicator of Recreational Ecosystem Services of Riparian Forests
We assumed the intensity of visits to be an indirect measure of user preference and willingness to spend time in this area. We inventoried the “traces” of user activity beyond the main walking track by mapping informal trails in the riparian forest, identified as linear damage in vegetation and soil being the effect of off-trail activity [61]. We used two indicators: Off trail density per patch (which better indicates the tendency of visitors to spatially penetrate the area and cause damage to the vegetation), and off trails soil compaction (which more describes the intensity of visits and can be related to the number of visitors who had used the trail). Mapping of informal trails was performed in years 2016 and 2017 in May–August in the field using a GPS device along the whole 20 km river section in the willow and forest stands. Linear tracks with visible vegetation losses and bare ground were inventoried. The density of tracks was presented as density in m/ha. In the central part of each inventoried trail section in the representative part, soil compaction was measured using a penetrometer (Eijkelkamp 06.01.SA, Giesbeek, the Netherlands) and presented in kN/cm2. The measurement was performed in all patches for at least 5 repetitions and the score averaged. Previous studies revealed that the main walking trail was visited by 136 to 687 people per day over the weekend and from 69 to 343 during weekdays [56]. A small proportion of the visitors, 1.1–11.5% walk off the main trail, which equates to around three thousand people/year who spend time beyond the main track within the natural riparian forest, most walk off the track only for a short period of time [56]. One-way ANOVA was used, followed by Tukey to test differences in forest type and vegetation parameters—tree stand composition, level of A. negundo density, and NDVI values, between the two examined riparian forest types—poplar and willow forest.
We further used General Linear Model to assess factors explaining intensity of visitor’s pressure, as a measure of recreational potential. We used averaged soil compaction per patch as a response variable. Factors included in the analysis as explanatory variables were: The dispersion of people on the off-trails; distance from the main path; distance to the main entrance; distance to the river; number of residents in the 500 m buffer, which reflects the distance that can be reached within a 5 min walk. The analysis of the impact of pathway compaction was performed separately for willow and poplar riparian forests, due to large differences in granulometric composition, in the first type the sand fraction was 26% (dusty clay) and in the second 79% (loose sand).
Spearman correlation was used to assess the relationship between the presence of A. negundo and the total vegetation cover at various heights (undergrowth, shrubs trees) and a total number of undergrowth species, as a measure of biodiversity. Statically significant differences were at p < 0.05. The analyses were performed in Statistica 10 program (Tulsa, Oklahoma, USA, StatSoft Inc.).
3. Results
3.1. Invasion Intensity into Different Forest Types
The forest stands in the investigated area were to a various extent invaded by A. negundo, and the intensity of invasion significantly differed between the two investigated forest stand types. Apart from the obvious difference in the share of indigenous species, the forests differed in terms of both volume and number of individuals of this invasive maple (Table 1). The ash-leaved maple was significantly more abundant in the willow forest than in the poplar, both in terms of number of individuals and the tree volume. In the willow forests (more frequently subject to flooding than the poplar) maples constituted nearly half of individuals (47.8%) and over one third in the poplar forests (33.5%), the difference in tree trunk number was however variable and no statistical difference was found (p = 0.09). The differences between the two forests and the invasive maple occurrence were also significant in terms of the volume of the trees expressed in their basal area. While the average basal area of ash-leaved maples per patch was approximately twice as big in the willow forests (245.9 cm2/ha) compared to poplar forests (157.1 cm2/ha; p = 0.01), it no longer prevailed in any of the stands in terms of volume, as its share in the total tree basal area was minimal and only accounted for 4.3% and 2% in willow and poplar forests, respectively, the differences were not significant. (Table 1). Those tree stands in which poplar was the dominant species and which were to a lesser extent invaded, were characterized by higher NDVI values, associated with larger biomass and vegetation fitness. NDVI also increased with the forest age, but the increase was found to be significant only in the willow forest (Table 1).

Table 1.
Differences in tree canopy structure, tree density and NDVI values between the willow and poplar forest stand and between the forests stands of different age, differences between the groups using ANOVA and post-hoc Tukey test at p < 0.05. Significant values are shown with asterisk, different groups shown using letters a and b.
The structure of the forests stands strongly depends on their age. Young willow stands are more highly invaded (73.6%) than older stands (37.1%), the percentage of individuals being 14.5% and 3.1% in terms of volume, respectively. The youngest willow stands (<10 years) have significantly higher share of invasive maples when compared to older patches in terms both the number of trunks (p = 0.00) and their volume (p = 0.00). The native tree species showed both increased numbers and increased volume in older stands (Table 1). In both willow and poplar forest stands up to 20 years of age, the ash-leaved maple is always the dominating tree species, while the older stands from 20 to 40 years of age showed only a small fraction of this species in terms of the basal area of trunks, which is at least five times lower than of the native trees. In willow stands a significant increase in numbers of invasive maples is noted in older stands when compared to younger ones, while both poplar and willows increase in volume. In poplar forests, the increase in numbers is not significant, while the willows decrease in numbers, the increase in volume is not homogenous (Table 1).
3.2. The Effect of A. negundo on Biodiversity
We found that the presence of A. negundo strongly modifies the structure of the forest stand at many levels. A. negundo is a main component of the shrub layer (2–5 m height) along with Sambucus nigra, Populus nigra, and Salix alba seedlings, hence the high correlation obtained (r = 0.708; Table 2). The presence of A. negundo contributes to a lower density of the canopy (r = −0.338) and high density of A. negundo is associated with a decline of the plant density of the undergrowth (r = −0.495). The development of the species in the canopy is also followed by a continuous decline of total number of plant species in undergrowth (r = −0.439).

Table 2.
The relationship between the presence of A. negundo and the total vegetation cover at various heights and biodiversity levels, expressed as number of species. Spearman’s correlation coefficients. Significant values shown with asterisk at p < 0.05.
3.3. Effect of A. negundo on Recreation Ecosystem Services
By linking Recreational Ecosystem Services (RES) to indicators describing cumulated effects of visitor activity throughout in area off the main track (mainly soil compaction), we found that a relationship between the invasive maple density and off–trail activity can be observed. Throughout the whole area there are no restrictions for walking off the main track and free penetration of the forest is allowed, although the main gravel track is easily distinguished. Off-trail activity is not desired, but is not by any way penalized. Whilst strictly speaking visitors should stick to the prepared pathways, the informal tracks indicate that they clearly are not doing so. In the willow forest, the relationship between the ash-leaved maple coverage and visitor activity is statistically significant and invasive maple basal area explained the variation in soil compaction in 21.46% in the willow forest, and in the poplar forest in 19.37% (Table 3). The inverse relationships between soil compaction and density of A. negundo allows us to conclude that off-trail visitor activity is reduced in highly invaded patches. Other factors explaining the variation in the compaction of the trails include the distance to the main track, which explained 7.6% in the willow stand and 6.9% of variation in the poplar stand (Table 3). The factors used in GLM explain in total for forest willow forest 29.0%—r = 0.53; p = 0.00 and in poplar forest in 30.5%—r = 0.55; p = 0.00.

Table 3.
The results of the General Linear Models (GLM) to assess the effect of factors contributing to recreational use of the riparian forests, soil compaction of trails used as a response variable, fixed effects in the models were factors contributing to the forest structure and location which might contribute to the recreational use of informal trails—stand age, average basal area of A. negundo, distance to the main path, stand age, NDVI, distance to nearest entrance, distance to the river, number of residents in a 500 m buffer, basal area of all trees, significant values shown with asterisk at p < 0.05.
4. Discussion
This study revealed significant alterations of the structure and biodiversity of riparian forests in a large city, subject to high recreational pressure when invaded by A. negundo from the herbaceous layer up to the canopy. The ash-leaved maple in such conditions always prevailed as the dominating species in younger stands, both in terms of number of individuals and the volume in both willow and poplar tree stands, while in tree stands of 30–40 years, the indigenous tree species were present in higher densities. In the conditions of Warsaw, the presence of invasive maple was associated with a selective negative impact on the patterns of how people move across the area, revealed in informal track density and soil compaction. It requires further studies to examine whether these relationships can be also found in other river valleys in urban areas, but it might be expected that subject to recreational pressure, similar phenomena can be observed. We found the willow forests where the share of ash-leaved maple was higher to be less frequently visited, while in poplar forests this relationship was less visible. The study showed the major effect of the invasive maple on both the biodiversity and recreation. We indicate that management implications for this species to optimize nature conservation and maintain recreation in such valuable areas.
With an increased demand from city residents for direct contact with nature [62], natural ecosystems such as riparian forests will become more frequently visited and gain more importance as places for recreation. Here, we found that willow stands invaded by A. negundo were less visited by people, while a reduction in visitation was not evident for poplar tree stands with a high maple density. A. negundo is perceived no differently to other trees by the public and is treated as an accepted component of the green space, more attractive than the view of the built-up areas [63]. Yet we found evidence of reduced usage of willow stands with a high ash-leaved maple content. The effect was more visible in the willow forests located closer to the water where the density of the trails and their compaction were negatively correlated to both number and volume of invasive maple. The use of poplar stands was less affected, the track density was only found to be smaller in sites where the number of individual ash-leaved maples was high. In the willow stands with high A. negundo, the activity of visitors on informal tracks was reduced by over one third. These areas are of a high importance to the visitors, as the presence of water in the recreational area has a great influence on the aesthetical judgment [64]. For many visitors, the presence or absence of invasive species may have little impact on recreation. A previous study found that many users accept spontaneous vegetation on grasslands providing it remains green [26], and many citizens may not recognize a species as invasive. Our findings were based on measurements of off-trail compaction, which are “traces” left by the visitors and may be affected by the soil difference between the two habitats or by proximity to water and user preferences for areas close to water. Also, differences in the “visibility” and easiness of penetration and herbaceous vegetation density, which might have affected the distribution of the informal tracks over the area.
In this study, A. negundo proved to be a widespread component of the riparian forest flora of Warsaw. The riparian forests in cities are reported to have 10–40% invasive tree species [39,65], and over the last 100 years an increase ash-leaved maple and other IAS in urban floras has been noted [66]. The presence of invasive tree species alters the composition of forests, leading to replacement of the riparian native species [67]. In extreme cases, in the riparian zones the invasion can lead to the development of homogenous communities of novel ecosystems little resembling the former riparian forests [68]. A. negundo is a pioneer species and easily spreads onto riparian areas, which constitute dispersal corridors to light-seeded plants [39,69]. This invasive maple is characterized by high germination rate under the tree canopy, and the long germination period contributes to the formation of dense young stands [70]. Abandoned areas are particularly attractive sites for the spread of A. negundo [39]. The share of this species in cities, mainly as street trees, in public parks and private backyards, averages 37% of the total trees, but can reach up to 80% [70]. In riparian areas this share is lower and is estimated as ca. 20% [39], which results from high competition of the native tree species.
Our study showed widespread invasion of A. negundo in riparian forests, but particularly acute in the younger forests stands. We also found major differences between the willow forests situated close to the river course and the poplar forests located further from the river. The willow forest may be more susceptible to invasion by A. negundo, where the density of shoots of the native willow was larger than the poplars in the neighboring habitat (Table 2). The river catchments are in general at risk of being subject to biological invasions [71], but the sites which are being regularly flooded are also more frequently subject to establishment of the seedlings from the seeds carried by water [72], and the correlation between the risk of invasion with the distance to the river course is previously recognized [39]. The willow forests compared to poplar forests, despite higher invasion rate, were characterized by lower NDVI, indicating higher biomass values in contrast to a study from Bulgaria where it was the more invaded sites in the riparian zones which had the highest canopy cover [73].
None of the patches examined was bereft of A. negundo, and comparison of the oldest stands with the youngest did not reveal trends of this species disappearing. Young tree stands up to the age of 20 years were characterized by numerous ash-leaved maple shoots of small basal area. In the older forest patches of age 20–40 years, the share of invasive maple is lower while the indigenous trees prevail both in terms of number of shoots and their basal area (Table 2). This development stage of the regenerating riparian forest is for A. negundo a moment of entering the survival phase, as its shoots grow in volume and develop under the canopy of other trees, but do not increase in numbers. A. negundo encounters biotic resistance in an intermediate successional niche when the indigenous species grow more rapidly [74]. The ash-leaved maple in older stands indicate A. negundo does stabilize at a low level, showing little need to undertake removal actions for this species. Removal of trees results in creating gaps which would be quickly filled by the invasive maple as it reproduces effectively from both shoots and rhyzomes [75]. The seedlings of ash-leaved maple are characterized by high tolerance and quick growth rate in the gaps [76]. These factors combine to make limiting the spread of A. negundo a difficult management challenge.
The presence of A. negundo strongly affects the structure of the tree stand, and differences in the herbaceous vegetation were noted. Woodlands with A. negundo had less dense canopy and more developed shrub layer and floristically poor herb layer, which indicates a loss of ecological value due to the presence of A. negundo [40,77,78]. The sites examined in this study were partially artificially created by river regulation and further developed due to natural processes (accumulation, succession). Increased penetration intensity by invasive species and degradation of indigenous plant communities is expected to be intensified by increasing pressure from recreational activities [79], and the future may be bleak for the remnants of natural forest in urban environments.
High demand for green areas and outdoor recreation in cites results in riparian areas being incorporated into the city’s green system, both the ones of high ecological value and the degraded ones [80]. In Warsaw, similarly to other European cities, the residents seek direct contact with nature, especially to the most valuable components of nature [7,81]. Areas along rivers, overgrown with dense vegetation of riparian forests and plentiful shore vegetation, are highly attractive to visitors [82]. Such natural ecosystems are exposed to high numbers of visitors and serve as recreation areas, but they are also subject to increased trampling and consequent biodiversity loss [80,83,84,85]. The preservation of biodiversity may then contradict the ability of residents to freely and actively use the space. It is not known how to manage degraded areas still having traces of natural ecosystems but with a high share of invasive species. Should they be protected as remnants of natural vegetation or a rather more flexible approach should be used, not removing invasive species, despite them posing a threat to biodiversity and to make it available to the public because of their contribution to recreation. Invasive species impede biodiversity, but also offer a multitude of other services to the residents [29,86], therefore their removal in cities, where they were introduced in the first place as ornamental plants, requires understanding of both the ecological process and taking into account the preferences of the public.
This study indicates that recreation can be influenced by the presence of the invasive A. negundo. However, if removal actions were to be undertaken, it is more desirable in the areas which are more intensively visited, the willow forests close to the water. Removal of high numbers of the invasive maple could result in a rapid decline of aesthetics and social approval of the more valuable areas by the water, as the public perceives the maple as superior to areas bereft of vegetation [44]. Recreational activity in such areas should rather be supported by creating additional infrastructure, as in protected areas, which could limit the pressure and boost indigenous riparian vegetation regeneration. From the point of the biodiversity preservation the ash-leaved maples should be removed [83], but considering the costs and constantly occurring disturbances in the area, it is expected that the maple will become an inseparable component of the city’s green spaces. The maple can be treated more gently and not be strongly controlled, the risk of invasion expansion should be taken into account, but so should its role in contributing to recreation and ecosystem services provisioning [29]. If removal of A. negundo is necessary, it should be carefully considered and if occurring should be spread over a longer period (over 30 years), allowing the indigenous plant communities to recover and regenerate and eliminate the invasive maple through natural biotic pressure [76].
5. Conclusions
- A. negundo is a permanent and abundant component of the urban riparian forests in the Vistula river valley in Warsaw, and it was found to be more abundant in willow forests stands than in poplar forests.
- The abundance of A. negundo was found to be significantly higher in younger stands than in older ones, the differences were manifested in both number of stems and their volume per plot.
- Occurrence of A. negundo in riparian forests negatively affects biodiversity, shrub layer, and herbaceous vegetation, the more invaded stands were poorer in species diversity.
- An increased share of A. negundo was found to be related to decreased activity of visitors in the forest, but the effect was slightly stronger in the willow forests. Presence of ash-leaved maple plays an important role in providing recreation possibilities for the city dwellers.
- Factors explaining the recreational activity by users in the riparian forests, expressed in soil compaction of informal tracks, were the distance from the main track and the volume of A. negundo trunks.
Author Contributions
Conceptualization, D.S.; Formal analysis, J.C.; Investigation, D.S., P.S., P.A. and J.C.; Methodology, P.S.; Supervision, R.J.H.; Validation, J.C. and R.J.H.; Writing – original draft, D.S., P.S. and R.J.H.
Funding
This research was partially funded by the “National Science Centre, Poland” under the contract agreement UMO-2017/25/B/ST10/02967.
Acknowledgments
We would like to thank to Mateusz Rudowicz and Michał Szczucki for their help in field measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tzoulas, K.; Korpela, K.; Venn, S.; Yli-Pelkonen, V.; Kazmierczak, A.; Niemela, J.; James, P. Promoting ecosystem and human health in urban areas using green infrastructure: A literature review. Landsc. Urban Plan. 2007, 81, 167–178. [Google Scholar] [CrossRef]
- Breuste, J.; Niemela, J.; Snep, R.P.H. Applying landscape ecological principles in urban environments. Landsc. Ecol. 2008, 23, 1139–1142. [Google Scholar] [CrossRef]
- Vandermeulen, V.; Verspecht, A.; Vermeire, B.; van Huylenbroeck, G.; Gellynck, X. The use of economic valuation to create public support for green infrastructure investments in urban areas. Landsc. Urban Plan. 2011, 103, 198–206. [Google Scholar] [CrossRef]
- Gómez-Baggethun, E.; Barton, D.N. Classifying and valuing ecosystem services for urban planning. Ecol. Econ. 2013, 86, 235–245. [Google Scholar] [CrossRef]
- Schäffler, A.; Swilling, M. Valuing green infrastructure in an urban environment under pressure—The Johannesburg Case. Ecol. Econ. 2013, 86, 246–257. [Google Scholar] [CrossRef]
- Breuste, J.; Rahimi, A. Many public urban parks, but who profits from them? The example of Tabriz, Iran. Ecol. Process. 2015, 4, 6. [Google Scholar] [CrossRef]
- Richards, D.R.; Warren, P.H.; Moggridge, H.L.; Maltby, L. Spatial variation in the impact of dragonflies and debris on recreational ecosystem services in a floodplain wetland. Ecosyst. Serv. 2015, 15, 113–121. [Google Scholar] [CrossRef]
- Shanahan, D.F.; Lin, B.B.; Gaston, K.J.; Bush, R.; Fuller, R.A. What is the role of trees and remnant vegetation in attracting people to urban parks? Landsc. Ecol. 2015, 30, 153–165. [Google Scholar] [CrossRef]
- Liquete, C.; Kleeschulte, S.; Dige, G.; Maes, J.; Grizzetti, B.; Olah, B.; Zulian, G. Mapping green infrastructure based on ecosystem services and ecological networks: A Pan-European case study. Environ. Sci. Policy 2016, 54, 268–280. [Google Scholar] [CrossRef]
- Pennington, R.T.; Lavin, M.; Oliveira-Filho, A. Woody plant diversity, evolution, and ecology in the tropics: Perspectives from seasonally dry tropical forests. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 437–457. [Google Scholar] [CrossRef]
- Haase, D. Holocene floodplains and their distribution in urban areas—Functionality indicators for their retention potentials. Landsc. Urban Plan. 2003, 66, 5–18. [Google Scholar] [CrossRef]
- Juutinen, A.; Koseniusc, A.K.; Ovaskainen, V. Estimating the benefits of recreation-oriented management in state-owned commercial forests in Finland: A choice experiment. J. For. Econ. 2014, 20, 396–412. [Google Scholar] [CrossRef]
- Bayley, P.B. Understanding large river: Floodplain ecosystems. BioScience 1995, 45, 153–158. [Google Scholar] [CrossRef]
- Barbier, E.B.; Thompson, J.R. The value of water: Floodplain versus large-scale irrigation benefits in northern Nigeria. Ambio 1998, 27, 434–440. [Google Scholar]
- Tockner, K.; Stanford, J.A. Riverine flood plains: Present state and future trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef]
- Grygoruk, M.; Mirosław-Świątek, D.; Chrzanowska, W.; Ignar, S. How much for water? Economic assessment and mapping of floodplain water storage as a catchment-scale ecosystem service of wetlands. Water 2013, 5, 1760–1779. [Google Scholar] [CrossRef]
- van Looy, K.; Tormos, T.; Souchon, Y.; Gilvear, D. Analyzing riparian zone ecosystem services bundles to instruct river management. Ecosyst. People 2017, 13, 330–341. [Google Scholar]
- Fitter, A.; Elmqvist, T.; Haines-Young, R.; Potschin, M.; Rinaldo, A.; Setälaä, H.; Stoll-Kleemann, S.; Zobel, M.; Murlis, J. An assessment of ecosystem services and biodiversity in Europe. Environ. Sci. Technol. 2010, 30, 1–28. [Google Scholar]
- Suding, K.N. Toward and area of restoration in ecology: Successes, failures and opportunities ahead. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 465–487. [Google Scholar] [CrossRef]
- Lachowycz, K.; Jones, A.P. Towards a better understanding of the relationship between greenspace and health: Development of a theoretical framework. Landsc. Urban Plan. 2013, 118, 62–69. [Google Scholar] [CrossRef]
- White, M.P.; Alcock, I.; Wheeler, B.W.; Depledge, M.H. Would you be happier living in a greener urban area? A Fixed-effects Analysis of Panel Data. Psychol. Sci. 2013, 24, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Alcock, I.; White, M.P.; Wheeler, B.W.; Fleming, L.E.; Depledge, M.H. Longitudinal effects on mental health of moving to greener and less green urban areas. Environ. Sci. Technol. 2014, 48, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Gascon, M.; Triguero-Mas, M.; Martinez, D.; Dadvand, P.; Forns, J.; Plasencia, A. Mental health benefits of long-term exposure to residential green and blue spaces: A systematic review. Int. J. Environ. Res. Public Health 2015, 12, 4354–4379. [Google Scholar] [CrossRef] [PubMed]
- Gascon, M.; Triguero-Mas, M.; Martínez, D. Residential green spaces and mortality: A systematic review. Environ. Int. 2016, 86, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Rupprecht, C.D.D.; Furuya, K. Residents’ perception of informal green space—A case study of Ichikawa City, Japan. Land 2018, 7, 102. [Google Scholar] [CrossRef]
- Sikorski, P.; Wińska-Krysiak, M.; Chormański, J.; Sikorska, D. Low-maintenance green tram tracks as a socially acceptable solution to greening a city. Urban For. Urban Green. 2018, 35, 148–164. [Google Scholar] [CrossRef]
- Charkes, H.; Dukes, J.S. Impacts of invasive species on ecosystem services. In Biological Invasions; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 217–237. [Google Scholar]
- Vilà, M.; Hulme, P.E. Non-native species, ecosystem services, and human well-being. In Impact of Biological Invasions on Ecosystem Services; Vilà, M., Hulme, P.E., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–14. [Google Scholar]
- Sladonja, B.; Poljuha, D.; Uzelac, M. Non-native invasive species as ecosystem service providers. In Ecosystem Services and Global Ecology; Hufnagel, L., Ed.; IntechOpen: London, UK, 2018; pp. 39–59. [Google Scholar]
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef]
- Antia, R.; Regoes, R.R.; Koella, J.C.; Bergstrom, C.T. The role of evolution in the emergence of infectious diseases. Nature 2003, 426, 658–660. [Google Scholar] [CrossRef]
- Pysek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Nienhuis, C.; Stout, J. Effectiveness of native bumblebees as pollinators of the alien invasive plant Impatiens glandulifera (Balsaminaceae) in Ireland. J. Pollinat. Ecol. 2009, 1, 1–11. [Google Scholar] [CrossRef]
- Preston, I.R.; le Maitre, D.C.; Blignaut, J.N.; Louw, L.; Palmer, C.G. Impact of invasive alien plants on water provision in selected catchments. Water SA 2018, 44, 719–729. [Google Scholar] [CrossRef]
- Greenwood, P.; Baumann, P.; Pulley, S.; Kuhn, N.J. The invasive alien plant, Impatiens glandulifera (Himalayan Balsam), and increased soil erosion: Causation or association? Case studies from a river system in Switzerland and the UK. J. Soils Sediment 2018, 18, 3463–3477. [Google Scholar] [CrossRef]
- Kapitza, K.; Zimmermann, H.; Martín-López, B.; von Wehrden, H. Research on the social perception of invasive species: A systematic literature review. NeoBiota 2019, 43, 47–68. [Google Scholar] [CrossRef]
- Newig, J.; Fritsch, O. Environmental governance: Participatory, multi-level—And effective? Environ. Policy Gov. 2009, 19, 197–214. [Google Scholar] [CrossRef]
- Schnitzler, J.; Benzler, J.; Altmann, D.; Mucke, I.; Krause, G. Survey on the population’s needs and the public health response during floods in Germany 2002. J. Public Health Manag. Pract. 2007, 13, 461–464. [Google Scholar] [CrossRef]
- Höfle, R.; Dullinger, S.; Essl, F. Different factors affect the local distribution, persistence and spread of alien tree species in floodplain forests. Basic Appl. Ecol. 2014, 15, 426–434. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Gdula, A.K.; Jagodziński, A.M. “The rich get richer” concept in riparian woody species—A case study of the Warta River Valley (Poznan, Poland). Urban For. Urban Green. 2015, 14, 107–114. [Google Scholar] [CrossRef]
- Rieger, I.; Kowarik, I.; Cherubini, P.; Cierjacks, A. A novel dendrochronological approach reveals drivers of carbon sequestration in tree species of riparian forests across spatiotemporal scales. Sci. Total Environ. 2017, 574, 1261–1275. [Google Scholar] [CrossRef]
- Foster, J.; Sandberg, L. Friends or foe? Invasive species and public green space in Toronto. Geogr. Rev. 2004, 94, 178–198. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Richardson, D.M.; Shackleton, C.M.; Bennett, B.; Crowley, S.L.; Dehnen-Schmutz, K.; Estevez, R.A.; Fischer, A.; Kueffer, C.; Kull, C.A.; et al. Explaining people’s perceptions of invasive alien species: A conceptual framework. J. Environ. Manag. 2018, 229, 10–26. [Google Scholar] [CrossRef]
- Potgieter, L.J.; Gaertner, M.; O’Farrell, P.J.; Richardson, D.M. Perceptions of impact: Invasive alien plants in the urban environment. J. Environ. Manag. 2018, 229, 76–87. [Google Scholar] [CrossRef]
- Hoyle, H.; Hitchmough, J.; Jorgensen, A. Attractive, climate-adapted and sustainable? Public perception of non-native planting in the designed urban landscape. Landsc. Urban Plan. 2017, 164, 49–63. [Google Scholar] [CrossRef]
- Sikorska, D.; Sikorski, P.; Richard, H.J. High biodiversity of green infrastructure does not contribute to recreational ecosystem services. Sustainability 2017, 9, 334. [Google Scholar] [CrossRef]
- Szymanowski, T. Kiedy zostały wprowadzone obce gatunki drzew do uprawy w Polsce? Rocz. Sekc. Dendrol. PTB 1960, 14, 81–99. [Google Scholar]
- Kowarik, I. Einführung und Ausbreitug Nichteinheimischer Gehölzarten in Berlin und Brandenburg; Botanischer Verein von Berlin und Brandenburg e.V.: Berlin, Germany, 1992. [Google Scholar]
- Pyšek, P.; Chytrý, M.; Pergl, J.; Sádlo, J.; Wild, J. Plant invasions in the Czech Republic: Current state, introduction dynamics, invasive species and invaded habitats. Preslia 2012, 84, 575–629. [Google Scholar]
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Plants of Foreign Origin in Poland with Particular Emphasis on Invasive Species; GDOŚ: Warszawa, Poland, 2012; pp. 1–197. (In Polish) [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 2010; Volume 2, pp. 1–500. [Google Scholar]
- Gudžinskas, Z. Conspectus of alien plant species of Lithuania. 8. Aceraceae, Balsaminaceae, Elaeagnaceae, Geraniaceae, Hippocastanaceae, Linaceae, Lythraceae, Onagraceae, Oxalidaceae, Rutaceae, and Vitaceae. Bot. Lith. 1998, 4, 363–377. [Google Scholar]
- Mędrzycki, P. NOBANIS—Invasive Alien Species Fact Sheet—Acer negundo. 2011. Available online: https://www.nobanis.org/globalassets/speciesinfo/a/acer-negundo/acer_negundo.pdf (accessed on 10 October 2019).
- Zisenis, M. Alien plant species: A real fear for urban ecosystems in Europe? Urban Ecosyst. 2015, 18, 355–370. [Google Scholar] [CrossRef]
- Degórska, B.; Degórski, M. Green infrastructure as a very important quality factor in urban areas—Warsaw case study. Europa XXI 2017, 32, 51–70. [Google Scholar] [CrossRef]
- Sikorski, P.; Parafjańczuk, S.; Wierzba, M.; Sikorska, D.; Borowski, J.; Kosić, I.V. The phenomenon of illegal dispersion in riparian forests under high tourist pressure. In Problems of Water Management in Forest, Urban and Non-Urbanized Areas; Kałuża, T., Strzeliński, P., Eds.; Wydawnictwo Naukowe Bogucki: Poznań, Poland, 2014; pp. 131–144. (In Polish) [Google Scholar]
- Matuszkiewicz, W.; Sikorski, P.; Szwed, W.; Wierzba, M.; Danielewicz, W.; Wysocki, C.; Kiciński, P. Vegetation of Poland. Illustrated Guide: Forests and Shrubs; PWN: Warszawa, Poland, 2012; pp. 189–207. (In Polish) [Google Scholar]
- Leuschner, C.; Ellenberg, H. Vegetation Ecology of Central Europe, 6th ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 652–688. [Google Scholar]
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017, 2017, 13536911. [Google Scholar] [CrossRef]
- Carlson, T.; Ripley, D. On the relationship between NDVI, fractional vegetation cover, and leaf area index. Remote Sens. Environ. 1997, 62, 241–252. [Google Scholar] [CrossRef]
- Coppes, J.; Braunisch, V. Managing visitors in nature areas: Where do they leave the trails? A spatial model. Wildl. Biol. 2013, 19, 1–12. [Google Scholar] [CrossRef]
- Abbott, A. City living marks the brain. Nature 2011, 474, 429. [Google Scholar] [CrossRef] [PubMed]
- Arnberger, A.; Schneider, I.E.; Ebenberger, M.; Eder, R.; Venette, R.C.; Snyder, S.A.; Gobster, P.H.; Choi, A.; Cottrell, S. Emerald ash borer impacts on visual preferences for urban forest recreation settings. Urban For. Urban Green. 2017, 27, 235–245. [Google Scholar] [CrossRef]
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; Cambridge University Press: New York, NY, USA, 1989; pp. 1–368. [Google Scholar]
- Schnitzler, A.; Hale, B.W.; Alsum, E.M. Examining native and exotic species diversity in European riparian forests. Biol. Conserv. 2007, 138, 146–156. [Google Scholar] [CrossRef]
- Kowarik, I. Novel urban ecosystems, biodiversity and conservation. Environ. Pollut. 2011, 159, 1974–1983. [Google Scholar] [CrossRef]
- Krevš, A.; Kučinskiene, A. Influence of invasive Acer negundo leaf litter on benthic microbial abundance and activity in the littoral zone of a temperate river in Lithuania. Knowl. Manag. Aquat. Ecosyst. 2017, 418, 26. [Google Scholar] [CrossRef][Green Version]
- Marozas, V.; Cekstere, G.; Laivins, M.; Straigyte, L. Comparison of neophyte communities of Robinia pseudoacacia L. and Acer negundo L. in the eastern Baltic Sea region cities of Riga and Kaunas. Urban For. Urban Green. 2015, 14, 826–834. [Google Scholar] [CrossRef]
- Säumel, I.; Kowarik, I. Urban rivers as dispersal corridors for primarily wind-dispersed invasive tree species. Landsc. Urban Plan. 2010, 94, 244–249. [Google Scholar] [CrossRef]
- Kostina, M.V.; Minkova, N.O.; Yasinskaya, O.I. Some biological features of Acer negundo L. in green plantations of Moscow. Russ. J. Biol. Invasions 2014, 5, 21–28. [Google Scholar] [CrossRef]
- Sunga, C.Y.; Li, M.-H.; Rogers, G.O.; Volder, A.; Wang, Z. Investigating alien plant invasion in urban riparian forests in a hot and semi-arid region. Landsc. Urban Plan. 2011, 100, 278–286. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar]
- Dyakov, N.; Zhelev, P. Alien species invasion and diversity of riparian forest according to environmental gradients and disturbance regime. Appl. Ecol. Environ. Res. 2013, 11, 249–272. [Google Scholar] [CrossRef]
- Saccone, P.; Girel, J.; Pages, J.P.; Brun, J.J.; Michalet, R. Ecological resistance to Acer negundo invasion in a European riparian forest: Relative importance of environmental and biotic drivers. Appl. Veg. Sci. 2013, 16, 184–192. [Google Scholar] [CrossRef]
- Merceron, N.R.; Lamarque, L.J.; Delzon, S.; Porté, A.J. Killing it softly: Girdling as an efficient eco-friendly method to locally remove invasive Acer negundo. Ecol. Rest. 2016, 34, 297–305. [Google Scholar] [CrossRef]
- Saccone, P.; Pagès, J.P.; Girel, J.; Brun, J.J.; Michalet, R. Acer negundo invasion along a successional gradient: Early direct facilitation by native pioneers and late indirect facilitation by conspecifics. New Phytol. 2010, 187, 831–842. [Google Scholar] [CrossRef]
- Pennington, D.N.; Hansel, J.R.; Gorchov, D.L. Urbanization and riparian forest woody communities: Diversity, composition, and structure within a metropolitan landscape. Biol. Conserv. 2010, 143, 182–194. [Google Scholar] [CrossRef]
- Straigytė, L.; Cekstere, G.; Laivins, M.; Marozas, V. The spread, intensity and invasiveness of the Acer negundo in Riga and Kaunas. Dendrobiology 2015, 74, 157–168. [Google Scholar] [CrossRef]
- Vakhlamova, T.; Rusterholz, H.P.; Kamkin, V.; Baur, B. Recreational use of urban and suburban forests affects plant diversity in a Western Siberian city. Urban For. Urban Green. 2016, 17, 92–103. [Google Scholar] [CrossRef]
- Sikorski, P. Influence of the Urban Park Nature on the Floristic Diversity of Undergrowth and Park Lawns; Wyd. Wieś Jutra: Warsaw, Poland, 2013; pp. 1–108. (In Polish) [Google Scholar]
- Mihalič, T. Performance of environmental resources of a tourist destination: Concept and application. J. Travel Res. 2013, 52, 614–630. [Google Scholar] [CrossRef]
- Kenwick, R.A.; Shammin, M.R.; Sullivanc, W.C. Preferences for riparian buffers. Landsc. Urban Plan. 2009, 91, 88–96. [Google Scholar] [CrossRef]
- Gonzalez, E.; Martínez-Fernandez, V.; Shafroth, P.B.; Sher, A.A.; Henry, A.L.; Garofano-Gomez, V.; Corenblit, D. Regeneration of Salicaceae riparian forests in the Northern Hemisphere: A new framework and management tool. J. Environ. Manag. 2018, 218, 374–387. [Google Scholar] [CrossRef]
- Cole, D.N.; Marion, J.L. Recreation impacts in some riparian forests of the Eastern United States. Environ. Manag. 1988, 12, 99–107. [Google Scholar] [CrossRef]
- Bötsch, Y.; Tablado, Z.; Scherl, D.; Kéry, M.; Graf, R.F.; Jenni, L. Effect of recreational trails on forest birds: Human presence matters. Front. Ecol. Evol. 2018, 6, 175. [Google Scholar] [CrossRef]
- Sharp, R.L.; Larson, L.R.; Green, G.T. Factors influencing public preferences for invasive alien species management. Biol. Conserv. 2011, 144, 2097–2104. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).