Application of a Partial Nitrogen Lab-Scale Sequencing Batch Reactor for the Treatment of Organic Wastewater and Its N2O Production Pathways, and the Microbial Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Start-Up and Operation of the PN-SBR
2.2. Analysis Methods for Water Quality Parameters and Gaseous and Dissolved N2O
2.3. Analysis of Site Preference Values
2.4. Analysis of the Bacterial Community and Core Bacterial Genera
2.5. Isolation, Phylogenetic Analysis, and Characteristics of Culturable Nitrifying Bacteria
2.6. Metagenomic Analysis of Functional Genes in the PN-SBR
3. Results and Discussion
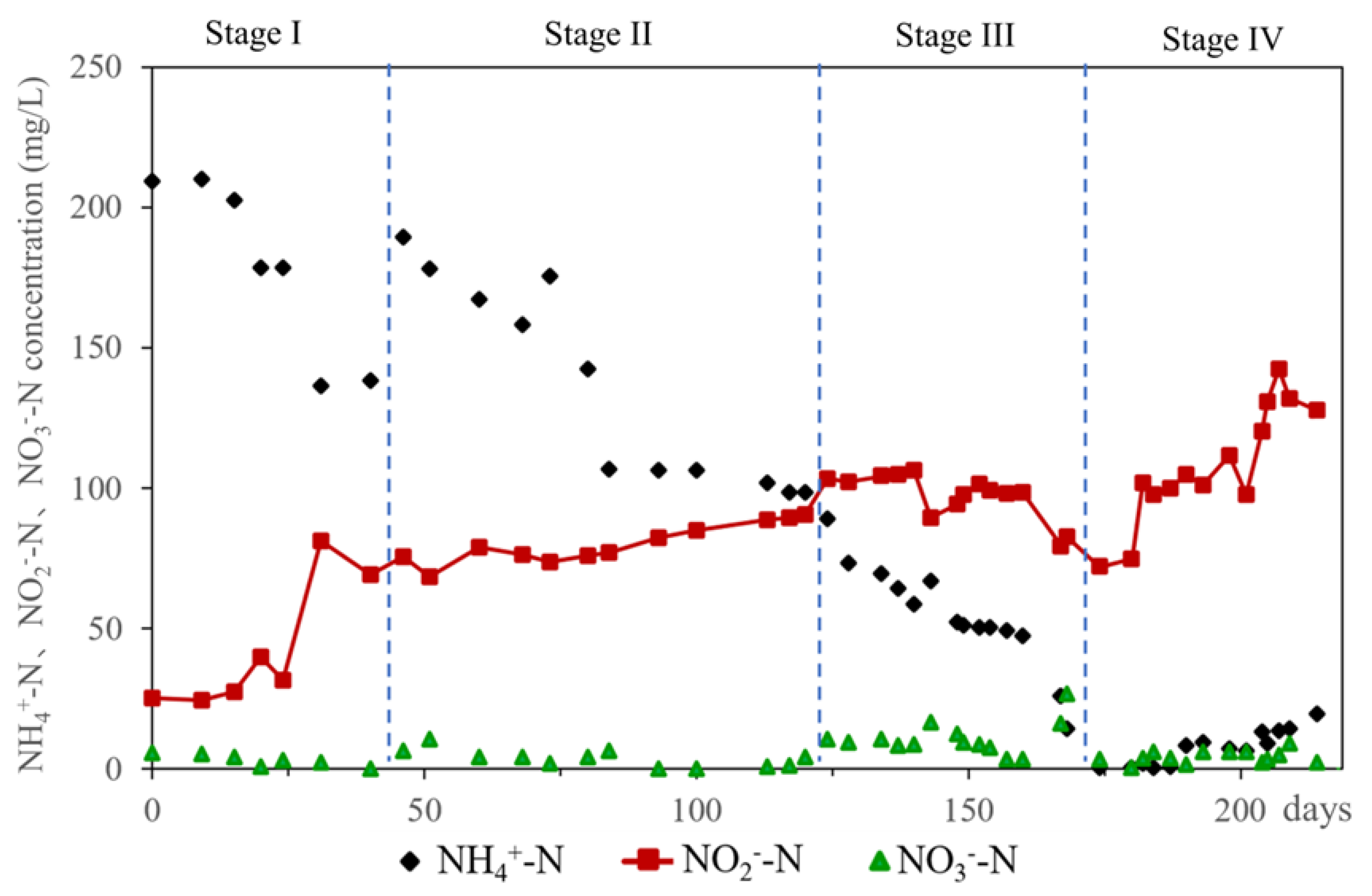
3.1. Successful Running of the PN-SBR with Synthetic Organic Wastewater
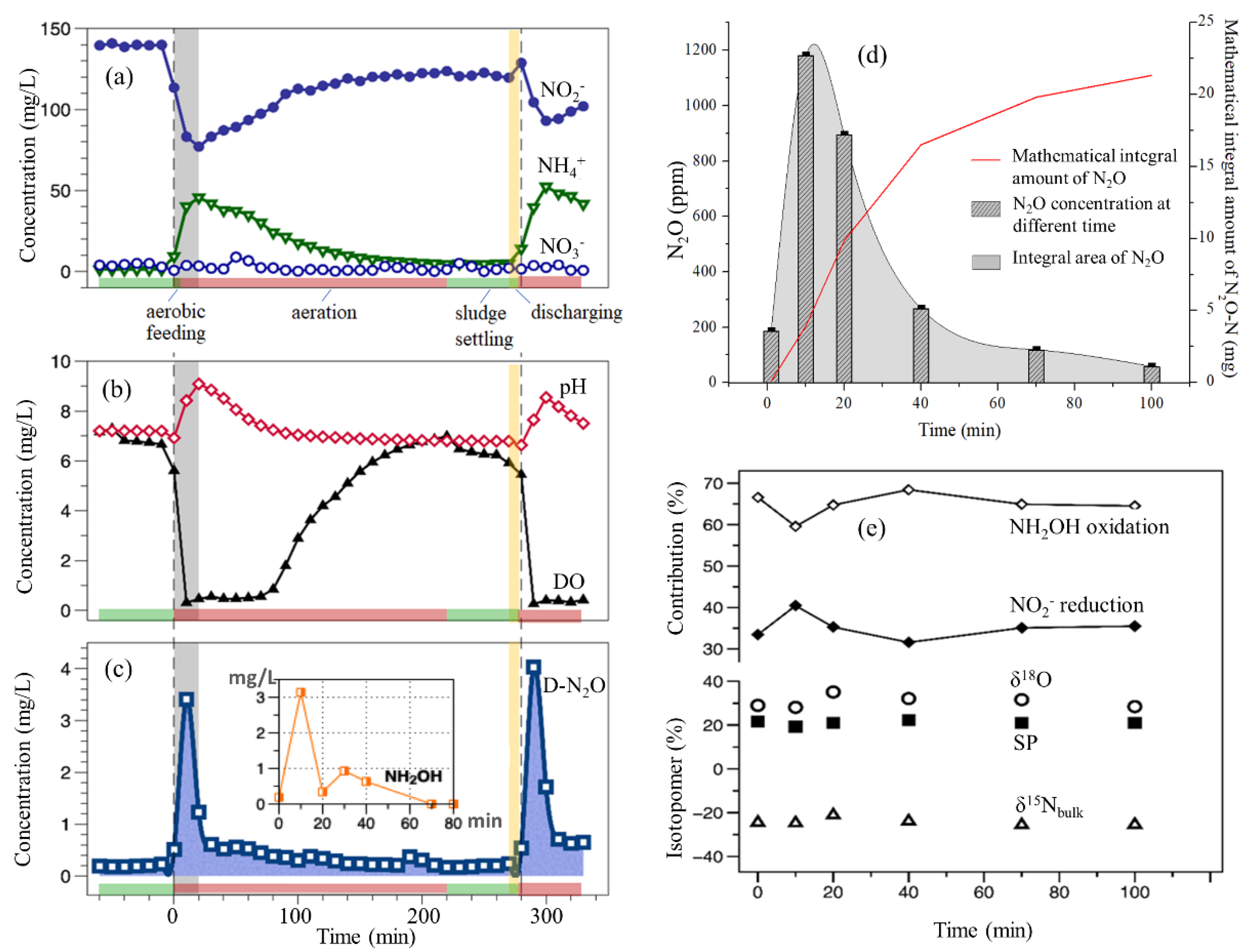
3.2. Performance of the PN-SBR in a Typical Cycle and D-N2O Releasing Patterns
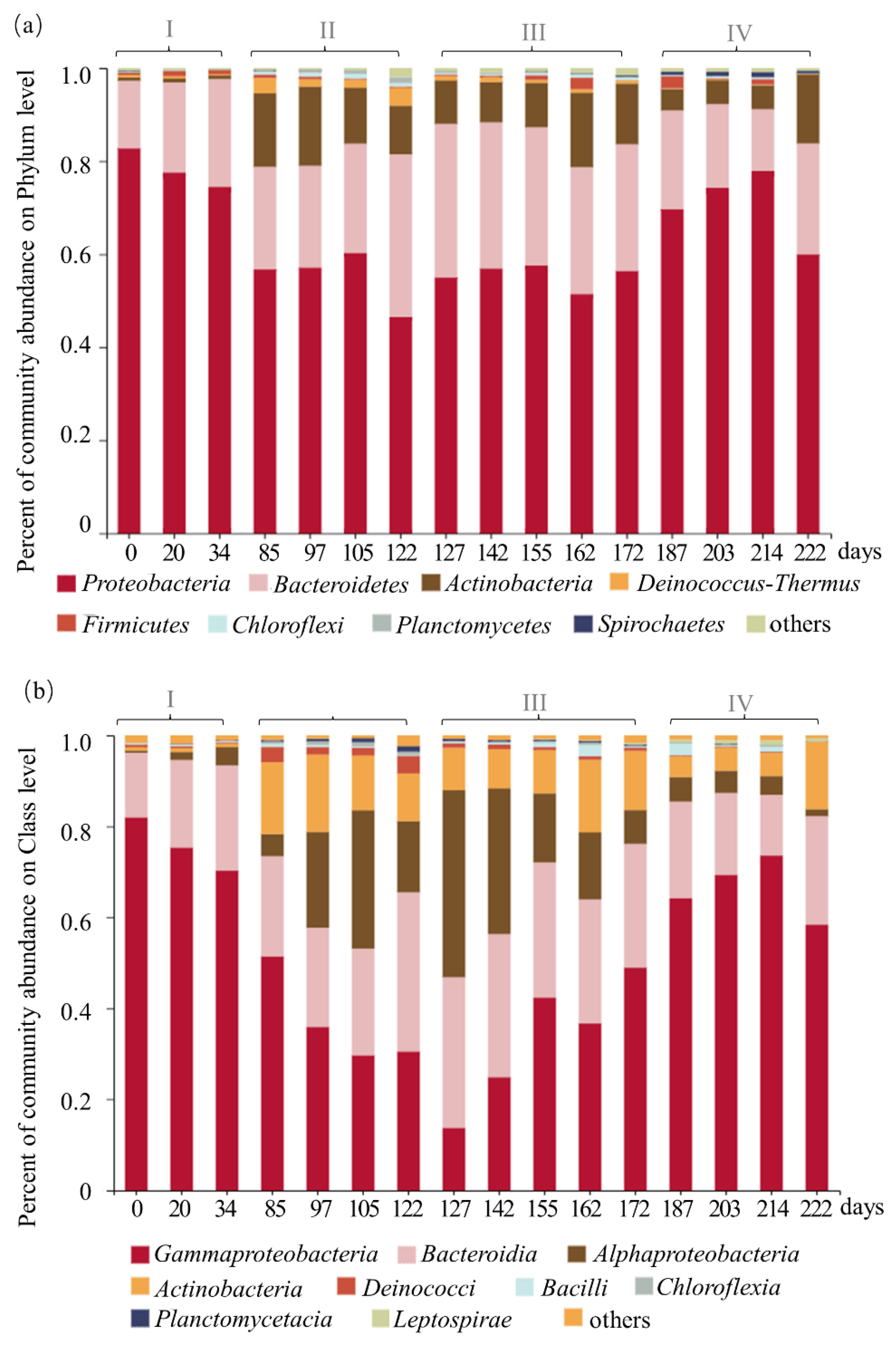
3.3. Succession of Microbial Community Structure in Different Stages of the PN-SBR
3.4. Correlation Network Analysis and Characteristics of Core Bacteria from the PN-SBR
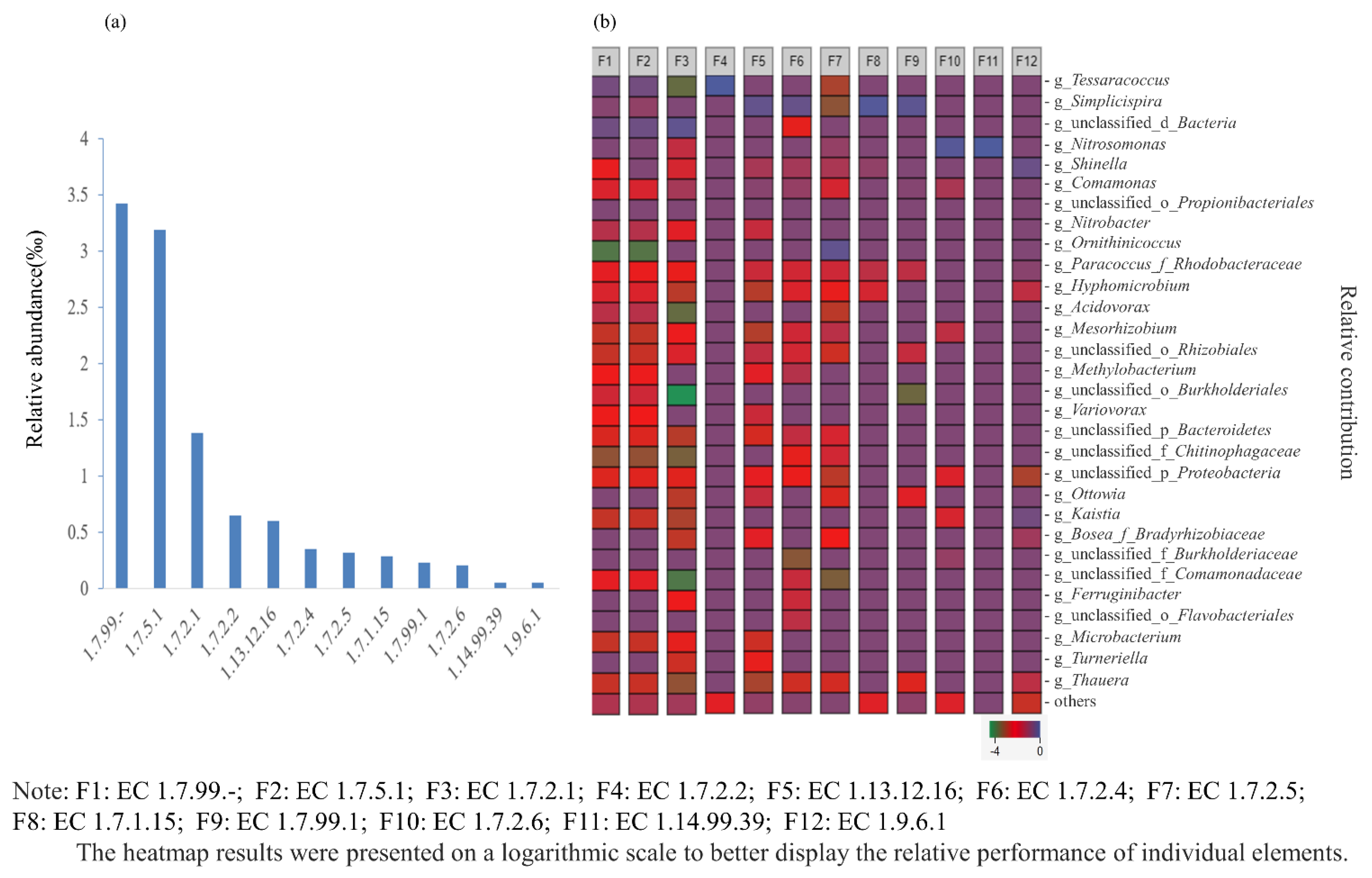
3.5. Metagenomic Analysis of Microbial Functional Genes in the PN-SBR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, D.; Ngo, H.H.; Guo, W.S.; Xu, W.Y.; Du, B.; Wei, Q. Partial nitrification granular sludge reactor as a pretreatment for anaerobic ammonium oxidation (Anammox): Achievement, performance and microbial community. Bioresour. Technol. 2018, 269, 25–31. [Google Scholar] [CrossRef]
- Chen, H.; Tu, Z.; Wu, S.; Yu, G.; Du, C.; Wang, H.; Yang, E.; Zhou, L.; Deng, B.; Wang, D.; et al. Recent advances in partial denitrification-anaerobic ammonium oxidation process for mainstream municipal wastewater treatment. Chemosphere 2021, 278, 130436. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barros, C.M.; Daelman, M.R.J.; Mampaey, K.E.; van Loosdrecht, M.C.M.; Volcke, E.I.P. Effect of aeration regime on N2O emission from partial nitritation-anammox in a full-scale granular sludge reactor. Water Res. 2015, 68, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Kartal, B.; Kuenen, J.G.; van Loosdrecht, M.C.M. Sewage Treatment with Anammox. Science 2010, 328, 702–703. [Google Scholar] [CrossRef]
- Soliman, M.; Eldyasti, A. Development of partial nitrification as a first step of nitrite shunt process in a Sequential Batch Reactor (SBR) using Ammonium Oxidizing Bacteria (AOB) controlled by mixing regime. Bioresour. Technol. 2016, 221, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Caballero, A.; Pijuan, M. N2O and NO emissions from a partial nitrification sequencing batch reactor: Exploring dynamics, sources and minimization mechanisms. Water Res. 2013, 47, 3131–3140. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, Z.; Jiang, J.; Wang, K.C.; Yu, S.Q.; Qiang, J.X.; Ming, Q.; An, Y.; Ye, J.F.; Wu, D.L. Partial nitrification performance and microbial community evolution in the membrane bioreactor for saline stream treatment. Bioresour. Technol. 2021, 320, 124419. [Google Scholar] [CrossRef]
- Okabe, S.; Oozawa, Y.; Hirata, K.; Watanabe, Y. Relationship between population dynamics of nitrifiers in biofilms and reactor performance at various C:N ratios. Water Res. 1996, 30, 1563–1572. [Google Scholar] [CrossRef]
- Oslislo, A.; Lewandowski, Z. Inhibition of nitrification in the packed-bed reactors by selected organic-compounds. Water Res. 1985, 19, 423–426. [Google Scholar] [CrossRef]
- Kim, S.-W.; Miyahara, M.; Fushinobu, S.; Wakagi, T.; Shoun, H. Nitrous oxide emission from nitrifying activated sludge dependent on denitrification by ammonia-oxidizing bacteria. Bioresour. Technol. 2010, 101, 3958–3963. [Google Scholar] [CrossRef]
- Liu, X.; Ni, S.-Q.; Guo, W.; Wang, Z.; Ahmad, H.A.; Gao, B.; Fang, X. N2O emission and bacterial community dynamics during realization of the partial nitrification process. RSC Adv. 2018, 8, 24305–24311. [Google Scholar] [CrossRef] [Green Version]
- Desloover, J.; Vlaeminck, S.E.; Clauwaert, P.; Verstraete, W.; Boon, N. Strategies to mitigate N2O emissions from biological nitrogen removal systems. Curr. Opin. Biotechnol. 2012, 23, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Massara, T.M.; Malamis, S.; Guisasola, A.; Antonio Baeza, J.; Noutsopoulos, C.; Katsou, E. A review on nitrous oxide (N2O) emissions during biological nutrient removal from municipal wastewater and sludge reject water. Sci. Total Environ. 2017, 596, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Song, Y.; Rathnayake, L.; Tumendelger, A.; Satoh, H.; Toyoda, S.; Yoshida, N.; Okabe, S. Identification of key nitrous oxide production pathways in aerobic partial nitrifying granules. Environ. Microbiol. 2014, 16, 3168–3180. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, R.M.L.D.; Oshiki, M.; Ishii, S.; Segawa, T.; Satoh, H.; Okabe, S. Effects of dissolved oxygen and pH on nitrous oxide production rates in autotrophic partial nitrification granules. Bioresour. Technol. 2015, 197, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Terada, A.; Sugawara, S.; Hojo, K.; Takeuchi, Y.; Riya, S.; Harper, W.F., Jr.; Yamamoto, T.; Kuroiwa, M.; Isobe, K.; Katsuyama, C.; et al. Hybrid Nitrous Oxide Production from a Partial Nitrifying Bioreactor: Hydroxylamine Interactions with Nitrite. Environ. Sci. Technol. 2017, 51, 2748–2756. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Peng, Y.; Wang, S. Short-cut nitrification of domestic wastewater in a pilot-scale A/O nitrogen removal plant. Bioprocess Biosyst. Eng. 2007, 30, 91–97. [Google Scholar] [CrossRef]
- Xiao, R.; Ni, B.-J.; Liu, S.; Lu, H. Impacts of organics on the microbial ecology of wastewater anammox processes: Recent advances and meta-analysis. Water Res. 2021, 191, 116817. [Google Scholar] [CrossRef]
- Wang, S.; Yu, H.; Su, Q.; Zuo, J. Exploring the role of heterotrophs in partial nitritation-anammox process treating thermal hydrolysis process—Anaerobic digestion reject water. Bioresour. Technol. 2021, 341, 125762. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Li, M.; Sang, W.; Wang, Y.; Wu, L.; Yang, Y. Bioaugmentation of sequencing batch reactor for aniline treatment during start-up period: Investigation of microbial community structure of activated sludge. Chemosphere 2020, 243, 125426. [Google Scholar] [CrossRef]
- Shapleigh, J.P. The Denitrifying Prokaryotes. In the Prokaryotes: Volume 2: Ecophysiology and Biochemistry; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 769–792. [Google Scholar]
- Guo, J.; Peng, Y.; Wang, S.; Ma, B.; Ge, S.; Wang, Z.; Huang, H.; Zhang, J.; Zhang, L. Pathways and Organisms Involved in Ammonia Oxidation and Nitrous Oxide Emission. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2213–2296. [Google Scholar] [CrossRef]
- Du, W.-L.; Huang, Q.; Miao, L.-L.; Liu, Y.; Liu, Z.-P. Association of running manner with bacterial community dynamics in a partial short-term nitrifying bioreactor for treatment of piggery wastewater with high ammonia content. AMB Express 2016, 6, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ai, G.-M.; Miao, L.-L.; Liu, Z.-P. Marinobacter strain NNA5, a newly isolated and highly efficient aerobic denitrifier with zero N2O emission. Bioresour. Technol. 2016, 206, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, S.; Mutobe, H.; Yamagishi, H.; Yoshida, N.; Tanji, Y. Fractionation of N2O isotopomers during production by denitrifier. Soil Biol. Biochem. 2005, 37, 1535–1545. [Google Scholar] [CrossRef]
- Sutka, R.L.; Ostrom, N.E.; Ostrom, P.H.; Breznak, J.A.; Gandhi, H.; Pitt, A.J.; Li, F. Distinguishing nitrous oxide production from nitrification and denitrification on the basis of isotopomer abundances. Appl. Environ. Microbiol. 2006, 72, 638–644. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, S.; Suzuki, Y.; Hattori, S.; Yamada, K.; Fujii, A.; Yoshida, N.; Kouno, R.; Murayama, K.; Shiomi, H. Isotopomer analysis of production and consumption mechanisms of N2O and CH4 in an advanced wastewater treatment system. Environ. Sci. Technol. 2011, 45, 917–922. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Bikbulatov, E.S.; Vereshchagin, V.M. Reagent for the determination of nitrites in the natural-water. Okeanologiya 1979, 19, 341–343. [Google Scholar]
- Koichiro, T.; Glen, S.; Sudhir, K. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar]
- Hallin, S.; Lydmark, P.; Kokalj, S.; Hermansson, M.; Sorensson, F.; Jarvis, A.; Lindgren, P.E. Community survey of ammonia-oxidizing bacteria in full-scale activated sludge processes with different solids retention time. J. Appl. Microbiol. 2005, 99, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Park, H.D.; Noguera, D.R. Evaluating the effect of dissolved oxygen on ammonia-oxidizing bacterial communities in activated sludge. Water Res. 2004, 38, 3275–3286. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Annachhatre, A.P. Assessment of partial nitrification reactor performance through microbial population shift using quinone profile, FISH and SEM. Bioresour. Technol. 2007, 98, 3602–3610. [Google Scholar] [CrossRef]
- Chandran, K.; Stein, L.Y.; Klotz, M.G.; van Loosdrecht, M.C.M. Nitrous oxide production by lithotrophic ammonia-oxidizing bacteria and implications for engineered nitrogen-removal systems. Biochem. Soc. Trans. 2011, 39, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Kira, O.; Shaviv, A.; Dubowski, Y. Direct tracing of NH3 and N2O emissions associated with urea fertilization approaches, using static incubation cells. Sci. Total Environ. 2019, 661, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Domingo-Felez, C.; Zhang, Z.; Blum, J.-M.; Jensen, M.M.; Smets, B.F. The effect of pH on N2O production in intermittently-fed nitritation reactors. Water Res. 2019, 156, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Liang, S.; Zhang, J.; Xie, H.; Miao, M.; Tian, L. N2O emission in a partial nitrification system: Dynamic emission characteristics and the ammonium-oxidizing bacteria community. Bioresour. Technol. 2013, 127, 400–406. [Google Scholar] [CrossRef]
- Soler-Jofra, A.; Picioreanu, C.; Yu, R.; Chandran, K.; van Loosdrecht, M.C.M.; Perez, J. Importance of hydroxylamine in abiotic N2O production during transient anoxia in planktonic axenic Nitrosomonas cultures. Chem. Eng. Sci. 2018, 335, 756–762. [Google Scholar] [CrossRef]
- Chua, F.J.D.; Sun, F.; Mukherjee, M.; Zhou, Y. Comparison of nitrous oxide emission between a partial and full nitrification enriched ammonia-oxidising culture. Chemosphere 2019, 220, 974–982. [Google Scholar] [CrossRef]
- Todt, D.; Dorsch, P. Mechanism leading to N2O production in wastewater treating biofilm systems. Rev. Environ. Sci. Biotechnol. 2016, 15, 355–378. [Google Scholar] [CrossRef]
- Stein, L.Y. Surveying N2O-producing pathways in bacteria. In Methods in Enzymology: Research on Nitrification and Related Processes, Volume 486, Part A; Klotz, M.G., Ed.; Academic Press: Pasadena, CA, USA, 2011; Volume 486, pp. 131–152. [Google Scholar]
- Ni, B.-J.; Yuan, Z. Recent advances in mathematical modeling of nitrous oxides emissions from wastewater treatment processes. Water Res. 2015, 87, 336–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Z.; Zhou, J.; Lin, Z.; Wang, Y.; Zhao, P.; Zhou, J.; Liu, S.; He, X. Effects of COD/TN ratio on nitrogen removal efficiency, microbial community for high saline wastewater treatment based on heterotrophic nitrification-aerobic denitrification process. Bioresour. Technol. 2020, 301, 122726. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Shi, Y.; Zeng, D.; Wu, G. Enhanced shortcut nitrogen removal and metagenomic analysis of functional microbial communities in a double sludge system treating ammonium-rich wastewater. Environ. Technol. 2020, 41, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Otte, S.; Schalk, J.; Kuenen, J.G.; Jetten, M.S. Hydroxylamine oxidation and subsequent nitrous oxide production by the heterotrophic ammonia oxidizer Alcaligenes faecalis. Appl. Microbiol. Biotechnol. 1999, 51, 255–261. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, X.C.; An, Q.; Huang, Y.S.; Lv, Q.H.; Dan, Q. N2O production from hydroxylamine oxidation and corresponding hydroxylamine oxidoreductase involved in a heterotrophic nitrifier A. faecalis strain NR. Bioprocess Biosyst. Eng. 2019, 42, 1983–1992. [Google Scholar] [CrossRef]




| Components (g/L) | Medium | |
|---|---|---|
| M1 | M2 | |
| (NH4)2SO4 | 0.66 | 0.33 |
| Sodium succinate | 4.72 | 1.0 |
| KH2PO4 | 0.5 | 0.5 |
| Na2HPO4 | 0.5 | 0.5 |
| MgSO4 | 0.1 | 0.1 |
| Glucose | 0 | 0.5 |
| Sodium pyruvate | 0 | 0.3 |
| Vitamin complex solution | 0 | 1 mL |
| Amino acids complex solution | 0 | 5 mL |
| Trace element solution | 2 mL | 1 mL |
| Index | Stage I | Stage II | Stage III | Stage IV |
|---|---|---|---|---|
| Shannon | 2.60 ± 0.08 | 3.09 ± 0.09 | 2.55 ± 0.19 | 1.95 ± 0.14 |
| Simpson | 0.13 ± 0.01 | 0.09 ± 0.01 | 0.16 ± 0.04 | 0.35 ± 0.07 |
| Chao | 206.48 ± 19.96 | 254.73 ± 10.92 | 216.16 ± 23.92 | 170.84 ± 17.07 |
| Strain | Taxonomy | NH4+-N Removal (%) | NO2−-N Formed (mg/L) | NO3−-N Formed (mg/L) |
|---|---|---|---|---|
| SBR-Zi-1 | Paracoccus | 96.3 ± 3.9 | 18.885 ± 3.183 | 1.718 ± 0.032 |
| SBR-SP13 | Comamonas | 61.7 ± 4.1 | 5.371 ± 0.989 | 0.921 ± 0.135 |
| SBR-SF4 | Acidovorax | 46.4 ± 2.8 | 6.543 ± 1.033 | 1.132 ± 0.019 |
| SBR-SC2 | Shinella | 50.3 ± 1.5 | 4.982 ± 0.686 | 0.964 ± 0.074 |
| SBR-S-SH9-1 | Propionibacteriaceae | 65.1 ± 5.7 | 13.786 ± 2.977 | 3.260 ± 0.145 |
| SBR-SF1-2 | Brevundimonas | 48.3 ± 2.4 | 7.317 ± 0,683 | 0.311 ± 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ma, B.; Liu, Z. Application of a Partial Nitrogen Lab-Scale Sequencing Batch Reactor for the Treatment of Organic Wastewater and Its N2O Production Pathways, and the Microbial Mechanism. Sustainability 2022, 14, 1457. https://doi.org/10.3390/su14031457
Liu Y, Ma B, Liu Z. Application of a Partial Nitrogen Lab-Scale Sequencing Batch Reactor for the Treatment of Organic Wastewater and Its N2O Production Pathways, and the Microbial Mechanism. Sustainability. 2022; 14(3):1457. https://doi.org/10.3390/su14031457
Chicago/Turabian StyleLiu, Ying, Boyan Ma, and Zhipei Liu. 2022. "Application of a Partial Nitrogen Lab-Scale Sequencing Batch Reactor for the Treatment of Organic Wastewater and Its N2O Production Pathways, and the Microbial Mechanism" Sustainability 14, no. 3: 1457. https://doi.org/10.3390/su14031457






