Spatial Analysis as a Tool for Plant Population Conservation: A Case Study of Tamarix chinensis in the Yellow River Delta, China
Abstract
:1. Introduction
2. Methodology
2.1. Study Area Description
2.2. Photogrammetry Workflow
2.3. Spatial Data Analysis
2.3.1. Analysis of Spatial Distribution Patterns
2.3.2. Spatial Autocorrelation Analysis
2.4. Spatial Regression Analysis
3. Results
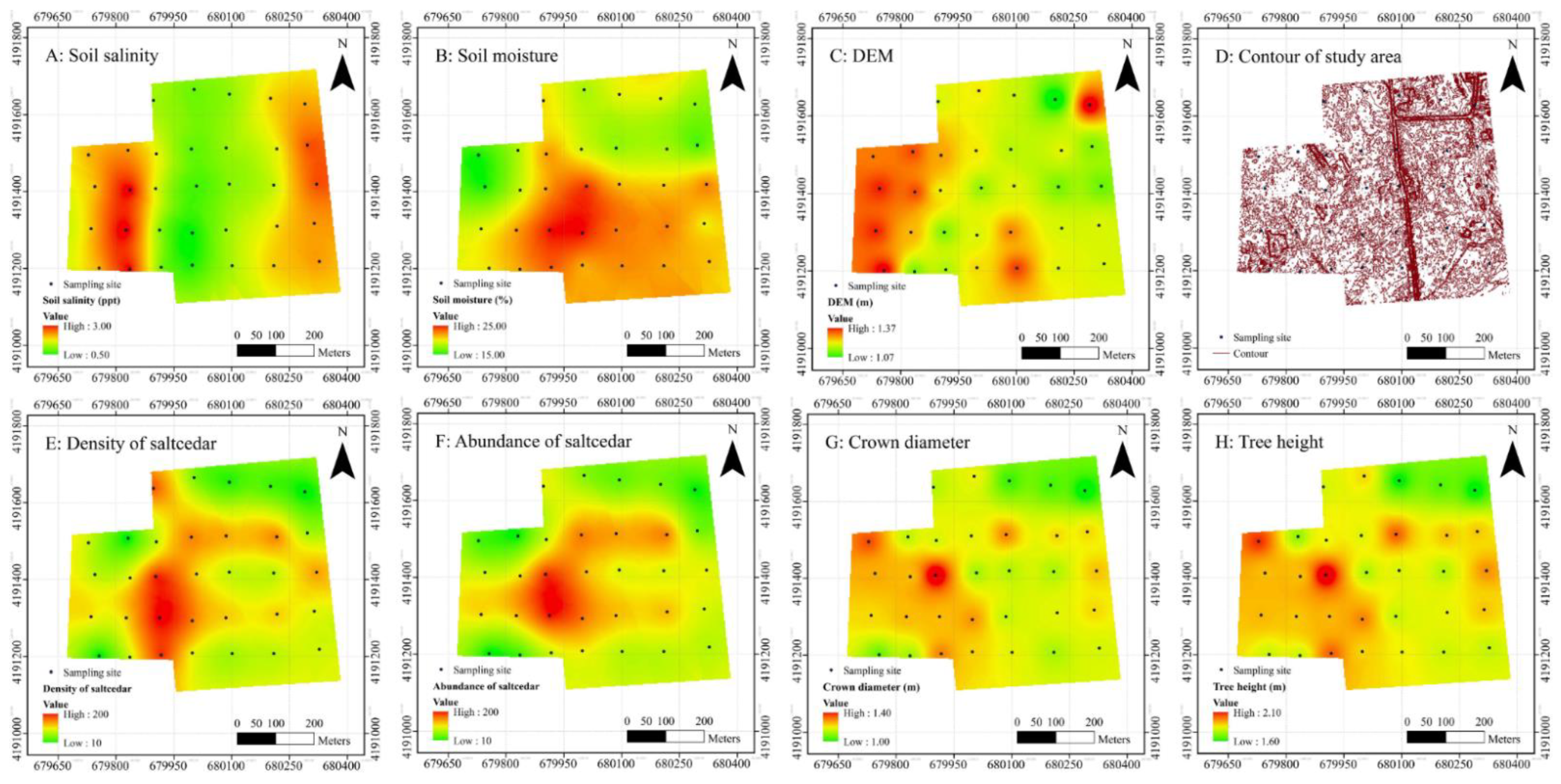
3.1. Descriptive Statistics and Spatial Distribution of Soil Properties and Saltcedar Features
3.1.1. Descriptive Statistics
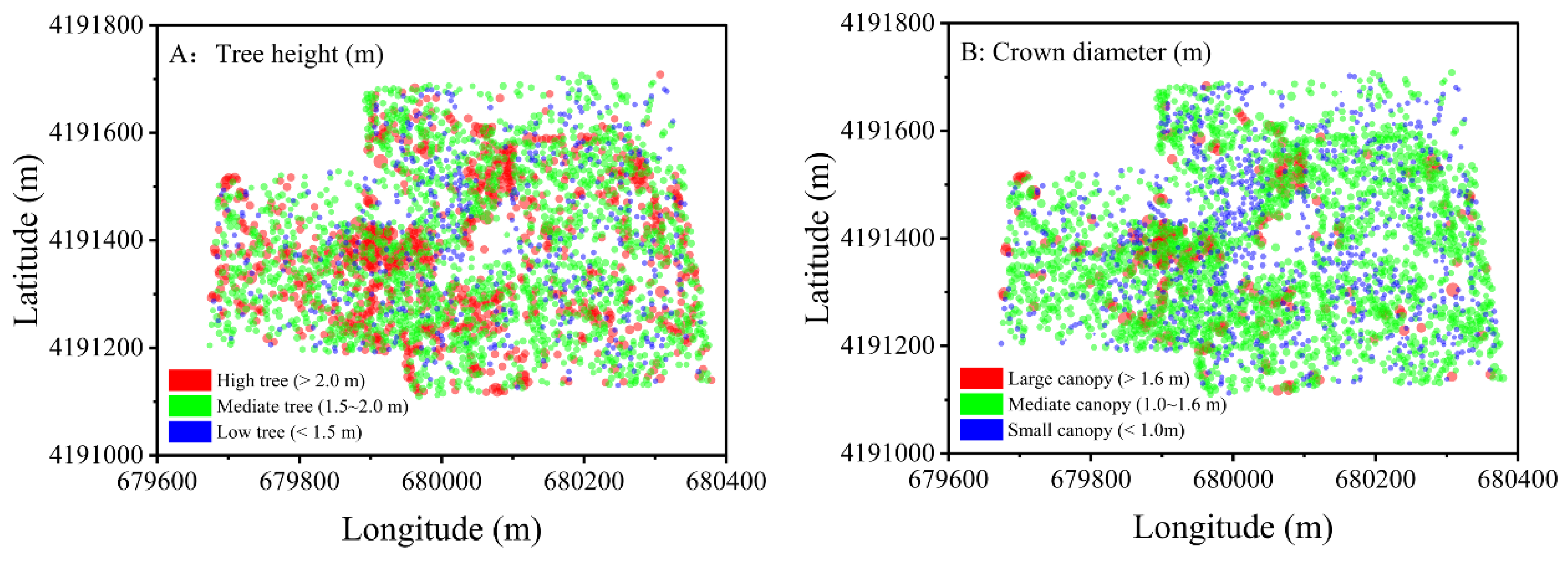
3.1.2. Spatial Distribution Patterns of Saltcedars
3.2. Spatial Autocorrelation Analysis of Soil and Saltcedar Variables
3.3. Quantification of Factors Influencing the Distribution of Saltcedars
4. Discussion
4.1. Spatial Pattern of Saltcedar Population and Mechanisms Driving Its Formation
4.2. Origin of Spatial Autocorrelation in Saltcedar Population
4.3. Implications for Ecological Management and Restoration of Saltcedar Population
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, M.; Wang, X.; Hui, C.; Yi, H.; Zhang, C.; Wu, X.; Bi, X.; Wang, Y.; Xiao, L.; Wang, D. Assembly of plant communities in coastal wetlands-the role of saltcedar Tamarix chinensis during early succession. J. Plant Ecol. 2015, 8, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Qi, M.; Sun, T.; Zhan, M.; Xue, S. Simulating dynamic vegetation changes in a tidal restriction area with relative stress tolerance curves. Wetlands 2015, 36, 31–43. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, T.; Zhu, M.; Qi, M.; Yang, W.; Shao, D. Salt marsh vegetation distribution patterns along groundwater table and salinity gradients in yellow river estuary under the influence of land reclamation. Ecol. Indic. 2018, 92, 82–90. [Google Scholar] [CrossRef]
- Cui, B.; Yang, Q.; Zhang, K.; Zhao, X.; You, Z. Responses of saltcedar (Tamarix chinensis) to water table depth and soil salinity in the Yellow River Delta, China. Plant Ecol. 2010, 209, 279–290. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.; Zhang, Q.; Li, J.; Zhang, Y.; Tu, C.; Ouyang, Z. Impact of water diversion on the hydrogeochemical characterization of surface water and groundwater in the Yellow River Delta. Appl. Geochem. 2014, 48, 83–92. [Google Scholar] [CrossRef]
- Sun, Q.; Lin, H.; Zhang, M.; Jiao, L.; Zhang, Y.; Yang, W.; Sun, T. Research progress on ecological restoration of coastal salt marsh. J. Beijing Norm. Univ. 2021, 57, 151–158. [Google Scholar]
- Jiao, L.; Li, F.; Liu, X.; Wang, S.; Zhou, Y. Fine-scale distribution patterns of Phragmites australis populations across an environmental gradient in the salt marsh wetland of Dunhuang, China. Sustainability 2020, 12, 1671. [Google Scholar] [CrossRef] [Green Version]
- Fullerton, A.H.; Steel, E.A.; Lange, I.; Caras, Y. Effects of spatial pattern and economic uncertainties on freshwater habitat restoration planning: A simulation exercise. Restor. Ecol. 2010, 18, 354–369. [Google Scholar] [CrossRef] [Green Version]
- Larson, A.J.; Churchill, D. Tree spatial patterns in fire-frequent forests of western North America, including mechanisms of pattern formation and implications for designing fuel reduction and restoration treatments. For. Ecol. Manag. 2012, 267, 74–92. [Google Scholar] [CrossRef]
- Viers, J.H.; Fremier, A.K.; Hutchinson, R.A.; Quinn, J.F.; Thorne, J.H.; Vaghti, M.G. Multiscale patterns of riparian plant diversity and implications for restoration. Restor. Ecol. 2012, 20, 160–169. [Google Scholar] [CrossRef]
- Wu, P.; Peng, X.; Yang, S.; Gao, Y.; Bai, F.; Yi, S.; Du, N.; Guo, W. Spatial distribution patterns and correlation of Tamarix chinensis population in coastal wetlands of Shandong, China. Chin. J. Plant Ecol. 2019, 43, 817–824. [Google Scholar]
- Liu, Y.; Liu, J.; Chen, Y.; Jing, S.; Feng, R.; Mao, G. Research on distribution patterns and population structure of Tamarix chinensis in the intertidal zone of coastal wetlands in Yellow River Delta. Ecol. Sci. 2017, 36, 153–158. [Google Scholar]
- Zhao, X.; Cui, B.; Sun, T.; Lv, J.; Lu, F. Analysis of spatial point pattern of Tamarix chinensis in different habitats. Ecol. Sci. 2011, 30, 142–148. [Google Scholar]
- He, Q.; Cui, B.; Hu, Q.; Yang, S.; Zhao, X. Fractal analysis on the distribution patterns of Tamarix chinensis under environmental gradients of different water table depth. Bull. Soil Water Conser. 2008, 28, 70–73. [Google Scholar]
- Chambers, L.G.; Osborne, T.Z.; Reddy, K.R. Effect of salinity-altering pulsing events on soil organic carbon loss along an intertidal wetland gradient: A laboratory experiment. Biogeochemistry 2013, 115, 363–383. [Google Scholar] [CrossRef]
- Bertness, M.D.; Callaway, R. Positive interactions in communities. Trends Ecol. Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef]
- Bertness, M.D.; Hacker, S.D. Physical stress and positive associations among marsh plants. Am. Nat. 1994, 144, 363–372. [Google Scholar] [CrossRef]
- Legendre, P.; Dale, M.R.T.; Fortin, M.J.; Gurevitch, J.; Hohn, M.; Myers, D. The consequences of spatial structure for the design and analysis of ecological field surveys. Ecography 2002, 25, 601–615. [Google Scholar] [CrossRef] [Green Version]
- Valcu, M.; Kempenaers, B. Spatial autocorrelation: An overlooked concept in behavioral ecology. Behav. Ecol. 2010, 21, 902–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kissling, W.D.; Carl, G. Spatial autocorrelation and the selection of simultaneous autoregressive models. Glob. Ecol. Biogeogr. 2008, 17, 59–71. [Google Scholar] [CrossRef]
- Kim, D. Modeling spatial and temporal dynamics of plant species richness across tidal creeks in a temperate salt marsh. Ecol. Indic. 2018, 93, 188–195. [Google Scholar] [CrossRef]
- Maheu-Giroux, M.; de Blois, S. Landscape ecology of Phragmites australis invasion in networks of linear wetlands. Landscape Ecol. 2006, 22, 285–301. [Google Scholar] [CrossRef]
- Badenhausser, I.; Gouat, M.; Goarant, A.; Cornulier, T.; Bretagnolle, V. Spatial autocorrelation in farmland grasshopper assemblages (Orthoptera: Acrididae) in western France. Environ. Entomol. 2012, 41, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P. Spatial autocorrelation: Trouble or new paradigm? Ecology 1993, 74, 1659–1673. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Z.; Guo, L. An evaluation of spatial autocorrelation and heterogeneity in the residuals of six regression models. For. Sci. 2009, 55, 533–548. [Google Scholar]
- Marrot, P.; Garant, D.; Charmantier, A.; Hadfield, J. Spatial autocorrelation in fitness affects the estimation of natural selection in the wild. Methods Ecol. Evol. 2015, 6, 1474–1483. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, T.; Moloney, K.A. Rings, circles, and null-models for point pattern analysis in ecology. Oikos 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Soberón, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Kneitel, J.M.; Chase, J.M. Trade-offs in community ecology: Linking spatial scales and species coexistence. Ecol. Lett. 2004, 7, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Cieszewski, C.J.; Strub, M.R.; Borders, B.E. Spatial regression modeling of tree height–diameter relationships. Can. J. For. Res. 2009, 39, 2283–2293. [Google Scholar] [CrossRef]
- Wang, Q.; Ni, J.; Tenhunen, J. Application of a geographically-weighted regression analysis to estimate net primary production of Chinese forest ecosystems. Glob. Ecol. Biogeogr. 2005, 14, 379–393. [Google Scholar] [CrossRef]
- Kim, D.; Shin, Y.H. Spatial autocorrelation potentially indicates the degree of changes in the predictive power of environmental factors for plant diversity. Ecol. Indic. 2016, 60, 1130–1141. [Google Scholar] [CrossRef]
- Lichstein, J.W.; Simons, T.R.; Shriner, S.A.; Franzreb, K.E. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 2002, 72, 445–463. [Google Scholar] [CrossRef]
- Wiegand, T.; Moloney, K.A. Handbook of Spatial Point-Pattern Analysis in Ecology; Chapman and Hall/CRC: New York, NY, USA, 2014. [Google Scholar]
- Rosenberg, M.S. The bearing correlogram: A new method of analyzing directional spatial autocorrelation. Geogr. Anal. 2000, 32, 267–278. [Google Scholar] [CrossRef]
- Dale, M.R.T.; Fortin, M.J. Spatial Analysis: A Guide for Ecologists; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Rosenberg, M.S.; Anderson, C.D. PASSaGE: Pattern Analysis, Spatial Statistics and Geographic Exegesis. Version 2. Methods Ecol. Evol. 2011, 2, 229–232. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, W.; Fu, B.; Wang, S.; Fan, H. Variability of Tamarix spp. characteristics in riparian plant communities are affected by soil properties and accessibility of anthropogenic disturbance in the lower reaches of Heihe River, China. For. Ecol. Manag. 2018, 410, 174–186. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, J.; Sun, T. Relations between the distribution of vegetation and environment in the Yellow River Delta and SPPA for Chinese tamarisk spatial distribution. J. Beijing For. Univ. 2009, 31, 29–36. [Google Scholar]
- Wu, Y.; Dai, L.; Wang, Y.; Xie, L.; Zhao, S.; Liu, Y.; Zhang, M.; Zhang, Z. Coexistence mechanisms of Tamarix chinensis and Suaeda salsa in the Yellow River Delta, China. Environ. Sci. Pollut. Res. 2020, 27, 26172–26181. [Google Scholar] [CrossRef]
- Pearce, C.M.; Smith, D.G. Saltcedar: Distribution, abundance, and dispersal mechanisms, northern Montana, USA. Wetlands 2003, 23, 215–228. [Google Scholar] [CrossRef]
- Di Tomaso, J.M. Impact, biology, and ecology of saltcedar (Tamarix spp.) in the southwestern United States. Weed Technol. 1998, 12, 326–336. [Google Scholar] [CrossRef]
- Weiner, J. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef] [Green Version]
- Reijers, V.C.; van den Akker, M.; Cruijsen, P.M.J.M.; Lamers, L.P.M.; van der Heide, T. Intraspecific facilitation explains the persistence of Phragmites australis in modified coastal wetlands. Ecosphere 2019, 10, e02842. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Yang, B.; Li, J.; Wang, R.; Liu, T.; Xiao, H. Characteristics of soil water and salt associated with Tamarix ramosissima communities during normal and dry periods in a semi-arid saline environment. Catena 2020, 193, 104661. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.J.; Anthelme, F.; et al. Facilitation in plant communities: The past, the present, and the future. J. Ecol. 2007, 96, 18–34. [Google Scholar] [CrossRef] [Green Version]
- Qi, M.; Sun, T.; Xue, S.; Yang, W.; Shao, D.; Martínez-López, J. Competitive ability, stress tolerance and plant interactions along stress gradients. Ecology 2018, 99, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Berger, U.; Yue, M.; Grimm, V. Asymmetric facilitation can reduce size inequality in plant populations resulting in delayed density-dependent mortality. Oikos 2016, 125, 1153–1161. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Dawazhaxi; Wang, B.; Li, Q.; Yu, Q.; Ou, X.; Ali, K. Spatial distribution patterns and intra-specific competition of pine (Pinus yunnanensis) in abandoned farmland under the Sloping Land Conservation Program. Ecol. Eng. 2019, 135, 17–27. [Google Scholar] [CrossRef]
- Wagner, H.H.; Fortin, M.J. Spatial analysis of landscapes: Concepts and statistics. Ecology 2005, 86, 1975–1987. [Google Scholar] [CrossRef] [Green Version]
- Beale, C.M.; Lennon, J.J.; Yearsley, J.M.; Brewer, M.J.; Elston, D.A. Regression analysis of spatial data. Ecol. Lett. 2010, 13, 246–264. [Google Scholar] [CrossRef]
- Terrones, A.; Moreno, J.; Agulló, J.C.; Villar, J.L.; Vicente, A.; Alonso, M.Á.; Juan, A. Influence of salinity and storage on germination of Tamarix taxa with contrasted ecological requirements. J. Arid Environ. 2016, 135, 17–21. [Google Scholar] [CrossRef]
- Overmars, K.P.; de Koning, G.H.J.; Veldkamp, A. Spatial autocorrelation in multi-scale land use models. Ecol. Model. 2003, 164, 257–270. [Google Scholar] [CrossRef]
- He, G.; Yuan, X.; Zhuang, Y.; Hu, H. An Integrated GNSS/LiDAR-SLAM pose estimation framework for large-scale map building in partially GNSS-denied environments. IEEE T. Instrum. Meas. 2021, 70, 1–9. [Google Scholar]
- Sleeman, J.C.; Boggs, G.S.; Radford, B.C.; Kendrick, G.A. Using agent-based models to aid reef restoration: Enhancing coral cover and topographic complexity through the spatial arrangement of coral transplants. Restor. Ecol. 2005, 13, 685–694. [Google Scholar] [CrossRef]
- Pringle, R.M.; Doak, D.F.; Brody, A.K.; Jocque, R.; Palmer, T.M. Spatial pattern enhances ecosystem functioning in an African savanna. PLoS Biol. 2010, 8, e1000377. [Google Scholar] [CrossRef]
- Stoll, P.; Prati, D. Intraspecific aggregation alters competitive interactions in experimental plant communities. Ecology 2001, 82, 319–327. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, T.; Gao, Y.; Li, B.; Fang, C.; Flory, S.L.; Zhao, B. Road effects on vegetation composition in a saline environment. J. Plant Ecol. 2012, 5, 206–218. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Cui, B.; Luo, M.; Qiu, D.; Shi, W.; Xie, C. Microtopographic structures facilitate plant recruitment across a saltmarsh tidal gradient. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2019, 29, 1336–1346. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, B.; Luo, M.; Shi, W. Designing microtopographic structures to facilitate seedling recruitment in degraded salt marshes. Ecol. Eng. 2018, 120, 266–273. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Lian, J.; Fan, Z.; Ouyang, X.; Ye, W. Seeing the forest from drones: Testing the potential of lightweight drones as a tool for long-term forest monitoring. Biol. Conserv. 2016, 198, 60–69. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef] [Green Version]


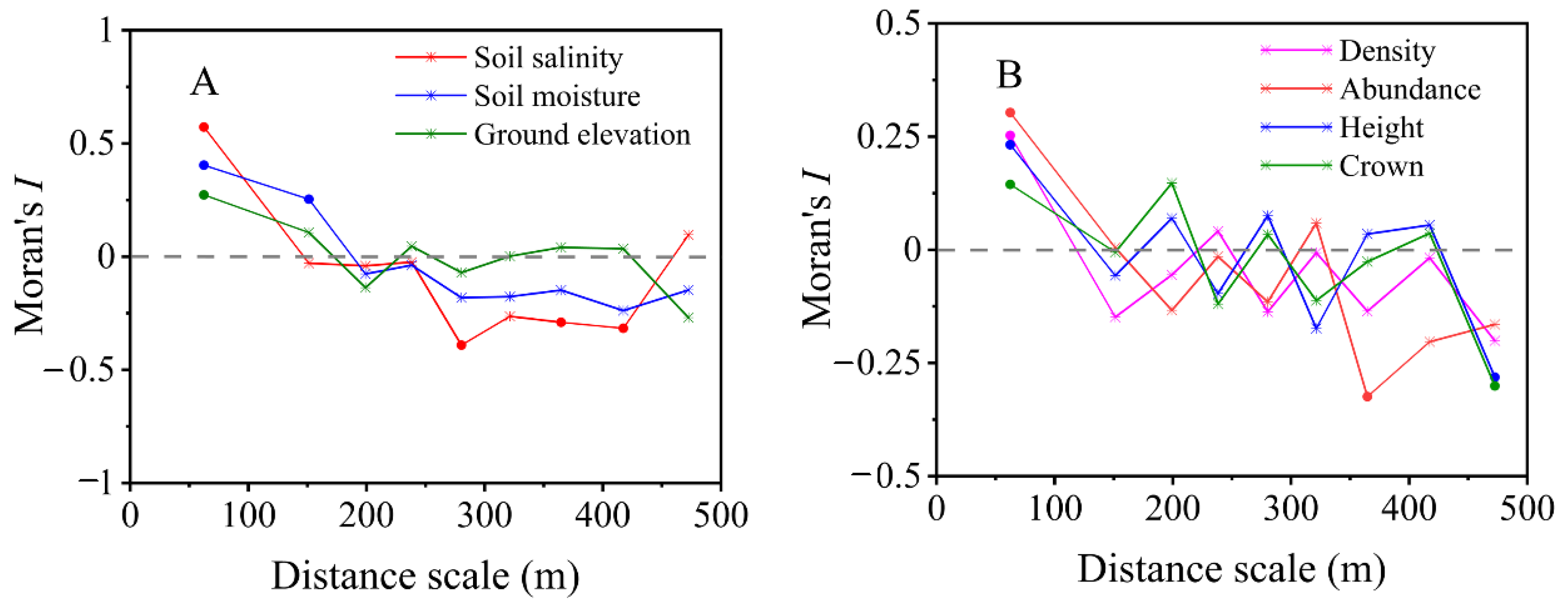

| Moran’s I | Zscore | p | |
|---|---|---|---|
| Soil salinity | 0.50 | 4.82 | p < 0.05 |
| Soil moisture | 0.42 | 4.00 | p < 0.05 |
| Ground elevation | 0.23 | 3.40 | p < 0.05 |
| Density | 0.19 | 2.26 | p < 0.05 |
| Abundance | 0.26 | 2.61 | p < 0.05 |
| Crown diameter | 0.27 | 2.76 | p < 0.05 |
| Tree height | 0.20 | 16.78 | p < 0.05 |
| Variable | Coefficient | SE | t-Statistic | Probability | |
|---|---|---|---|---|---|
| OLS | Constant | −0.01 | 0.12 | 0.00 | 0.98 |
| Zmoisture | 0.37 | 0.13 | 2.87 | 0.00 ** | |
| Zsalinity | −0.26 | 0.13 | −2.02 | 0.04 * | |
| Zcrown | 0.57 | 0.12 | 4.64 | 0.00 ** | |
| Goodness of fit | R2 = 0.52, LIK = −32.36, AIC = 76.95, SC = 78.71 | ||||
| SLM | Constant | 0.04 | 0.11 | 0.38 | 0.71 |
| Zmoisture | 0.41 | 0.13 | 3.17 | 0.00 ** | |
| Zsalinity | −0.29 | 0.12 | −2.39 | 0.02 * | |
| Zcrown | 0.57 | 0.11 | 4.99 | 0.00 ** | |
| ρ | −0.23 | 0.24 | −0.94 | 0.35 | |
| Goodness of fit | R2 = 0.58, LIK = −32.10, AIC = 74.21, SC = 81.69 | ||||
| SEM | Constant | 0.02 | 0.10 | 0.16 | 0.87 |
| Zmoisture | 0.36 | 0.11 | 3.14 | 0.00 ** | |
| Zsalinity | −0.27 | 0.11 | −2.39 | 0.02 * | |
| Zcrown | 0.55 | 0.11 | 4.86 | 0.00 ** | |
| λ | −0.14 | 0.30 | −0.45 | 0.65 | |
| Goodness of fit | R2 = 0.57, LIK = −32.01, AIC = 72.61, SC = 78.60 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, L.; Zhang, Y.; Sun, T.; Yang, W.; Shao, D.; Zhang, P.; Liu, Q. Spatial Analysis as a Tool for Plant Population Conservation: A Case Study of Tamarix chinensis in the Yellow River Delta, China. Sustainability 2021, 13, 8291. https://doi.org/10.3390/su13158291
Jiao L, Zhang Y, Sun T, Yang W, Shao D, Zhang P, Liu Q. Spatial Analysis as a Tool for Plant Population Conservation: A Case Study of Tamarix chinensis in the Yellow River Delta, China. Sustainability. 2021; 13(15):8291. https://doi.org/10.3390/su13158291
Chicago/Turabian StyleJiao, Le, Yue Zhang, Tao Sun, Wei Yang, Dongdong Shao, Peng Zhang, and Qiang Liu. 2021. "Spatial Analysis as a Tool for Plant Population Conservation: A Case Study of Tamarix chinensis in the Yellow River Delta, China" Sustainability 13, no. 15: 8291. https://doi.org/10.3390/su13158291







