A Retrospective Comparison of Water Quality Treatment in a Bioretention Cell 16 Years Following Initial Analysis
Abstract
:1. Introduction
2. Methodology
2.1. Site Description
2.2. Monitoring
2.3. Data Analysis
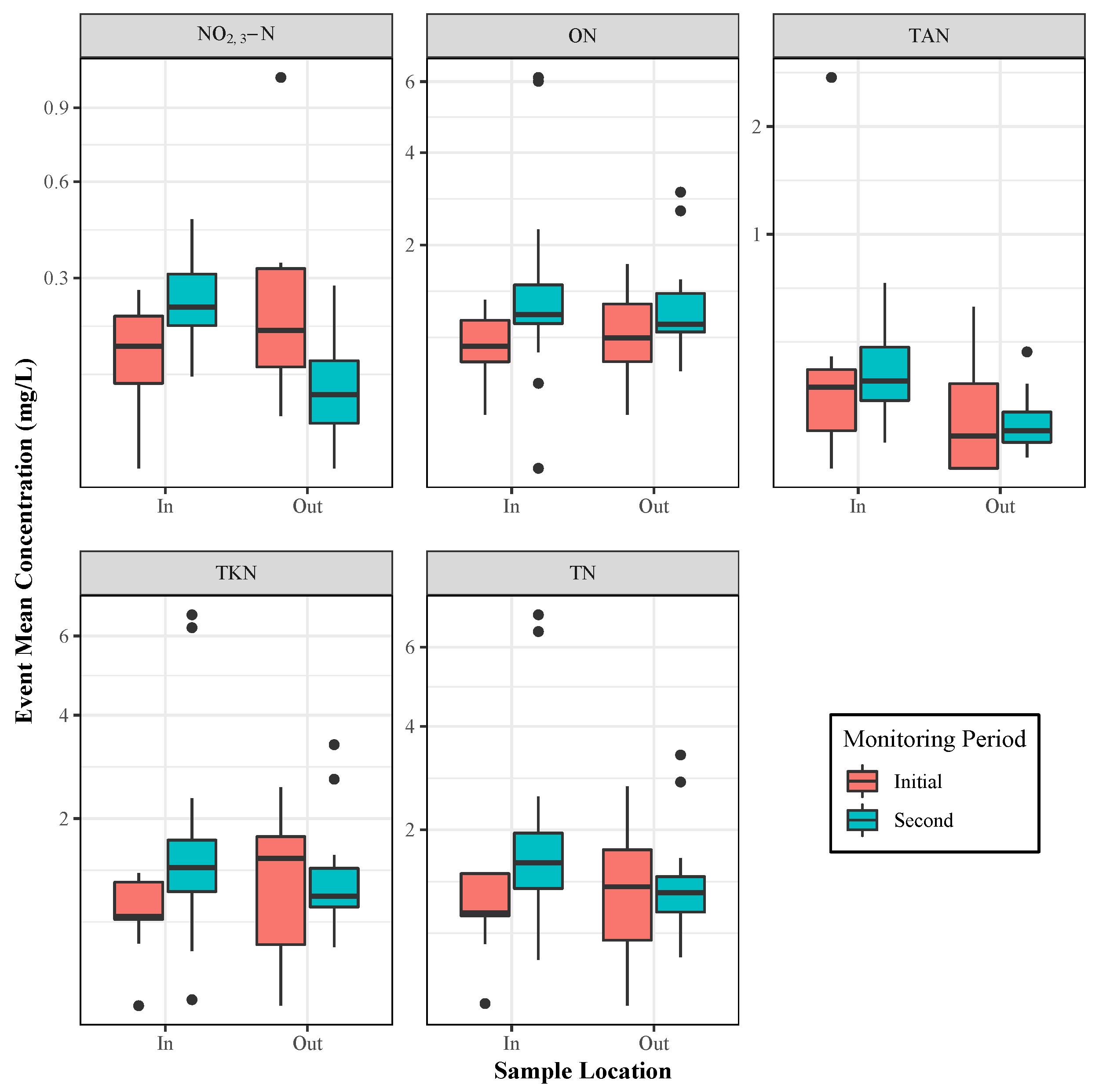
3. Results and Discussion
3.1. Nitrate
3.2. Phosphorus
3.3. Regulatory Implications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, A.P. Green Engineering Principles Promote Low-impact Development. Environ. Sci. Technol. 2005, 39, 338A–344A. [Google Scholar] [CrossRef] [Green Version]
- Dietz, M.E. Low Impact Development Practices: A Review of Current Research and Recommendations for Future Directions. Water Air Soil Pollut. 2007, 186, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Line, D.E.; Brown, R.A.; Hunt, W.F.; Lord, W.G. Effectiveness of LID for Commercial Development in North Carolina. J. Environ. Eng. 2012, 138, 680–688. [Google Scholar] [CrossRef]
- Wilson, C.E.; Hunt, W.F.; Winston, R.J.; Smith, P. Comparison of Runoff Quality and Quantity from a Commercial Low-Impact and Conventional Development in Raleigh, North Carolina. J. Environ. Eng. 2015, 141, 05014005. [Google Scholar] [CrossRef]
- Bratieres, K.; Fletcher, T.; Deletic, A.; Zinger, Y. Nutrient and sediment removal by stormwater biofilters: A large-scale design optimisation study. Water Res. 2008, 42, 3930–3940. [Google Scholar] [CrossRef]
- Coffman, L.S.; Green, R.; Clar, M.; Bitter, S. Development of Bioretention Practices for Stormwater Management. J. Water Manag. Model. 1994, 6062, 23–42. [Google Scholar] [CrossRef]
- Davis, A.P.; Hunt, W.F.; Traver, R.G.; Clar, M. Bioretention Technology: Overview of Current Practice and Future Needs. J. Environ. Eng. 2009, 135, 109–117. [Google Scholar] [CrossRef]
- Davis, A.P.; Traver, R.G.; Hunt, W.F.; Lee, R.; Brown, R.A.; Olszewski, J.M. Hydrologic Performance of Bioretention Storm-Water Control Measures. J. Hydrol. Eng. 2012, 17, 604–614. [Google Scholar] [CrossRef]
- Hunt, W.F.; Davis, A.P.; Traver, R.G. Meeting Hydrologic and Water Quality Goals through Targeted Bioretention Design. J. Environ. Eng. 2012, 138, 698–707. [Google Scholar] [CrossRef]
- Lucas, W.C.; Greenway, M. Nutrient Retention in Vegetated and Nonvegetated Bioretention Mesocosms. J. Irrig. Drain. Eng. 2008, 134, 613–623. [Google Scholar] [CrossRef]
- Komlos, J.; Traver, R.G. Long-Term Orthophosphate Removal in a Field-Scale Storm-Water Bioinfiltration Rain Garden. J. Environ. Eng. 2012, 138, 991–998. [Google Scholar] [CrossRef]
- Johnson, J.P.; Hunt, W.F. Evaluating the spatial distribution of pollutants and associated maintenance requirements in an 11 year-old bioretention cell in urban Charlotte, NC. J. Environ. Manag. 2016, 184, 363–370. [Google Scholar] [CrossRef]
- Willard, L.L.; Wynn-Thompson, T.; Krometis, L.H.; Neher, T.P.; Badgley, B.D. Does It Pay to be Mature? Evaluation of Bioretention Cell Performance Seven Years Postconstruction. J. Environ. Eng. 2017, 143, 04017041. [Google Scholar] [CrossRef]
- Hunt, W.F.; Jarrett, A.R.; Smith, J.T.; Sharkey, L.J. Evaluating Bioretention Hydrology and Nutrient Removal at Three Field Sites in North Carolina. J. Irrig. Drain. Eng. 2006, 132, 600–608. [Google Scholar] [CrossRef] [Green Version]
- Soil Survey Staff. Web Soil Survey. 2018. Available online: https://websoilsurvey.sc.egov.usda.gov/ (accessed on 18 April 2018).
- Malcom, H.R. Elements of Urban Stormwater Design; North Carolina State University: Raleigh, NC, USA, 1989. [Google Scholar]
- Standard Practice for Sampling with a Scoop; ASTM Standard D5633; Technical Report; ASTM: West Conshohocken, PA, USA, 2012. [CrossRef]
- Li, H.; Davis, A.P. Urban Particle Capture in Bioretention Media. I: Laboratory and Field Studies. J. Environ. Eng. 2008, 134, 409–418. [Google Scholar] [CrossRef]
- NOAA NCEI. NCDC Climate Normals; NOAA NCEI: Washington, DC, USA, 2018.
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Davis, A.P. Field Performance of Bioretention: Water Quality. Environ. Eng. Sci. 2007, 24, 1048–1064. [Google Scholar] [CrossRef]
- Davis, A.P.; Shokouhian, M.; Sharma, H.; Minami, C. Water Quality Improvement through Bioretention Media: Nitrogen and Phosphorus Removal. Water Environ. Res. 2006, 78, 284–293. [Google Scholar] [CrossRef]
- Dietz, M.E.; Clausen, J.C. A Field Evaluation of Rain Garden Flow and Pollutant Treatment. Water Air Soil Pollut. 2005, 167, 123–138. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Davis, A.P. Water Quality Improvement through Reductions of Pollutant Loads Using Bioretention. J. Environ. Eng. 2009, 135, 567–576. [Google Scholar] [CrossRef]
- Brown, R.A.; Hunt, W.F. Improving bioretention/biofiltration performance with restorative maintenance. Water Sci. Technol. 2012, 65, 361. [Google Scholar] [CrossRef]
- McNett, J.K.; Hunt, W.F.; Osborne, J.A. Establishing Storm-Water BMP Evaluation Metrics Based upon Ambient Water Quality Associated with Benthic Macroinvertebrate Populations. J. Environ. Eng. 2010, 136, 535–541. [Google Scholar] [CrossRef]
- Hunt, W.F.; Smith, J.T.; Jadlocki, S.J.; Hathaway, J.M.; Eubanks, P.R. Pollutant Removal and Peak Flow Mitigation by a Bioretention Cell in Urban Charlotte, N.C. J. Environ. Eng. 2008, 134, 403–408. [Google Scholar] [CrossRef]
- Passeport, E.; Hunt, W.F.; Line, D.E.; Smith, R.A.; Brown, R.A. Field Study of the Ability of Two Grassed Bioretention Cells to Reduce Storm-Water Runoff Pollution. J. Irrig. Drain. Eng. 2009, 135, 505–510. [Google Scholar] [CrossRef]
- Merriman, L.S.; Hunt, W.F. Maintenance versus Maturation: Constructed Storm-Water Wetland’s Fifth-Year Water Quality and Hydrologic Assessment. J. Environ. Eng. 2014, 140, 05014003. [Google Scholar] [CrossRef]
- Payne, E.G.I.; Fletcher, T.D.; Russell, D.G.; Grace, M.R.; Cavagnaro, T.R.; Evrard, V.; Deletic, A.; Hatt, B.E.; Cook, P.L.M. Temporary Storage or Permanent Removal? The Division of Nitrogen between Biotic Assimilation and Denitrification in Stormwater Biofiltration Systems. PLoS ONE 2014, 9, e90890. [Google Scholar] [CrossRef] [Green Version]
- Read, J.; Wevill, T.; Fletcher, T.; Deletic, A. Variation among plant species in pollutant removal from stormwater in biofiltration systems. Water Res. 2008, 42, 893–902. [Google Scholar] [CrossRef]
- Turk, R.P.; Kraus, H.T.; Hunt, W.F.; Carmen, N.B.; Bilderback, T.E. Nutrient Sequestration by Vegetation in Bioretention Cells Receiving High Nutrient Loads. J. Environ. Eng. 2017, 143, 06016009. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, H.; Zhang, Y. Soil microbial activities and carbon and nitrogen fixation. Res. Microbiol. 2003, 154, 393–398. [Google Scholar] [CrossRef]
- Dunn, R.M.; Mikola, J.; Bol, R.; Bardgett, R.D. Influence of microbial activity on plant–microbial competition for organic and inorganic nitrogen. Plant Soil 2006, 289, 321–334. [Google Scholar] [CrossRef]
- Birgand, F.; Skaggs, R.W.; Chescheir, G.M.; Gilliam, J.W. Nitrogen Removal in Streams of Agricultural Catchments—A Literature Review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 381–487. [Google Scholar] [CrossRef]
- Kim, H.; Seagren, E.A.; Davis, A.P. Engineered bioretention for removal of nitrate from stormwater runoff. Water Environ. Res. 2003, 75, 355–367. [Google Scholar] [CrossRef]
- Seitzinger, S.; Harrison, J.A.; Böhlke, J.K.; Bouwman, A.F.; Lowrance, R.; Peterson, B.; Tobias, C.; Drecht, G.V. Denitrification Across Landscapes and Waterscapes: A Synthesis. Ecol. Appl. 2006, 16, 2064–2090. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils; Pearson Prentice Hall: Columbus, OH, USA, 2008. [Google Scholar]
- Peterson, I.J.; Igielski, S.; Davis, A.P. Enhanced Denitrification in Bioretention Using Woodchips as an Organic Carbon Source. J. Sustain. Water Built Environ. 2015, 1, 04015004. [Google Scholar] [CrossRef]
- Hsieh, C.h.; Davis, A.P.; Needelman, B.A. Bioretention Column Studies of Phosphorus Removal from Urban Stormwater Runoff. Water Environ. Res. 2007, 79, 177–184. [Google Scholar] [CrossRef]
- Lammers, R.W.; Bledsoe, B.P. What role does stream restoration play in nutrient management? Crit. Rev. Environ. Sci. Technol. 2017, 47, 335–371. [Google Scholar] [CrossRef]
- Hatt, B.E.; Fletcher, T.D.; Deletic, A. Hydraulic and Pollutant Removal Performance of Fine Media Stormwater Filtration Systems. Environ. Sci. Technol. 2008, 42, 2535–2541. [Google Scholar] [CrossRef]
- N.C. DEQ. North Carolina Stormwater Control Measure Credit Document; North Carolina Department of Environmental Quality: Raleigh, NC, USA, 2017.
- Davis, A.P.; Traver, R.G.; Hunt, W.F. Improving Urban Stormwater Quality: Applying Fundamental Principles. J. Contemp. Water Res. Educ. 2010, 146, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Blecken, G.T.; Hunt, W.F.; Al-Rubaei, A.M.; Viklander, M.; Lord, W.G. Stormwater control measure (SCM) maintenance considerations to ensure designed functionality. Urban Water J. 2017, 14, 278–290. [Google Scholar] [CrossRef]





| Characteristic | Chapel Hill BRC |
|---|---|
| Year constructed | 2001 |
| Underlying soil | Clay, clay loam, and silty clay |
| 2002–2003 drainage area (m) | 607 |
| 2017–2018 drainage area (m) | 1133 |
| Imperviousness | 100% |
| BRC surface area (m) | 90 |
| Bowl storage depth (cm) | 10 |
| Media depth (m) | 1.2 |
| Median infiltration rate (cm/h) | 3.3–7.6 |
| Original media P-index | 4–12 (3.7–11.1 mg Mehlich-3 P/kg) |
| Underdrain type | Conventional (no IWS) |
| Vegetative cover | Perennial grasses, trees, shrubs |
| Analyte | Method |
|---|---|
| NO3-N | EPA Method 353.2 |
| TAN | Standard Method 4500-NH3 G |
| TKN | EPA Method 351.2 |
| ON | TKN–TAN |
| TN | NO3-N + TKN |
| TP | Standard Method 4500-P F |
| OP | EPA Method 365.1 |
| PBP | TP–OP |
| Pollutant | Initial Monitoring Period | Second Monitoring Period | ||||
|---|---|---|---|---|---|---|
| EMC In | EMC Out | Change | EMC In | EMC Out | Change | |
| (mg/L) | (%) | (mg/L) | (%) | |||
| TN | 0.89 | 1.23 | +37.6 * | 1.51 | 1.12 | −25.8 * |
| TKN | 0.74 | 1.41 | +90.5 * | 1.29 | 0.95 | −26.4 |
| TAN | 0.17 | 0.05 | −70.6 | 0.19 | 0.06 | −68.4 * |
| NO3-N | 0.15 | 0.18 | +20.0 * | 0.23 | 0.08 | −67.4 * |
| ON | 0.56 | 0.70 | +25.0 * | 0.95 | 0.84 | −12.1 |
| TP | 0.14 | 0.17 | +21.4 | 0.14 | 0.09 | −39.3 * |
| OP | 0.07 | 0.05 | −28.6 | 0.02 | 0.03 | +50.0 |
| PBP | 0.04 | 0.04 | 0.0 | 0.11 | 0.04 | −63.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, J.P.; Hunt, W.F. A Retrospective Comparison of Water Quality Treatment in a Bioretention Cell 16 Years Following Initial Analysis. Sustainability 2019, 11, 1945. https://doi.org/10.3390/su11071945
Johnson JP, Hunt WF. A Retrospective Comparison of Water Quality Treatment in a Bioretention Cell 16 Years Following Initial Analysis. Sustainability. 2019; 11(7):1945. https://doi.org/10.3390/su11071945
Chicago/Turabian StyleJohnson, Jeffrey P., and William F. Hunt. 2019. "A Retrospective Comparison of Water Quality Treatment in a Bioretention Cell 16 Years Following Initial Analysis" Sustainability 11, no. 7: 1945. https://doi.org/10.3390/su11071945





