Industrial-Scale Hydrothermal Carbonization of Agro-Industrial Digested Sludge: Filterability Enhancement and Phosphorus Recovery
Abstract
:1. Introduction
2. Materials and Methods
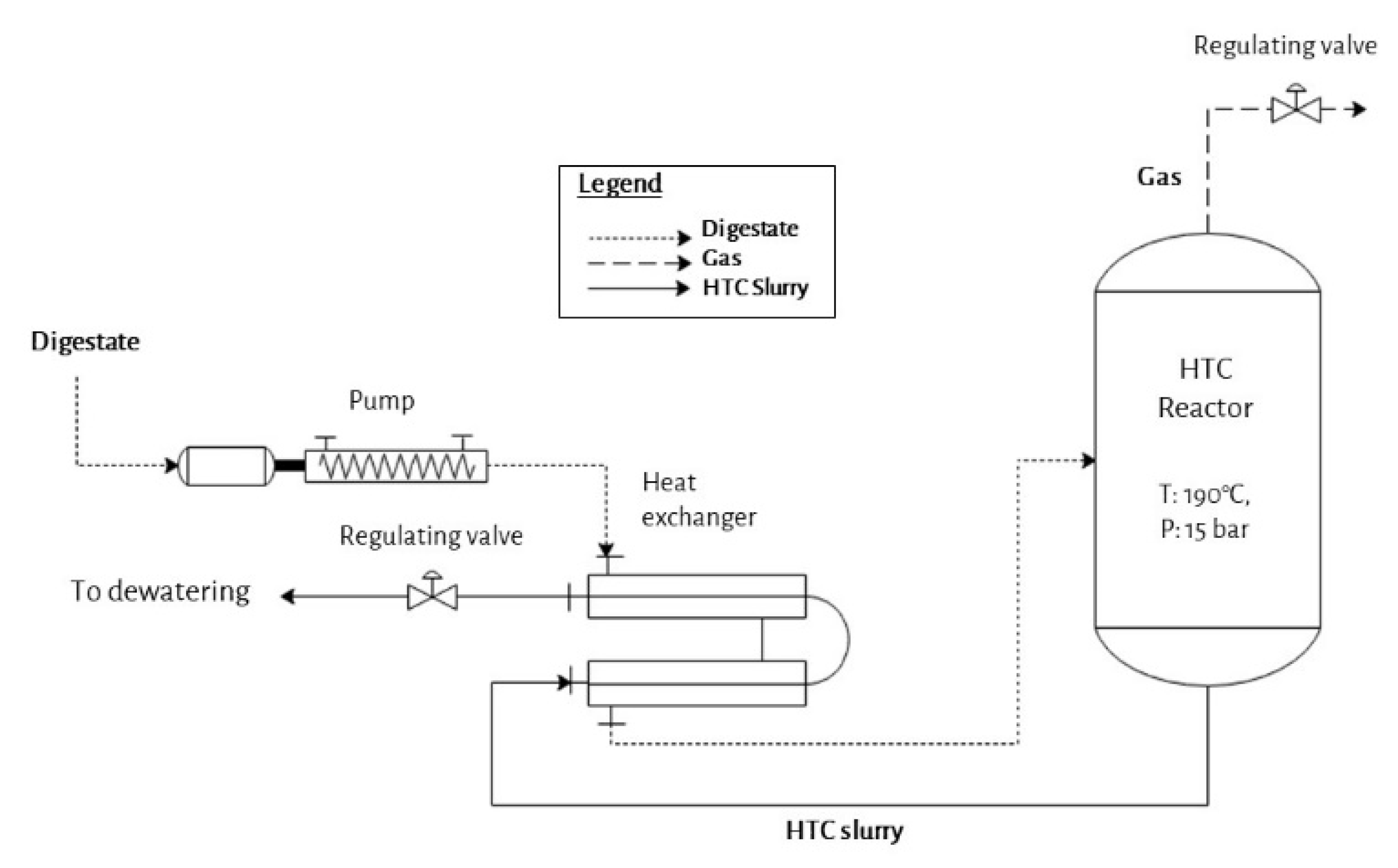
2.1. C-700 Continuous Operating HTC Plant of Carborem Srl
2.2. HTC Slurries Preparation and Conditioning
2.2.1. HTC Slurries Preparation
2.2.2. Conditioning of HTC Residues
2.3. Filtration Tests
2.4. Phosphorus Recovery
2.5. Analytical Characterization of Digestate, HTC Residues and Struvite
3. Results
3.1. Digested Sludge and HTC Slurry Properties and Characteristics
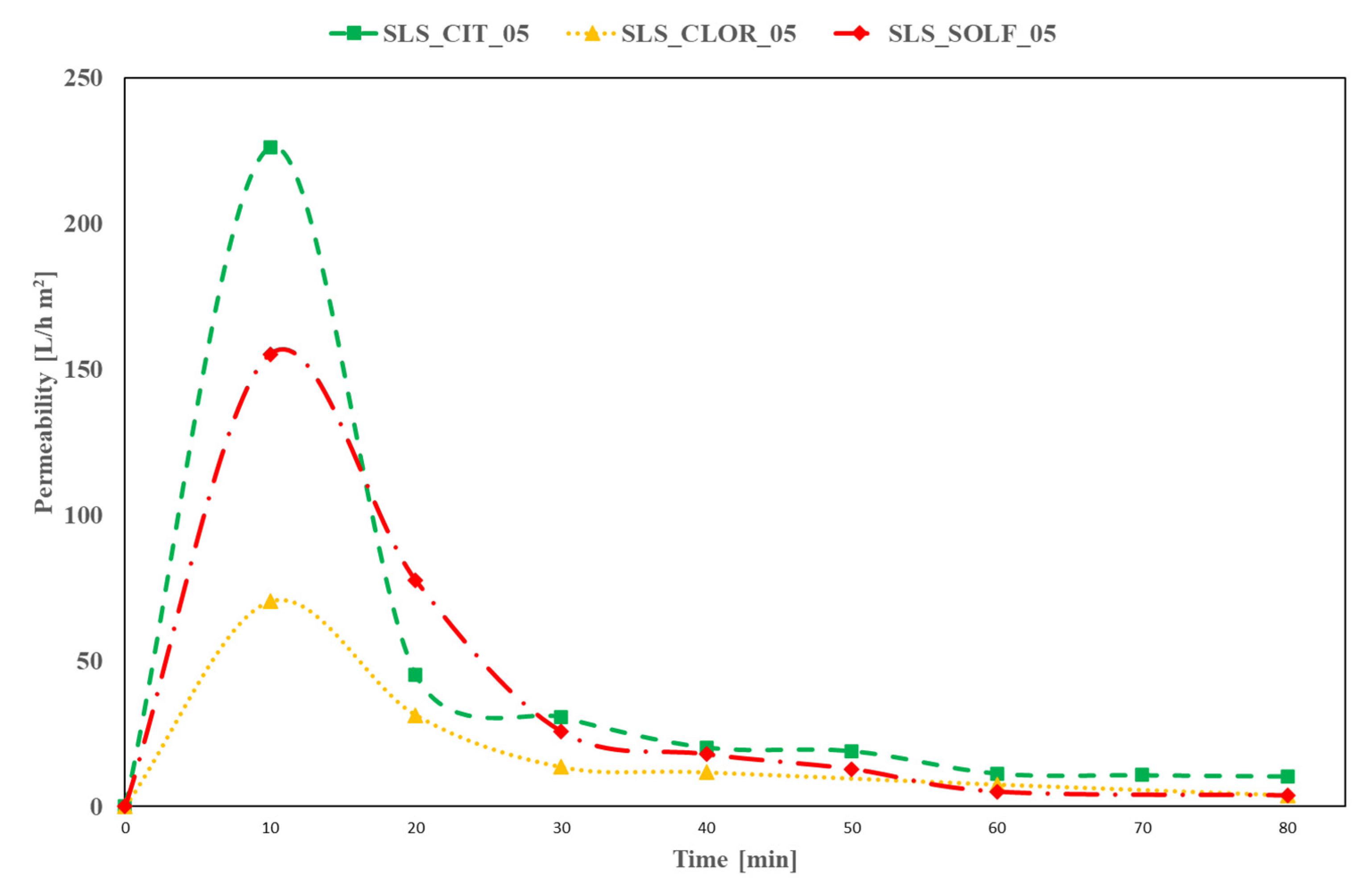
3.2. SL and SLS Residues Filtration Experiment Results
3.3. SL and SLHC Conditioned Solid Characteristics
3.4. Phosphorus Recovery from HTC Liquid Residues
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Obersteiner, G.; Scherhaufer, S. Chapter 12—Environmental impact of food waste. In Environmental Impact of Agro-Food Industry and Food Consumption; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 261–283. ISBN 978-0-12-821363-6. [Google Scholar]
- Suthar, S. Recycling of agro-industrial sludge through vermitechnology. Ecol. Eng. 2010, 36, 1028–1036. [Google Scholar] [CrossRef]
- Zahedi, S.; Solera, R.; Pérez, M. An eco-friendly way to valorize winery wastewater and sewage sludge: Anaerobic co-digestion. Biomass Bioenergy 2020, 142, 105779. [Google Scholar] [CrossRef]
- Shi, W.; Healy, M.G.; Ashekuzzaman, S.M.; Daly, K.; Leahy, J.J.; Fenton, O. Dairy processing sludge and co-products: A review of present and future re-use pathways in agriculture. J. Clean. Prod. 2021, 314, 128035. [Google Scholar] [CrossRef]
- Tait, S.; Harris, P.W.; McCabe, B.K. Biogas recovery by anaerobic digestion of Australian agro-industry waste: A review. J. Clean. Prod. 2021, 299, 126876. [Google Scholar] [CrossRef]
- Pascual, J.A.; Morales, A.B.; Ayuso, L.M.; Segura, P.; Ros, M. Characterisation of sludge produced by the agri-food industry and recycling options for its agricultural uses in a typical Mediterranean area, the Segura River basin (Spain). Waste Manag. 2018, 82, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F.M. Risk assessment of heavy metal toxicity through contaminated vegetables from waste water irrigated area of Varanasi, India. Trop. Ecol. 2010, 51, 375–387. [Google Scholar]
- Mosquera-Losada, M.R.; Muñoz-Ferreiro, N.; Rigueiro-Rodríguez, A. Agronomic characterisation of different types of sewage sludge: Policy implications. Waste Manag. 2010, 30, 492–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanlari, A.; Doğuş Tuncer, A.; Sözen, A.; Şirin, C.; Gungor, A. Energetic, environmental and economic analysis of drying municipal sewage sludge with a modified sustainable solar drying system. Sol. Energy 2020, 208, 787–799. [Google Scholar] [CrossRef]
- Li, Z.; Lu, H.; Ren, L.; He, L. Experimental and modeling approaches for food waste composting: A review. Chemosphere 2013, 93, 1247–1257. [Google Scholar] [CrossRef]
- González-Arias, J.; Carnicero, A.; Sánchez, M.E.; Martínez, E.J.; López, R.; Cara-Jiménez, J. Management of off-specification compost by using co-hydrothermal carbonization with olive tree pruning. Assessing energy potential of hydrochar. Waste Manag. 2021, 124, 224–234. [Google Scholar] [CrossRef]
- Aymerich, E.; Esteban-Gutiérrez, M.; Sancho, L. Analysis of the stability of high-solids anaerobic digestion of agro-industrial waste and sewage sludge. Bioresour. Technol. 2013, 144, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Maragkaki, A.E.; Vasileiadis, I.; Fountoulakis, M.; Kyriakou, A.; Lasaridi, K.; Manios, T. Improving biogas production from anaerobic co-digestion of sewage sludge with a thermal dried mixture of food waste, cheese whey and olive mill wastewater. Waste Manag. 2018, 71, 644–651. [Google Scholar] [CrossRef]
- Maragkaki, A.E.; Fountoulakis, M.; Gypakis, A.; Kyriakou, A.; Lasaridi, K.; Manios, T. Pilot-scale anaerobic co-digestion of sewage sludge with agro-industrial by-products for increased biogas production of existing digesters at wastewater treatment plants. Waste Manag. 2017, 59, 362–370. [Google Scholar] [CrossRef]
- Morais, N.W.S.; Coelho, M.M.H.; Silva, A.D.S.E.; Silva, F.S.S.; Ferreira, T.J.T.; Pereira, E.L.; Santos, A.B.D. Biochemical potential evaluation and kinetic modeling of methane production from six agro-industrial wastewaters in mixed culture. Environ. Pollut. 2021, 280, 116876. [Google Scholar] [CrossRef]
- Ivanchenko, A.; Yelatontsev, D.; Savenkov, A. Anaerobic co-digestion of agro-industrial waste with cheese whey: Impact of centrifuge comminution on biogas release and digestate agrochemical properties. Biomass Bioenergy 2021, 147, 106010. [Google Scholar] [CrossRef]
- Tamburini, E.; Gaglio, M.; Castaldelli, G.; Fano, E.A. Biogas from Agri-Food and Agricultural Waste Can Appreciate Agro-Ecosystem Services: The Case Study of Emilia Romagna Region. Sustainability 2020, 12, 8392. [Google Scholar] [CrossRef]
- Glowacka, A.; Szostak, B.; Klebaniuk, R. Effect of Biogas Digestate and Mineral Fertilisation on the Soil Properties and Yield and Nutritional Value of Switchgrass Forage. Agronomy 2020, 10, 490. [Google Scholar] [CrossRef] [Green Version]
- Möller, K. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Kupper, T.; Bürge, D.; Bachmann, H.J.; Güsewell, S.; Mayer, J. Heavy metals in source-separated compost and digestates. Waste Manag. 2014, 34, 867–874. [Google Scholar] [CrossRef]
- European Committee. EU Report on Critical Raw Materials List; European Commission, COM(2017) 490, 13.9.2017. Available online: https://ec.europa.eu/transparency/documents-register/detail?ref=COM(2017)490&lang=en (accessed on 19 August 2021).
- Beggio, G.; Schievano, A.; Bonato, T.; Hennebert, P.; Pivato, A. Statistical analysis for the quality assessment of digestates from separately collected organic fraction of municipal solid waste (OFMSW) and agro-industrial feedstock. Should input feedstock to anaerobic digestion determine the legal status of digestate? Waste Manag. 2019, 87, 546–558. [Google Scholar] [CrossRef]
- Corden, C.; Bougas, K.; Cunningham, E.; Tyrer, D.; Kreißig, J.; Zetti, E.; Gamero, E.; Wildey, R.; Crookes, M. Digestate and Compost as Fertilisers: Risk Assessment and Risk Management Options; Woods Environment and Infrastructure Solutions: London, UK, 2019. [Google Scholar]
- European Commission. Directive 2006/123/EC on services in the internal market. In EU Regulation of E-Commerce; Edward Elgar Publishing: Cheltenham, UK, 2017; pp. 128–145. [Google Scholar]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal carbonization as a valuable tool for energy and environmental applications: A review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Messineo, A.; Mäkelä, M.; Barr, M.R.; Volpe, R.; Corrado, C.; Fiori, L. Reactivity of cellulose during hydrothermal carbonization of lignocellulosic biomass. Fuel Process. Technol. 2020, 206, 106456. [Google Scholar] [CrossRef]
- Picone, A.; Volpe, M.; Giustra, M.G.; Bella, G.D.; Messineo, A. Hydrothermal Carbonization of Lemon Peel Waste: Preliminary Results on the Effects of Temperature during Process Water Recirculation. Appl. Syst. Innov. 2021, 4, 19. [Google Scholar] [CrossRef]
- Picone, A.; Volpe, M.; Messineo, A. Process Water Recirculation during Hydrothermal Carbonization of Waste Biomass: Current Knowledge and Challenges. Energies 2021, 14, 2962. [Google Scholar] [CrossRef]
- Hitzl, M.; Corma, A.; Pomares, F.; Renz, M. The hydrothermal carbonization (HTC) plant as a decentral biorefinery for wet biomass. Catal. Today 2015, 257, 154–159. [Google Scholar] [CrossRef]
- Mäkelä, M.; Benavente, V.; Fullana, A. Hydrothermal carbonization of lignocellulosic biomass: Effect of process conditions on hydrochar properties. Appl. Energy 2015, 155, 576–584. [Google Scholar] [CrossRef]
- Reza, M.T.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J. Hydrothermal carbonization of loblolly pine: Reaction chemistry and water balance. Biomass Convers. Biorefinery 2014, 4, 311–321. [Google Scholar] [CrossRef]
- Benavente, V.; Calabuig, E.; Fullana, A. Upgrading of moist agro-industrial wastes by hydrothermal carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Heidari, M.; Regmi, B.; Salaudeen, S.; Arku, P.; Thimmannagari, M.; Dutta, A. Hydrothermal carbonization of fruit wastes: A promising technique for generating hydrochar. Energies 2018, 11, 2022. [Google Scholar] [CrossRef] [Green Version]
- Volpe, M.; Codignole, F.; Nepu, L.; Toufiq, S.M.; Maryanne, R.; Volpe, R.; Messineo, A. Enhancement of energy and combustion properties of hydrochar via citric acid catalysed secondary char production. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Saha, N.; Volpe, M.; Fiori, L.; Volpe, R.; Messineo, A.; Reza, M.T. Cationic dye adsorption on hydrochars of winery and citrus juice industries residues: Performance, mechanism, and thermodynamics. Energies 2020, 13, 4686. [Google Scholar] [CrossRef]
- Lin, J.; Mariuzza, D.; Volpe, M.; Fiori, L.; Ceylan, S.; Goldfarb, L. Integrated thermochemical conversion process for valorizing mixed agricultural and dairy waste to nutrient-enriched biochars and biofuels. Bioresour. Technol. 2021, 328, 124765. [Google Scholar] [CrossRef]
- Wu, K.; Gao, Y.; Zhu, G.; Zhu, J.; Yuan, Q.; Chen, Y.; Cai, M.; Feng, L. Characterization of dairy manure hydrochar and aqueous phase products generated by hydrothermal carbonization at different temperatures. J. Anal. Appl. Pyrolysis 2017, 127, 335–342. [Google Scholar] [CrossRef]
- Saha, N.; Saba, A.; Saha, P.; McGaughy, K.; Franqui-Villanueva, D.; Orts, W.J.; Hart-Cooper, W.M.; Toufiq Reza, M. Hydrothermal carbonization of various paper mill sludges: An observation of solid fuel properties. Energies 2019, 12, 858. [Google Scholar] [CrossRef] [Green Version]
- Mäkelä, M.; Benavente, V.; Fullana, A. Hydrothermal carbonization of industrial mixed sludge from a pulp and paper mill. Bioresour. Technol. 2016, 200, 444–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merzari, F.; Lucian, M.; Volpe, M.; Andreottola, G.; Fiori, L. Hydrothermal carbonization of biomass: Design of a bench-Scale reactor for evaluating the heat of reaction. Chem. Eng. Trans. 2018, 65, 43–48. [Google Scholar]
- Hitzl, M.; Mendez, A.; Owsianiak, M.; Renz, M. Making hydrochar suitable for agricultural soil: A thermal treatment to remove organic phytotoxic compounds. J. Environ. Chem. Eng. 2018, 6, 7029–7034. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Merzari, F.; Wüst, D.; Kruse, A.; Andreottola, G.; Fiori, L. Hydrothermal carbonization coupled with anaerobic digestion for the valorization of the organic fraction of municipal solid waste. Bioresour. Technol. 2020, 314, 123734. [Google Scholar] [CrossRef]
- Merzari, F.; Goldfarb, J.; Andreottola, G.; Mimmo, T.; Volpe, M.; Fiori, L. Hydrothermal carbonization as a strategy for sewage sludge management: Influence of process withdrawal point on hydrochar properties. Energies 2020, 13, 2809. [Google Scholar] [CrossRef]
- Tasca, A.L.; Puccini, M.; Gori, R.; Corsi, I.; Galletti, A.M.R.; Vitolo, S. Hydrothermal carbonization of sewage sludge: A critical analysis of process severity, hydrochar properties and environmental implications. Waste Manag. 2019, 93, 1–13. [Google Scholar] [CrossRef]
- Xu, Z.X.; Song, H.; Li, P.J.; He, Z.X.; Wang, Q.; Wang, K.; Duan, P.G. Hydrothermal carbonization of sewage sludge: Effect of aqueous phase recycling. Chem. Eng. J. 2020, 387, 123410. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Holdich, R.G.; Wheatley, A.D.; Martin, S.J.; Shama, G. Hydrothermal Carbonization of Primary Sewage Sludge and Synthetic Faeces: Effect of Reaction Temperature and Time on Filterability. Environ. Prog. Sustain. Energy 2015, 34, 1279–1290. [Google Scholar] [CrossRef] [Green Version]
- Volpe, M.; Fiori, L.; Merzari, F.; Messineo, A.; Andreottola, G. Hydrothermal carbonization as an efficient tool for sewage sludge valorization and phosphorous recovery. Chem. Eng. Trans. 2020, 80, 199–204. [Google Scholar]
- Becker, G.C.; Wüst, D.; Köhler, H.; Lautenbach, A.; Kruse, A. Novel approach of phosphate-reclamation as struvite from sewage sludge by utilising hydrothermal carbonization. J. Environ. Manag. 2019, 238, 119–125. [Google Scholar] [CrossRef]
- Marin-Batista, J.D.; Mohedano, A.F.; Rodriguez, J.J.; Rubia, M.A.D.L. Energy and Phosphorous Recovery Through Hydrothermal Carbonization of Digested Sewage Sludge. Waste Manag. 2020, 105, 566–574. [Google Scholar] [CrossRef]
- Gerner, G.; Meyer, L.; Wanner, R.; Keller, T. Sewage Sludge Treatment by Hydrothermal Carbonization: Fuel Production. Energies 2021, 14, 2697. [Google Scholar] [CrossRef]
- Ma, J.Z.; Zhang, M.; Liu, Z.G.; Wang, M.; Sun, Y.; Zheng, W.K.; Lu, H. Copper-based-zinc-boron foliar fertilizer improved yield, quality, physiological characteristics, and microelement concentration of celery (Apium graveolens L.). Environ. Pollut. Bioavailab. 2019, 31, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M.; Singh, S.; Ross, A.B. Fate of inorganic material during hydrothermal carbonisation of biomass: Influence of feedstock on combustion behaviour of hydrochar. Fuel 2016, 169, 135–145. [Google Scholar] [CrossRef]
- Reza, M.; Lynam, J.G.; Helal Uddin, M.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Volpe, M.; Wüst, D.; Merzari, F.; Lucian, M.; Andreottola, G.; Kruse, A.; Fiori, L. One stage olive mill waste streams valorisation via hydrothermal carbonisation. Waste Manag. 2018, 80, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.; Baxter, L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Xue, J.; Chellappa, T.; Ceylan, S.; Goldfarb, J.L. Enhancing biomass + coal Co-firing scenarios via biomass torrefaction and carbonization: Case study of avocado pit biomass and Illinois No. 6 coal. Renew. Energy 2018, 122, 152–162. [Google Scholar] [CrossRef]
- Rruff Database Sample ID: R050511. Available online: https://rruff.info/struvite/display=default/R050511 (accessed on 20 May 2021).





| Sample | Total Mass (kg) | TS % | TS (kg) | pH | TOC wt% db | N Tot wt% db | P wt% db | P (kg) | P Rec. wt% |
|---|---|---|---|---|---|---|---|---|---|
| DS | 1000 | 5.1 | 51.0 | 7.0 | 26.0 | 3.6 | 2.3 | 1.17 | - |
| SL | 933 | 3.9 | 36.4 | 7.0 | 24.5 | 3.1 | 3.1 | 1.14 | 97.3 |
| SLHC | 131 | 27.7 | 36.3 | 7.1 | 29.3 | 2.8 | 3.1 | 1.10 | 94.0 |
| HTC Liquid | 1175 | <0.01 | 0 | 7.0 | - | 1370 (1) | 76.5 (1) | 0.09 | −7.7 (2,3) |
| Sample | Cd (mg/kg) | Hg (mg/kg)] | Ni (mg/kg) | Pb (mg/kg) | Cu (mg/kg) | Zn (mg/kg) |
|---|---|---|---|---|---|---|
| DS | <0.12 | <0.20 | 17.2 | 6.4 | 440 | 232 |
| SL | 0.18 | 0.21 | 22.8 | 11.7 | 830 | 301 |
| SLHC | <0.12 | 0.32 | 10.3 | 19.4 | 650 | 309 |
| Sample | TS wt% | TOC wt% | MY * wt% | P wt% | Hg (mg/kg) | Ni (mg/kg) | Pb (mg/kg) | Cu (mg/kg) | Zn (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| SL | 5.1 | 26.0 | - | 3.10 | 0.21 | 22.8 | 11.7 | 830.0 | 301.0 |
| HC_CIT_30_CELL_40 | 41.0 | 26.1 | 89.3 | 0.10 | 0.10 | 6.7 | 0.9 | 24.6 | 13.6 |
| HC_CIT_30_PERL_20 | 54.7 | 20.3 | 85.5 | 0.06 | 0.34 | 3.1 | 1.2 | 57.0 | 20.6 |
| HC_SOLF_30_PERL_20 | 42.9 | 18.3 | 87.3 | 0.24 | 0.12 | 11.4 | 1.4 | 34.1 | 24.8 |
| SLHC | 27.7 | 29.3 | - | 3.10 | 0.32 | 10.3 | 19.4 | 650.0 | 309.0 |
| SLHC_SOLF_05 | 42.8 | 24.4 | 87.5 | 0.14 | 0.11 | 4.1 | 2.2 | 91.2 | 22.0 |
| SLHC_CLOR_05 | 38.8 | 26.8 | 80.0 | 0.39 | 0.16 | 3.7 | 2.0 | 97.1 | 37.9 |
| SLHC_CIT_05 | 44.0 | 35.8 | 83.3 | 0.13 | 0.18 | 8.4 | 1.9 | 93.8 | 36.3 |
| Sample | Msample (g) | P (g) | TS wt% | Pcake wt% db | Pcake (g) | PFiltrate (g) | Prec-Filtrate wt% |
|---|---|---|---|---|---|---|---|
| SL | 7500 | 9.1 | 3.9 | - | - | - | - |
| SL_CIT_30_CELL_40 | 8025 | 9.1 | 10.2 | 0.10 | 0.56 | 8.51 | 93.8 |
| SL_CIT_30_Perl_20 | 7875 | 9.1 | 8.5 | 0.06 | 0.24 | 8.83 | 97.4 |
| SL_SOLF_30_Perl_20 | 7875 | 9.1 | 8.5 | 0.24 | 0.97 | 8.09 | 89.3 |
| SLHC | 1600 | 13.7 | 27.7 | - | - | - | - |
| SLS_SOLF_05 | 3650 | 13.7 | 12.1 | 0.39 | 1.51 | 12.23 | 89.0 |
| SLS_CLOR_05 | 3640 | 13.7 | 12.2 | 0.14 | 0.50 | 13.24 | 96.4 |
| SLS_CIT_05 | 3715 | 13.7 | 11.9 | 0.13 | 0.48 | 13.26 | 96.5 |
| Sample | VM wt% | FC wt% | Ash wt% | HHV (MJ/kg) |
|---|---|---|---|---|
| DS | 41.7 | 9.9 | 48.4 | 11.33 |
| SLHC | 39.6 | 10.6 | 49.8 | 11.27 |
| HC_CIT_30_CELL_40 | 52.0 | 9.5 | 38.5 | 11.32 |
| HC_CIT_30_PERL_20 | 31.5 | 9.4 | 59.1 | 9.38 |
| HC_SOLF_30_PERL_20 | 28.0 | 5.1 | 66.9 | 7.20 |
| SLHC_SOLF_05 | 46.1 | 6.0 | 47.9 | 11.19 |
| SLHC_CLOR_05 | 40.2 | 13.1 | 46.7 | 12.20 |
| SLHC_CIT_05 | 50.7 | 16.8 | 32.5 | 17.59 |
| Sample | pH | N (NH4+) (g/L) | P (g/L) |
|---|---|---|---|
| L_CIT_30_CELL_40 | 4.0 | 0.60 | 1.278 |
| L_CIT_30_PERL_20 | 3.4 | 0.72 | 1.236 |
| L_SOLF_30_PERL_20 | 4.4 | 0.73 | 1.168 |
| SLL_SOLF_05 | 2.3 | 3.78 | 4.954 |
| SLL_CLOR_05 | 2.6 | 3.21 | 4.858 |
| SLL_CIT_05 | 3.2 | 3.36 | 4.611 |
| Sample | P (mol/L) | Struvite (g/L) | Struvite (mol/L) | P Rec Struvite wt% |
|---|---|---|---|---|
| L_CIT_30_CELL_40 | 0.041 | 5.15 | 0.021 | 50.8% |
| L_CIT_30_PERL_20 | 0.040 | 5.64 | 0.023 | 57.5% |
| L_SOLF_30_PERL_20 | 0.038 | 5.15 | 0.021 | 55.6% |
| SLL_SOLF_05 | 0.160 | 38.53 | 0.157 | 97.9% |
| SLL_CLOR_05 | 0.157 | 33.13 | 0.135 | 85.9% |
| SLL_CIT_05 | 0.149 | 33.30 | 0.136 | 90.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucian, M.; Merzari, F.; Gubert, M.; Messineo, A.; Volpe, M. Industrial-Scale Hydrothermal Carbonization of Agro-Industrial Digested Sludge: Filterability Enhancement and Phosphorus Recovery. Sustainability 2021, 13, 9343. https://doi.org/10.3390/su13169343
Lucian M, Merzari F, Gubert M, Messineo A, Volpe M. Industrial-Scale Hydrothermal Carbonization of Agro-Industrial Digested Sludge: Filterability Enhancement and Phosphorus Recovery. Sustainability. 2021; 13(16):9343. https://doi.org/10.3390/su13169343
Chicago/Turabian StyleLucian, Michela, Fabio Merzari, Michele Gubert, Antonio Messineo, and Maurizio Volpe. 2021. "Industrial-Scale Hydrothermal Carbonization of Agro-Industrial Digested Sludge: Filterability Enhancement and Phosphorus Recovery" Sustainability 13, no. 16: 9343. https://doi.org/10.3390/su13169343







