Remediation of Emerging Heavy Metals from Water Using Natural Adsorbent: Adsorption Performance and Mechanistic Insights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of AWSB
2.2. Adsorption Experiments
2.3. Adsorption Isotherms
2.4. Thermodynamics
2.5. Kinetics
3. Results and Discussion
3.1. Characterizations of AWSB
3.2. Adsorption Performance
3.2.1. Effect of the Initial Metal Concentration
3.2.2. Effect of Contact Time
3.2.3. Effect of pH
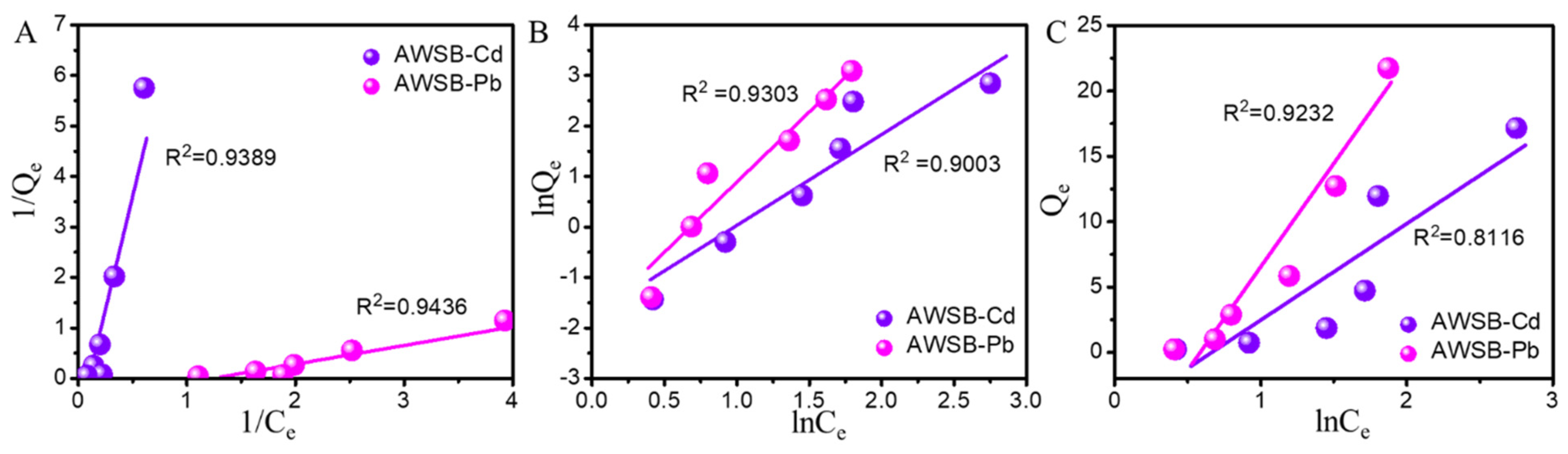
3.2.4. Adsorption Isotherms
3.2.5. Adsorption Thermodynamics
3.2.6. Adsorption Kinetics
3.3. Insights of the Adsorption Mechanism
- (a)
- SEM images (Figure 7A) for post-adsorption of AWSB indicated the adsorption of Cd and Pb ions in the internal pores ultimately making the surface area of biochar well-arranged and least porous. The increased carbon content of the biochar due to pyrolysis thereby increases the adsorption area for the metals. The Cd and Pb adsorption on the AWSB surface were confirmed by using SEM-EDX (Figure 7B). The EDX results revealed that the Pb percentage on AWSB of the pre-adsorbed sample was 0% and increased to 21.52% after adsorption (Table 1). Similarly, the percentage of adsorption for Cd on AWSB surface for pre and post adsorption samples was increased from 8.56% to 19.18%. A high percentage of Si, K, and Ca was also observed on biochar surfaces in EDX analysis. These results strongly evidenced and confirmed the adsorption of heavy metals on AWSB surface where Pb results illustrate the strong adsorption on biochar surface. When Cd and Pb are adsorbed, the AWSB surface has certain particles or minerals crystal on it, showing that Cd and Pb related compounds are produced between Cd, Pb, and biochar, according to SEM spectra.
- (b)
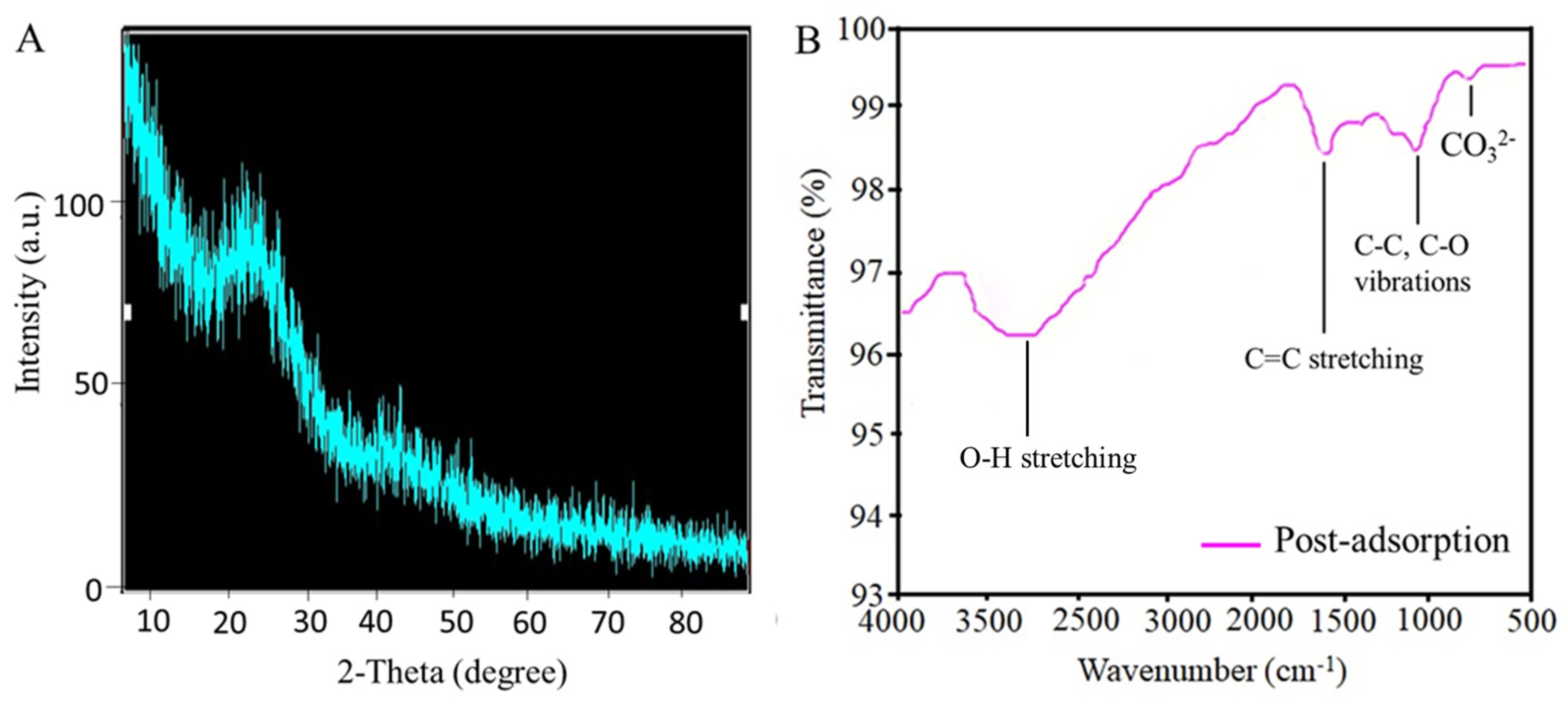
- XRD pattern of post-adsorbed Cd and Pb on AWSB is presented in Figure 8A. Post-adsorption of Cd(NO3)2, Pb(NO3)2, and pH controlling agent (HCl and NaOH), these XRD peaks become broader and stronger at about 20° which corresponds to SiO2 which are in-line with the EDX results [29,33]. The change in the structure of biochar after Cd and Pb adsorption accelerates the precipitation process at the time of adsorption and facilitates the surface precipitation which is an important adsorption mechanism for these metals. The new crystal peak was emerged at 42° after Cd and Pb adsorption on AWSB corresponds to CdCO3 and PbCO3 presence. The shape and characterization of CO32− was consistent with the previous study [52].
- (c)
- Biochar metal cations (Ca2+, Mg2+, K+) are stored by complexation (e.g., —COOM, —R—O—M with —OH and COOH) or precipitation or by electrostatic attraction. During the adsorption process, Cd and Pb are exchanged through these cations in solution through surface complexation by metal exchange process, co-precipitation, or by an electrostatic exchange. Our findings are also supported by previous literature that the concentration of cations and anions decreased after heavy metal adsorption on biochar [53].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
References
- Li, A.Y.; Deng, H.; Jiang, Y.H.; Ye, C.H.; Yu, B.G.; Zhou, X.L. Superefficient Removal of Heavy Metals from Wastewater by Mg- Loaded Biochars: Adsorption Characteristics and Removal Mechanisms. Langmuir 2020, 36, 9160–9174. [Google Scholar] [CrossRef]
- Chai, J.; Au, P.; Mubarak, N.M.; Khalid, M.; Ng, W.P. Adsorption of Heavy Metal from Industrial Wastewater onto Low-Cost Malaysian Kaolin Clay–Based Adsorbent. Environ. Sci. Pollut. Res. 2020, 27, 13949–13962. [Google Scholar] [CrossRef]
- Yu, W.; Hu, J.; Yu, Y.; Ma, D.; Gong, W.; Qiu, H.; Hu, Z.; Gao, H. Facile Preparation of Sulfonated Biochar for Highly Efficient Removal of Toxic Pb(II) and Cd(II) from Wastewater. Sci. Total Environ. 2021, 750, 141545. [Google Scholar] [CrossRef]
- Hassan, A.; Khan, A.; Nawaz, I.; Yousaf, S.; Sabir, A.; Iqbal, M. Soil Amendments Enhanced the Growth of Nicotiana Alata L. and Petunia Hydrida L by Stabilizing Heavy Metals from Wastewater. J. Environ. Manag. 2019, 242, 46–55. [Google Scholar] [CrossRef]
- Poo, K.; Son, E.; Chang, J.; Ren, X.; Choi, Y.; Chae, K. Biochars Derived from Wasted Marine Macro-Algae (Saccharina Japonica and Sargassum Fusiforme) and Their Potential for Heavy Metal Removal in Aqueous Solution. J. Environ. Manag. 2018, 206, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, T.; Hsu, P.; Xie, J.; Zhao, J.; Liu, K.; Sun, J.; Xu, J.; Tang, J.; Ye, Z.; et al. Direct/Alternating Current Electrochemical Method for Removing and Recovering Heavy Metal from Water Using Graphene Oxide Electrode. ACS Nano 2019, 13, 6431–6437. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhang, Q. Investigating the Sorption Behavior of Cadmium from Aqueous Solution by Potassium Permanganate-Modified Biochar : Quantify Mechanism and Evaluate the Modification Method. Environ. Sci. Pollut. Res. 2018, 25, 8330–8339. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, S. Removal of Cadmium in Aqueous Solution Using Wheat Straw Biochar : Effect of Minerals and Mechanism. Environ. Sci. Pollut. Res. 2018, 25, 8688–8700. [Google Scholar] [CrossRef] [PubMed]
- Pino, L.; Vargas, C.; Schwarz, A.; Borquez, R. Influence of Operating Conditions on the Removal of Metals and Sulfate from Copper Acid Mine Drainage by Nanofiltration. Chem. Eng. J. 2018, 345, 114–125. [Google Scholar] [CrossRef]
- Gay, A.; Pal, P.; Banat, F.; Abu, M. Separation and Purification Technology Enhanced Removal of Mixed Metal Ions from Aqueous Solutions Using Flotation by Colloidal Gas Aphrons Stabilized with Sodium Alginate. Sep. Purif. Technol. 2018, 202, 103–110. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Ju, M. Preparation and Modification of Biochar Materials and Their Application in Soil Remediation. Appl. Sci. 2019, 9, 1365. [Google Scholar] [CrossRef] [Green Version]
- Technol, W.S.; Heidelberg, S.B. Biosorption (Removing) of Cd(II), Cu(II) and Methylene Blue Using Biochar Produced by Different Pyrolysis Conditions of Beech and Spruce Sawdust. Wood Sci. Technol. 2017, 51, 1321–1338. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Cai, J.; Zhang, X.; Zhang, J.; Shao, J. Evaluation and Prediction of Cadmium Removal from Aqueous Solution by Phosphate-Modified Activated Bamboo Biochar. Energy Fuels 2018, 23, 4467–4477. [Google Scholar] [CrossRef]
- Ni, B.; Huang, Q.; Wang, C.; Ni, T.; Sun, J.; Wei, W. Competitive Adsorption of Heavy Metals in Aqueous Solution onto Biochar Derived from Anaerobically Digested Sludge. Chemosphere 2019, 219, 351–357. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Gao, Y.; Li, A. Facile Synthesis of Nano ZnO/ZnS Modified Biochar by Directly Pyrolyzing of Zinc Contaminated Corn Stover for Pb(II), Cu(II) and Cr(VI) Removals. Waste Manag. 2018, 79, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Nartey, O.D.; Zhao, B. Biochar Preparation, Characterization, and Adsorptive Capacity and Its Effect on Bioavailability of Contaminants: An Overview. Adv. Mater. Sci. Eng. 2014, 2014, 12. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Chemosphere Physicochemical and Sorptive Properties of Biochars Derived from Woody and Herbaceous Biomass. Chemosphere 2015, 134, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Govumoni, S.P.; Koti, S.; Kothagouni, S.Y.; Venkateshwar, S. Evaluation of Pretreatment Methods for Enzymatic Saccharification of Wheat Straw for Bioethanol Production. Carbohydr. Polym. 2013, 91, 646–650. [Google Scholar] [CrossRef]
- Rodriguez-gomez, D.; Lehmann, L.; Schultz-jensen, N.; Bjerre, A.B.; Hobley, T.J. Examining the Potential of Plasma-Assisted Pretreated Wheat Straw for Enzyme Production by Trichoderma Reesei. Appl. Biochem. Biotechnol. 2012, 166, 2051–2063. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C. Di; Alvira, D.; Greco, G.; González, B.; Manyà, J.J. Physically Activated Wheat Straw-Derived Biochar for Biomass Pyrolysis Vapors Upgrading with High Resistance against Coke Deactivation. Fuel 2019, 255, 115807. [Google Scholar] [CrossRef]
- Anto, S.; Sudhakar, M.P.; Shan, T.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Activation Strategies for Biochar to Use as an Efficient Catalyst in Various Applications. Fuel 2021, 285, 119205. [Google Scholar] [CrossRef]
- Plaza, M.G.; González, A.S.; Pis, J.J.; Rubiera, F.; Pevida, C. Production of Microporous Biochars by Single-Step Oxidation: Effect of Activation Conditions on CO2 Capture. Appl. Energy 2014, 114, 551–562. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Xu, F.; Xie, Y.; Wang, C.; Zhang, A.; Li, L.; Xu, H. Science of the Total Environment Effect of Modi Fi Ed Coconut Shell Biochar on Availability of Heavy Metals and Biochemical Characteristics of Soil in Multiple Heavy Metals Contaminated Soil. Sci. Total Environ. 2018, 645, 702–709. [Google Scholar] [CrossRef]
- Zhi-liang, C.; Jian-qiang, Z.; Ling, H.; Zhi-hui, Y.; Zhao-jun, L.I.; Min-chao, L.I.U. Removal of Cd and Pb with Biochar Made from Dairy Manure at Low Temperature. J. Integr. Agric. 2019, 18, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Ellis, N.; Dehkhoda, A.M. A Novel Method to Tailor the Porous Structure of KOH-Activated Biochar and Its Application in Capacitive Deionization and Energy Storage. Biomass Bioenergy 2016, 87, 107–121. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Luo, L.; Deng, S.; Shi, G.; Zhang, S. Sorption of Tetracycline on H3PO4 Modified Biochar Derived from Rice Straw and Swine Manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Huang, Q.; Chi, Y.; Yan, J. Effect of ZnCl2-Activated Biochar on Catalytic Pyrolysis of Mixed Waste Plastics for Producing Aromatic-Enriched Oil. Waste Manag. 2018, 81, 128–137. [Google Scholar] [CrossRef]
- Boshir, M.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Bioresource Technology Progress in the Preparation and Application of Modified Biochar for Improved Contaminant Removal from Water and Wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef]
- Naeem, M.A.; Imran, M.; Amjad, M.; Abbas, G.; Tahir, M.; Murtaza, B.; Zakir, A.; Shahid, M.; Bulgariu, L.; Ahmad, I. Batch and Column Scale Removal of Cadmium from Water Using Raw and Acid Activated Wheat. Water 2019, 11, 1438. [Google Scholar] [CrossRef] [Green Version]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of Heavy Metals from Aqueous Solution by Biochars Derived from Anaerobically Digested Biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Idrees, M.; Al-wabel, M.I.; Ahmad, M.; Hina, K. Sorption of Cr(III) from Aqueous Media via Naturally Functionalized Microporous Biochar : Mechanistic Study. Microchem. J. 2019, 144, 242–253. [Google Scholar] [CrossRef]
- Chen, Y.; Ho, S.; Wang, D.; Wei, Z.; Chang, J.; Ren, N. Lead Removal by a Magnetic Biochar Derived from Persulfate-ZVI Treated Sludge Together with One-Pot Pyrolysis. Bioresour. Technol. 2018, 247, 463–470. [Google Scholar] [CrossRef]
- Wu, Q.; Xian, Y.; He, Z.; Zhang, Q.; Wu, J.; Yang, G.; Zhang, X. Adsorption Characteristics of Pb(II) Using Biochar Derived from Spent Mushroom Substrate. Sci. Rep. 2019, 9, 15999. [Google Scholar] [CrossRef] [Green Version]
- Idrees, M.; Batool, S.; Ullah, H.; Hussain, Q.; Al-wabel, M.I.; Ahmad, M.; Hussain, A.; Riaz, M.; Sik, Y.; Kong, J. Adsorption and Thermodynamic Mechanisms of Manganese Removal from Aqueous Media by Biowaste-Derived Biochars. J. Mol. Liq. 2018, 266, 373–380. [Google Scholar] [CrossRef]
- Hamza, I.A.A.; Martincigh, B.S.; Ngila, J.C.; Nyamori, V.O. Adsorption Studies of Aqueous Pb(II) onto a Sugarcane Bagasse/Multi-Walled Carbon Nanotube Composite. Phys. Chem. Earth 2013, 66, 157–166. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, G.; Wei, D.; Yan, T.; Xue, X.; Shi, S.; Wei, Q. Preparation and Utilization of Anaerobic Granular Sludge-Based Biochar for the Adsorption of Methylene Blue from Aqueous Solutions. J. Mol. Liq. 2014, 198, 334–340. [Google Scholar] [CrossRef]
- Bashir, S.; Zhu, J.; Fu, Q.; Hu, H. Comparing the Adsorption Mechanism of Cd by Rice Straw Pristine and KOH-Modified Biochar. Environ. Sci. Pollut. Res. 2018, 25, 11875–11883. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ma, L.Q.; Li, Y. Characteristics and Mechanisms of Hexavalent Chromium Removal by Biochar from Sugar Beet Tailing. J. Hazard. Mater. 2011, 190, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Logam, P.; Kumbahan, A. Removal of Heavy Metals from Wastewater Using Date Palm as a Biosorbent: A Comparative Review. Sains Malays. 2018, 47, 35–49. [Google Scholar]
- Forghani, M.; Azizi, A.; Jamal, M.; Asadi, L. Adsorption of Lead(II) and Chromium(VI) from Aqueous Environment onto Metal-Organic Framework MIL-100 (Fe): Synthesis, Kinetics, Equilibrium and Thermodynamics. J. Solid State Chem. 2020, 291, 121636. [Google Scholar] [CrossRef]
- Usman, A.; Sallam, A.; Zhang, M.; Vithanage, M. Sorption Process of Date Palm Biochar for Aqueous Cd(II) Removal : Efficiency and Mechanisms. Water Air Soil Pollut. 2016, 227, 449. [Google Scholar] [CrossRef]
- El-yazeed, W.S.A.; El-reash, Y.G.A.; Elatwy, L.A.; Ahmed, A.I. Facile Fabrication of Bimetallic Fe-Mg MOF for the Synthesis of Xanthenes and Removal of Heavy Metal. RSC Adv. 2020, 10, 9693–9703. [Google Scholar] [CrossRef] [Green Version]
- Coşkun, Y.İ.; Aksuner, N.; Yanik, J. Sandpaper Wastes as Adsorbent for the Removal of Brilliant Green and Malachite Green Dye. Acta Chimica Slovenica 2019, 66, 402–413. [Google Scholar] [CrossRef]
- Mittal, H.; Babu, R.; Dabbawala, A.A.; Alhassan, S.M. Low-Temperature Synthesis of Magnetic Carbonaceous Materials Coated with Nanosilica for Rapid Adsorption of Methylene Blue. ACS Omega 2020, 5, 6100–6112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wne, R.; Leahy, J.J.; Hayes, M.H.B.; Kwapin, W. Kinetic and Adsorptive Characterization of Biochar in Metal Ions Removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Matsumoto, Y.; Machida, M. Adsorption of Zn(II) and Cd(II) Ions onto Magnesium and Activated Carbon Composite in Aqueous Solution. Appl. Surf. Sci. 2010, 256, 1619–1623. [Google Scholar] [CrossRef]
- Xu, D.; Tan, X.; Chen, C.; Wang, X. Removal of Pb(II) from Aqueous Solution by Oxidized Multiwalled Carbon Nanotubes. J. Hazard. Mater. 2008, 154, 407–416. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gómez-Serrano, V.; Gong, H. Sorption of Arsenic, Cadmium, and Lead by Chars Produced from Fast Pyrolysis of Wood and Bark during Bio-Oil Production. J. Colloid Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.S. Removal of Lead from Water Using Biochars Prepared from Hydrothermal Liquefaction of Biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef]
- Park, J.; Sik, Y.; Kim, S.; Cho, J.; Heo, J.; Delaune, R.D.; Seo, D. Competitive Adsorption of Heavy Metals onto Sesame Straw Biochar in Aqueous Solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef]
- Chi, T.; Zuo, J.; Liu, F. Performance and Mechanism for Cadmium and Lead Adsorption from Water and Soil by Corn Straw Biochar. Front. Environ. Sci. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Shan, R.; Yan, L.; Yang, K.; Hao, Y.; Du, B. Adsorption of Cd(II) by Mg–Al–CO3- and Magnetic Fe3O4/Mg–Al–CO3-Layered Double Hydroxides: Kinetic, Isothermal, Thermodynamic and Mechanistic Studies. J. Hazard. Mater. 2015, 299, 42–49. [Google Scholar] [CrossRef]
- Cui, X.; Fang, S.; Yao, Y.; Li, T.; Ni, Q.; Yang, X.; He, Z. Science of the Total Environment Potential Mechanisms of Cadmium Removal from Aqueous Solution by Canna Indica Derived Biochar. Sci. Total Environ. 2016, 562, 517–525. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, X.; Shi, L.; Li, J.; Li, S.; Lü, J.; Li, Y. Efficient Removal of Lead from Solution by Celery-Derived Biochars Rich in Alkaline Minerals. Bioresour. Technol. 2017, 235, 185–192. [Google Scholar] [CrossRef]
- Keiluweit, M.; Kleber, M. Molecular-Level Interactions in Soils and Sediments : The Role of Aromatic π-Systems. Environ. Sci. Technol. 2009, 43, 3421–3429. [Google Scholar] [CrossRef]
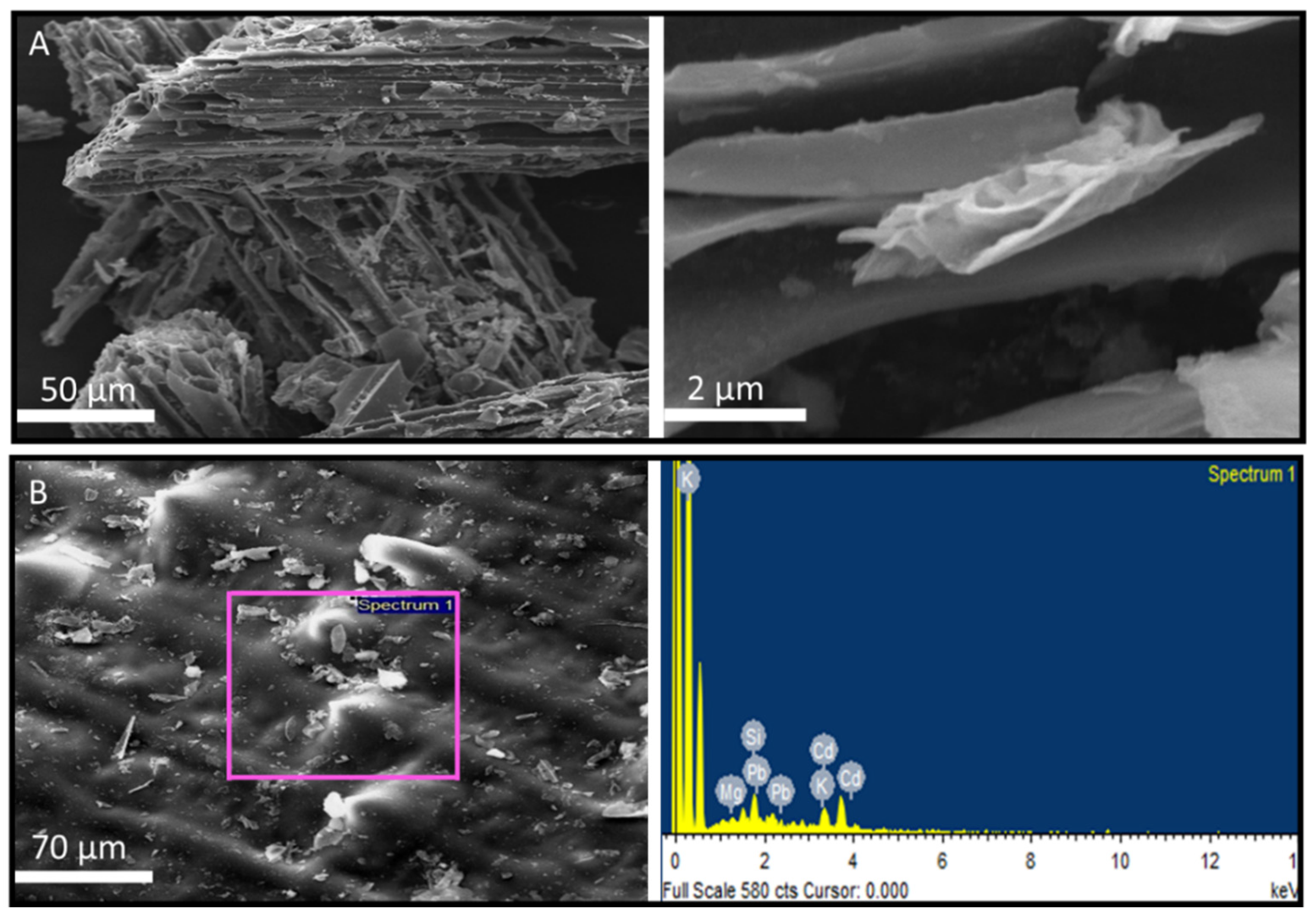





| Element | WSB | AWSB | Post-Adsorption | |||
|---|---|---|---|---|---|---|
| Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | |
| K | 35.20 | 30.53 | 35.84 | 37.00 | - | - |
| Mg | - | - | 5.74 | 9.54 | - | - |
| Si | 42.75 | 51.62 | 32.74 | 47.05 | - | - |
| Ca | 20.85 | 17.65 | - | - | 8.62 | 9.09 |
| Al | - | - | - | - | 50.67 | 79.32 |
| Cd | - | - | 8.56 | 3.07 | 19.18 | 7.21 |
| Pb | 1.21 | 0.20 | - | - | 21.52 | 4.39 |
| Heavy Metal | Temperature (K) | Langmuir Model | Freundlich Model | Temkin Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| KL (L/mg) | Qm (mg/g) | R2 | KF [(mg/g) (L/g)n] | n | R2 | B (J/mol) | A (L/g) | R2 | ||
| Cd | 298 | 1.048 | 9.285 | 0.939 | 0.725 | 1.949 | 0.900 | 318.1 | 0.188 | 0.811 |
| 310 | 1.103 | 9.009 | 0.928 | 0.702 | 1.954 | 0.913 | 335.8 | 0.176 | 0.807 | |
| 322 | 0.635 | 3.724 | 0.911 | 0.705 | 1.954 | 0.909 | 348.6 | 0.177 | 0.804 | |
| Pb | 298 | 0.736 | 0.621 | 0.943 | 0.498 | 2.865 | 0.930 | 169.9 | 0.269 | 0.923 |
| 310 | 0.161 | 0.191 | 0.935 | 0.513 | 2.884 | 0.924 | 179.6 | 0.267 | 0.903 | |
| 322 | 0.028 | 0.172 | 0.926 | 0.519 | 2.895 | 0.919 | 196.5 | 0.269 | 0.815 | |
| Heavy Metal | Temperature (K) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol K) |
|---|---|---|---|---|
| Cd | 298 | −35.44 | −1.90 | 3.49 |
| 310 | −36.46 | |||
| 322 | −37.67 | |||
| Pb | 298 | −32.04 | 112.42 | 119.26 |
| 310 | −33.28 | |||
| 322 | −34.47 |
| Adsorbent | Kinetic Models | Parameters | Cd | Pb |
|---|---|---|---|---|
| AWSB | Pseudo 1st order | qe (Exp) mg/g | 6.064 | 6.921 |
| qe (Cal) mg/g | 7.644 | 7.872 | ||
| K1 | 0.005 | 0.004 | ||
| h | 0.268 | 0.267 | ||
| R2 | 0.983 | 0.993 | ||
| Pseudo 2nd order | qe (Exp) mg/g | 7.53 | 8.142 | |
| qe (Cal) mg/g | 8.85 | 9.03 | ||
| K2 | 0.004 | 0.003 | ||
| h | 0.277 | 0.272 | ||
| R2 | 0.984 | 0.996 | ||
| Power function | kf | −0.233 | −0.090 | |
| b | 1.530 | 1.893 | ||
| R2 | 0.782 | 0.839 | ||
| Intraparticle diffusion | kdif | 0.620 | 0.988 | |
| c | 0.269 | 0.262 | ||
| R2 | 0.814 | 0.839 |
| Adsorbents | Adsorbents | Quantity Adsorbed (mg/g) | Reference |
|---|---|---|---|
| Cd | Out-grassed magnesium composite (Mg-AC) | 0.094 | Yanagisawa et al. [42] |
| BC-300 | 4.41 | Usman et al. [48] | |
| Dairy manure | 0.28 | Xu et al. [49] | |
| Oak | 0.05 | Mohan et al. [50] | |
| Pine | 0.01 | Mohan et al. [50] | |
| Anaerobically digested sludge Adsorption | 0.44 | Ni et al. [51] | |
| AWSB | 8.85 | Present work | |
| Pb | Oxidized MWCNTs Bagasse | 2.06 | Xu et al. [49] |
| Digested whole sugar beet | 0.20 | Inyang et al. [52] | |
| Rice husk | 0.01 | Liu and Zhang [53] | |
| Sesame straw | 0.49 | Park et al. [54] | |
| Pinewood | 0.02 | Liu and Zhang [53] | |
| Anaerobically digested sludge Adsorption | 0.61 | Ni et al. [51] | |
| AWSB | 9.06 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.N.; Ullah, H.; Naeem, S.; Uddin, J.; Hamid, Y.; Ahmad, W.; Ding, J. Remediation of Emerging Heavy Metals from Water Using Natural Adsorbent: Adsorption Performance and Mechanistic Insights. Sustainability 2021, 13, 8817. https://doi.org/10.3390/su13168817
Khan MN, Ullah H, Naeem S, Uddin J, Hamid Y, Ahmad W, Ding J. Remediation of Emerging Heavy Metals from Water Using Natural Adsorbent: Adsorption Performance and Mechanistic Insights. Sustainability. 2021; 13(16):8817. https://doi.org/10.3390/su13168817
Chicago/Turabian StyleKhan, Mehak Nawaz, Hidayat Ullah, Sundas Naeem, Jalal Uddin, Yasir Hamid, Waqar Ahmad, and Jia Ding. 2021. "Remediation of Emerging Heavy Metals from Water Using Natural Adsorbent: Adsorption Performance and Mechanistic Insights" Sustainability 13, no. 16: 8817. https://doi.org/10.3390/su13168817






