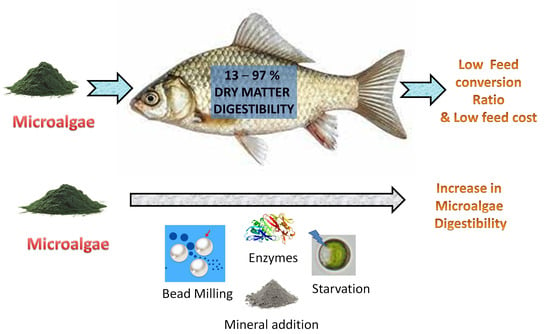
Nutrients and Energy Digestibility of Microalgal Biomass for Fish Feed Applications
Abstract
:1. Introduction
2. Factors Contributing to Digestion of Microalgae
3. Methods to Improve the Digestibility of Microalgae
4. Nutrient Digestibility
4.1. Dry Matter Digestibility
4.2. Protein and Amino Acid Digestibility
4.3. Lipid and Fatty Acid Digestibility
4.4. Carbohydrate Digestibility
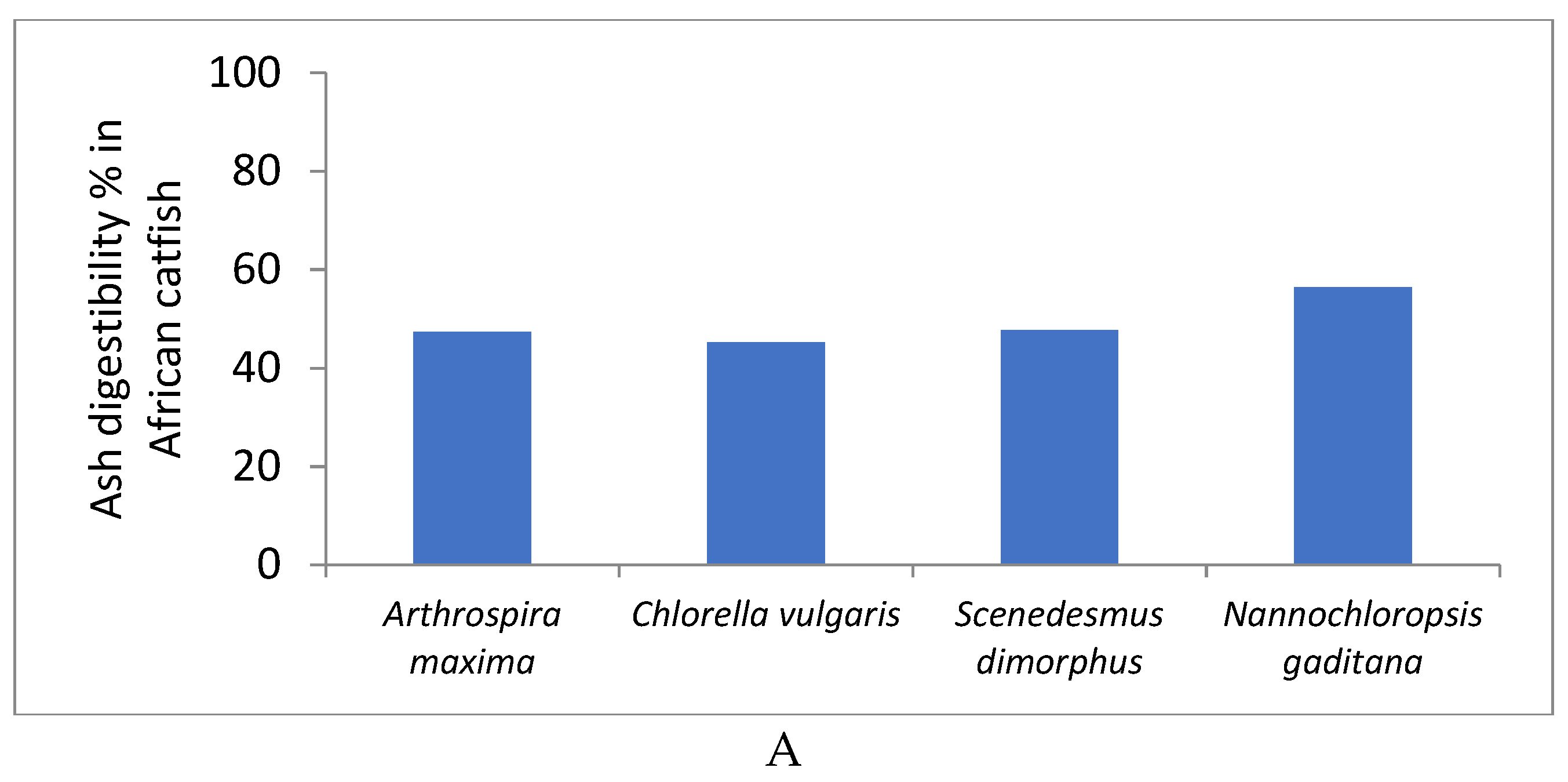
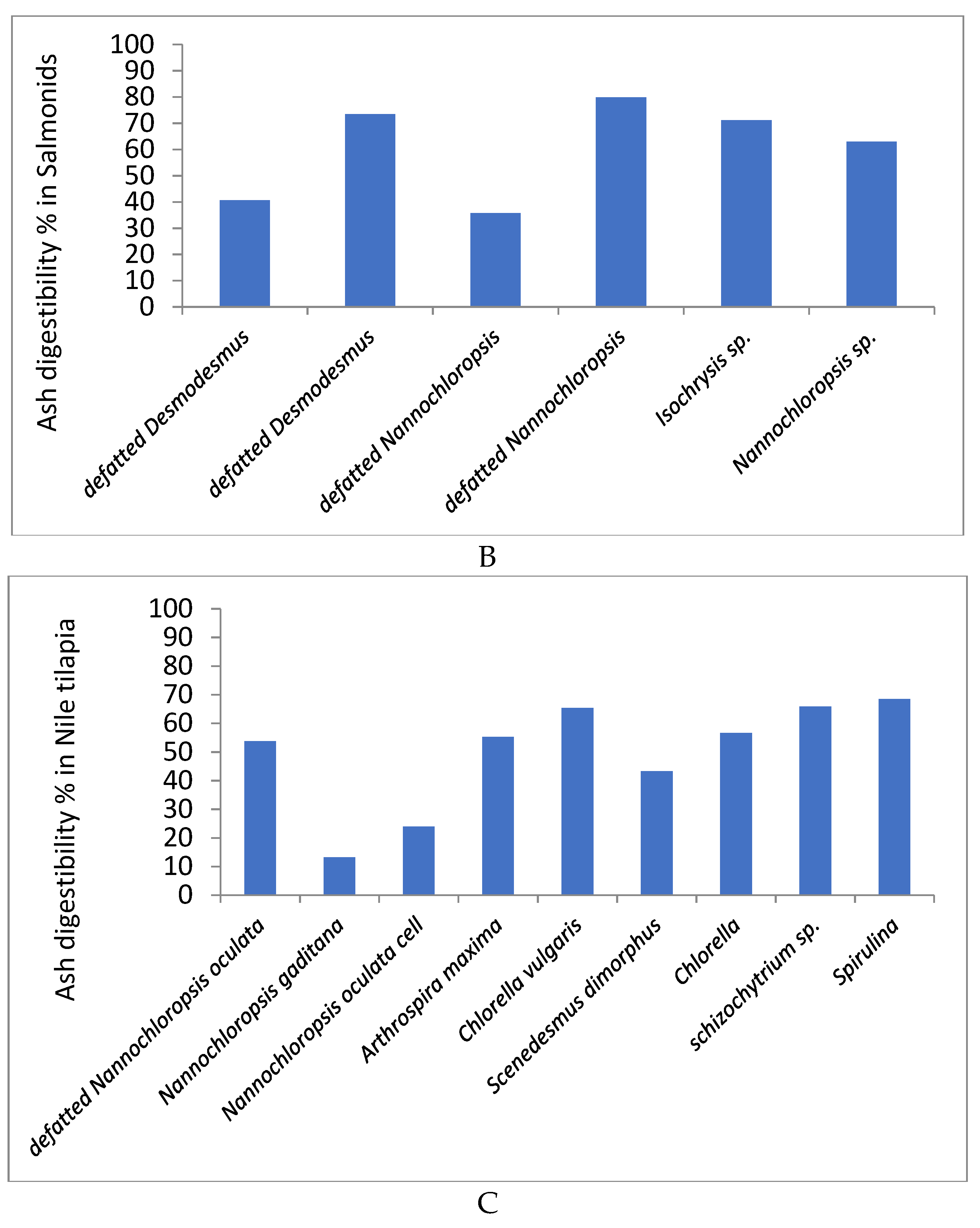
4.5. Ash (Mineral) Digestibility
5. Energy Digestibility
6. Digestibility of Individual Microalgal Species
7. Perspective and Future Direction
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, W. 21 Microalgae for Aquaculture. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Wiley: Hoboken, NJ, USA, 2004; p. 380. [Google Scholar]
- Fleurence, J.; Morançais, M.; Dumay, J.; Decottignies, P.; Turpin, V.; Munier, M.; Garcia-Bueno, N.; Jaouen, P. What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends Food Sci. Technol. 2012, 27, 57–61. [Google Scholar] [CrossRef]
- Nagappan, S.; Das, P.; AbdulQuadir, M.; Thaher, M.; Khan, S.; Mahata, C.; Al-Jabri, H.; Vatland, A.K.; Kumar, G. Potential of microalgae as a sustainable feed ingredient for aquaculture. J. Biotechnol. 2021, 341, 1–20. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Glencross, B.D.; Booth, M.; Allan, G.L. A feed is only as good as its ingredients–a review of ingredient evaluation strategies for aquaculture feeds. Aquac. Nutr. 2007, 13, 17–34. [Google Scholar] [CrossRef]
- Sarker, P.; Gamble, M.; Kelson, S.; Kapuscinski, A. Nile tilapia (Oreochromis niloticus) show high digestibility of lipid and fatty acids from marine Schizochytrium sp. and of protein and essential amino acids from freshwater Spirulina sp. feed ingredients. Aquac. Nutr. 2016, 22, 109–119. [Google Scholar] [CrossRef]
- Sarker, P.K.; Kapuscinski, A.R.; Lanois, A.J.; Livesey, E.D.; Bernhard, K.P.; Coley, M.L. Towards sustainable aquafeeds: Complete substitution of fish oil with marine microalga Schizochytrium sp. improves growth and fatty acid deposition in juvenile Nile tilapia (Oreochromis niloticus). PLoS ONE 2016, 11, e0156684. [Google Scholar]
- Cardinaletti, G.; Messina, M.; Bruno, M.; Tulli, F.; Poli, B.; Giorgi, G.; Chini-Zittelli, G.; Tredici, M.; Tibaldi, E. Effects of graded levels of a blend of Tisochrysis lutea and Tetraselmis suecica dried biomass on growth and muscle tissue composition of European sea bass (Dicentrarchus labrax) fed diets low in fish meal and oil. Aquaculture 2018, 485, 173–182. [Google Scholar] [CrossRef]
- Kiron, V.; Sørensen, M.; Huntley, M.; Vasanth, G.K.; Gong, Y.; Dahle, D.; Palihawadana, A.M. Defatted biomass of the microalga, Desmodesmus sp., can replace fishmeal in the feeds for Atlantic salmon. Front. Mar. Sci 2016, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.; Kaushik, S. Nutritional energetics in fish: Energy and protein utilization in rainbow trout (Salmo gairdneri). Asp. Food Prod. Consum. Energy Values 1990, 61, 132–172. [Google Scholar]
- Burr, G.; Barrows, F.; Gaylord, G.; Wolters, W. Apparent digestibility of macro-nutrients and phosphorus in plant-derived ingredients for Atlantic salmon, Salmo salar and Arctic charr, Salvelinus alpinus. Aquac. Nutr. 2011, 17, 570–577. [Google Scholar] [CrossRef]
- Sørensen, M.; Berge, G.M.; Reitan, K.I.; Ruyter, B. Microalga Phaeodactylum tricornutum in feed for Atlantic salmon (Salmo salar)—Effect on nutrient digestibility, growth and utilization of feed. Aquaculture 2016, 460, 116–123. [Google Scholar] [CrossRef]
- McGoogan, B.B.; Reigh, R.C. Apparent digestibility of selected ingredients in red drum (Sciaenops ocellatus) diets. Aquaculture 1996, 141, 233–244. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Reigh, R.C. Apparent digestibility of selected feedstuffs in diets for hybrid striped bass (Morone saxatilis♀ x Morone chrysops♂). Aquaculture 1995, 138, 313–322. [Google Scholar] [CrossRef]
- Wilson, R.P. Channel Catfish, Ictalurus punctatus. In Handbook of Nutrient Requirements of Finfish; CRC Press: Boca Raton, FL, USA, 2017; pp. 35–54. [Google Scholar]
- Schmitz, O.; Greuel, E.; Preffer, E. Digestibility of crude protein and organic matter of potential sources of dietary protein for eels (Anguilla anguilla, L.). Aquaculture 1984, 41, 21–30. [Google Scholar] [CrossRef]
- Teuling, E.; Schrama, J.W.; Gruppen, H.; Wierenga, P.A. Effect of cell wall characteristics on algae nutrient digestibility in Nile tilapia (Oreochromis niloticus) and African catfish (Clarus gariepinus). Aquaculture 2017, 479, 490–500. [Google Scholar] [CrossRef]
- Teuling, E.; Wierenga, P.A.; Agboola, J.O.; Gruppen, H.; Schrama, J.W. Cell wall disruption increases bioavailability of Nannochloropsis gaditana nutrients for juvenile Nile tilapia (Oreochromis niloticus). Aquaculture 2019, 499, 269–282. [Google Scholar] [CrossRef]
- Hossain, M.; Jauncey, K. Studies on the protein, energy and amino acid digestibility of fish meal, mustard oilcake, linseed and sesame meal for common carp (Cyprinus carpio L.). Aquaculture 1989, 83, 59–72. [Google Scholar] [CrossRef]
- Allan, G.L.; Parkinson, S.; Booth, M.A.; Stone, D.A.; Rowland, S.J.; Frances, J.; Warner-Smith, R. Replacement of fish meal in diets for Australian silver perch, Bidyanus bidyanus: I. Digestibility of alternative ingredients. Aquaculture 2000, 186, 293–310. [Google Scholar] [CrossRef]
- Gong, Y.; Guterres, H.; Huntley, M.; Sørensen, M.; Kiron, V. Digestibility of the defatted microalgae Nannochloropsis sp. and Desmodesmus sp. when fed to Atlantic salmon, Salmo salar. Aquac. Nutr. 2018, 24, 56–64. [Google Scholar] [CrossRef]
- Sørensen, M.; Gong, Y.; Bjarnason, F.; Vasanth, G.K.; Dahle, D.; Huntley, M.; Kiron, V. Nannochloropsis oceania-derived defatted meal as an alternative to fishmeal in Atlantic salmon feeds. PLoS ONE 2017, 12, e0179907. [Google Scholar] [CrossRef]
- Vizcaíno, A.; López, G.; Sáez, M.; Jiménez, J.; Barros, A.; Hidalgo, L.; Camacho-Rodríguez, J.; Martínez, T.; Cerón-García, M.; Alarcón, F. Effects of the microalga Scenedesmus almeriensis as fishmeal alternative in diets for gilthead sea bream, Sparus aurata, juveniles. Aquaculture 2014, 431, 34–43. [Google Scholar] [CrossRef]
- Niccolai, A.; Zittelli, G.C.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef] [Green Version]
- Palinska, K.A.; Krumbein, W.E. Perforation patterns in the peptidoglycan wall of filamentous cyanobacteria. J. Phycol. 2000, 36, 139–145. [Google Scholar] [CrossRef]
- Marshall, R.; McKinley, S.; Pearce, C.M. Effects of nutrition on larval growth and survival in bivalves. Rev. Aquac. 2010, 2, 33–55. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Skrede, A.; Mydland, L.; Ahlstrøm, Ø.; Reitan, K.; Gislerød, H.; Øverland, M. Evaluation of microalgae as sources of digestible nutrients for monogastric animals. Anim. Feed Sci. Technol. 2011, 20, 131–142. [Google Scholar] [CrossRef]
- Nuño, K.; Villarruel-López, A.; Puebla-Pérez, A.; Romero-Velarde, E.; Puebla-Mora, A.; Ascencio, F. Effects of the marine microalgae Isochrysis galbana and Nannochloropsis oculata in diabetic rats. J. Funct. Foods 2013, 5, 106–115. [Google Scholar] [CrossRef]
- Rodehutscord, M.; Borchert, F.; Gregus, Z.; Pfeffer, E. Availability and utilisation of free lysine in rainbow trout (Oncorhynchus mykiss): 2. Comparison of l-lysine· HCl and l-lysine sulphate. Aquaculture 2000, 187, 177–183. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Custódio, M.; Batista, S.; Fernandes, H.; Kiron, V. Defatted microalgae (Nannochloropsis sp.) from biorefinery as a potential feed protein source to replace fishmeal in European sea bass diets. Fish Physiol. Biochem. 2019, 45, 1067–1081. [Google Scholar] [CrossRef]
- Bitou, N.; Ninomiya, M.; Tsujita, T.; Okuda, H. Screening of lipase inhibitors from marine algae. Lipids 1999, 34, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Batista, S.; Pintado, M.; Marques, A.; Abreu, H.; Silva, J.L.; Jessen, F.; Tulli, F.; Valente, L.M. Use of technological processing of seaweed and microalgae as strategy to improve their apparent digestibility coefficients in European seabass (Dicentrarchus labrax) juveniles. J. Appl. Phycol. 2020, 32, 3429–3446. [Google Scholar] [CrossRef]
- Sarker, P.K.; Kapuscinski, A.R.; Vandenberg, G.W.; Proulx, E.; Sitek, A.J.; Thomsen, L. Towards sustainable and ocean-friendly aquafeeds: Evaluating a fish-free feed for rainbow trout (Oncorhynchus mykiss) using three marine microalgae species. Elem. Sci. Anthrop. 2020, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Sinha, A.K.; Kumar, V.; Makkar, H.P.; De Boeck, G.; Becker, K. Non-starch polysaccharides and their role in fish nutrition–A review. Food Chem. 2011, 127, 1409–1426. [Google Scholar] [CrossRef]
- Norambuena, F.; Hermon, K.; Skrzypczyk, V.; Emery, J.A.; Sharon, Y.; Beard, A.; Turchini, G.M. Algae in fish feed: Performances and fatty acid metabolism in juvenile Atlantic salmon. PLoS ONE 2015, 10, e0124042. [Google Scholar] [CrossRef] [Green Version]
- Sarker, P.K.; Kapuscinski, A.R.; Bae, A.Y.; Donaldson, E.; Sitek, A.J.; Fitzgerald, D.S.; Edelson, O.F. Towards sustainable aquafeeds: Evaluating substitution of fishmeal with lipid-extracted microalgal co-product (Nannochloropsis oculata) in diets of juvenile Nile tilapia (Oreochromis niloticus). PLoS ONE 2018, 13, e0201315. [Google Scholar] [CrossRef] [PubMed]
- Domozych, D.; Ciancia, M.; Fangel, J.U.; Mikkelsen, M.D.; Ulvskov, P.; Willats, W.G. The cell walls of green algae: A journey through evolution and diversity. Front. Plant Sci. 2012, 3, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karapanagiotidis, I.T.; Bell, M.V.; Little, D.C.; Yakupitiyage, A. Replacement of dietary fish oils by alpha-linolenic acid-rich oils lowers omega 3 content in tilapia flesh. Lipids 2007, 42, 547–559. [Google Scholar] [CrossRef]
- Wee, K. Aquaculture Nutrition Research in Australia. In Proceedings of the Aquaculture Nutrition Workshop Salamander Bay, New South Wales, Australia, 15–17 April 1991. [Google Scholar]
- Falge, R.; Schpanof, L.; Jurss, K. Amylase, esterase and protease activity in the intestine content of rainbow trout Salmo gairdneri Rich., after feeding with feed containing different amounts of starch and protein. J. Ichthyol. 1978, 18, 283–287. [Google Scholar]
- Becker, W. 18 Microalgae In Human and Animal Nutrition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Wiley Online Library: Hoboken, NJ, USA, 2004; Volume 312. [Google Scholar]
- Encarnação, P.; de Lange, C.; Rodehutscord, M.; Hoehler, D.; Bureau, W.; Bureau, D.P. Diet digestible energy content affects lysine utilization, but not dietary lysine requirements of rainbow trout (Oncorhynchus mykiss) for maximum growth. Aquaculture 2004, 235, 569–586. [Google Scholar] [CrossRef]
- Mišurcová, L. Seaweeds digestibility and Methods Used for Digestibility Determination. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2012. [Google Scholar]
- Duval, B.; Shetty, K.; Thomas, W.H. Phenolic compounds and antioxidant properties in the snow alga Chlamydomonas nivalis after exposure to UV light. J. Appl. Phycol. 1999, 11, 559–566. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Li, H.-B.; Cheng, K.-W.; Wong, C.-C.; Fan, K.-W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Carpenter, K.; Booth, V. In Damage to lysine in food processing: Its measurement and its significance. Nutr. Abstr. Rev. 1973, 43, 423–451. [Google Scholar]
- Opstvedt, J.; Miller, R.; Hardy, R.W.; Spinelli, J. Heat-induced changes in sulfhydryl groups and disulfide bonds in fish protein and their effect on protein and amino acid digestibility in rainbow trout (Salmo gairdneri). J. Agric. Food Chem. 1984, 32, 929–935. [Google Scholar] [CrossRef]
- Evans, D.; Claiborne, J. The Physiology of Fishes; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Shi, Z.; Li, X.-Q.; Chowdhury, M.K.; Chen, J.-N.; Leng, X.-J. Effects of protease supplementation in low fish meal pelleted and extruded diets on growth, nutrient retention and digestibility of gibel carp, Carassius auratus gibelio. Aquaculture 2016, 460, 37–44. [Google Scholar] [CrossRef]
- Guedes, A.C.; Sousa-Pinto, I.; Malcata, F.X. Application of Microalgae Protein to Aquafeed. In Handbook of Marine Microalgae; Elsevier: Amsterdam, The Netherlands, 2015; pp. 93–125. [Google Scholar]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Berge, G.; Hatlen, B.; Odom, J.; Ruyter, B. Physical treatment of high EPA Yarrowia lipolytica biomass increases the availability of n-3 highly unsaturated fatty acids when fed to Atlantic salmon. Aquac. Nutr. 2013, 19, 110–121. [Google Scholar] [CrossRef]
- Gong, Y.; Bandara, T.; Huntley, M.; Johnson, Z.I.; Dias, J.; Dahle, D.; Sørensen, M.; Kiron, V. Microalgae Scenedesmus sp. as a potential ingredient in low fishmeal diets for Atlantic salmon (Salmo salar L.). Aquaculture 2019, 501, 455–464. [Google Scholar] [CrossRef]
- Shene, C.; Monsalve, M.T.; Vergara, D.; Lienqueo, M.E.; Rubilar, M. High pressure homogenization of Nannochloropsis oculata for the extraction of intracellular components: Effect of process conditions and culture age. Eur. J. Lipid Sci. Technol. 2016, 118, 631–639. [Google Scholar] [CrossRef]
- Maehre, H.K.; Edvinsen, G.K.; Eilertsen, K.-E.; Elvevoll, E.O. Heat treatment increases the protein bioaccessibility in the red seaweed dulse (Palmaria palmata), but not in the brown seaweed winged kelp (Alaria esculenta). J. Appl. Phycol. 2016, 28, 581–590. [Google Scholar] [CrossRef] [Green Version]
- McMillan, J.R.; Watson, I.A.; Ali, M.; Jaafar, W. Evaluation and comparison of algal cell disruption methods: Microwave, waterbath, blender, ultrasonic and laser treatment. Appl. Energy 2013, 103, 128–134. [Google Scholar] [CrossRef]
- Kousoulaki, K.; Mørkøre, T.; Nengas, I.; Berge, R.; Sweetman, J. Microalgae and organic minerals enhance lipid retention efficiency and fillet quality in Atlantic salmon (Salmo salar L.). Aquaculture 2016, 451, 47–57. [Google Scholar] [CrossRef]
- MišurCoVá, L.; KráčMar, S.; KLeJduS, B.; VaCeK, J. Nitrogen content, dietary fiber, and digestibility in algal food products. Czech J. Food Sci. 2010, 28, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Douglas, M.W.; Parsons, C.M.; Bedford, M.R. Effect of various soybean meal sources and Avizyme on chick growth performance and ileal digestible energy. J. Appl. Poult. Res. 2000, 9, 74–80. [Google Scholar] [CrossRef]
- Cowieson, A.; Ravindran, V.; Selle, P. Influence of dietary phytic acid and source of microbial phytase on ileal endogenous amino acid flows in broiler chickens. Poult. Sci. 2008, 87, 2287–2299. [Google Scholar] [CrossRef]
- Fu, C.C.; Hung, T.C.; Chen, J.Y.; Su, C.H.; Wu, W.T. Hydrolysis of microalgae cell walls for production of reducing sugar and lipid extraction. Bioresour. Technol. 2010, 101, 8750–8754. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, A.; Sarker, P.K.; Bureau, D.P.; Chouinard, Y.; Vandenberg, G.W. Apparent digestibility of macronutrients and fatty Acids from microalgae (Schizochytrium sp.) fed to rainbow trout (Oncorhynchus mykiss): A potential candidate for fish oil substitution. Animals 2021, 11, 456. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Chowdhury, M.K.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Yasumaru, F.; Lemos, D. In vitro prediction of digestible protein content of marine microalgae (Nannochloropsis granulata) meals for Pacific white shrimp (Litopenaeus vannamei) and rainbow trout (Oncorhynchus mykiss). Algal Res. 2017, 21, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Agboola, J.O.; Teuling, E.; Wierenga, P.A.; Gruppen, H.; Schrama, J.W. Cell wall disruption: An effective strategy to improve the nutritive quality of microalgae in African catfish (Clarias gariepinus). Aquac. Nutr. 2019, 25, 783–797. [Google Scholar] [CrossRef] [Green Version]
- Nagappan, S.; Devendran, S.; Tsai, P.-C.; Jayaraman, H.; Alagarsamy, V.; Pugazhendhi, A.; Ponnusamy, V.K. Metabolomics integrated with transcriptomics and proteomics: Evaluation of systems reaction to nitrogen deficiency stress in microalgae. Process Biochem. 2020, 91, 1–14. [Google Scholar] [CrossRef]
- Wilson, R.P.; John, E.H. Protein and amino acid requirements of fishes. Annu. Rev. Nutr. 1986, 6, 225–244. [Google Scholar] [CrossRef]
- Guimarães, I.; Pezzato, L.E.; Barros, M.M. Amino acid availability and protein digestibility of several protein sources for Nile tilapia, Oreochromis niloticus. Aquac. Nutr. 2008, 14, 396–404. [Google Scholar] [CrossRef]
- Reitan, K.I.; Rainuzzo, J.R.; Olsen, Y. Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J. Phycol. 1994, 30, 972–979. [Google Scholar] [CrossRef]
- Sørensen, M.; Penn, M.; El-Mowafi, A.; Storebakken, T.; Chunfang, C.; Øverland, M.; Krogdahl, Å. Effect of stachyose, raffinose and soya-saponins supplementation on nutrient digestibility, digestive enzymes, gut morphology and growth performance in Atlantic salmon (Salmo salar, L). Aquaculture 2011, 314, 145–152. [Google Scholar] [CrossRef]
- Refstie, S.; Storebakken, T.; Roem, A.J. Feed consumption and conversion in Atlantic salmon (Salmo salar) fed diets with fish meal, extracted soybean meal or soybean meal with reduced content of oligosaccharides, trypsin inhibitors, lectins and soya antigens. Aquaculture 1998, 162, 301–312. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Sundby, A.; Olli, J.J. Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) digest and metabolize nutrients differently. Effects of water salinity and dietary starch level. Aquaculture 2004, 229, 335–360. [Google Scholar] [CrossRef]
- Grisdale-Helland, B.; Helland, S. Replacement of protein by fat and carbohydrate in diets for Atlantic salmon (Salmo salar) at the end of the freshwater stage. Aquaculture 1997, 152, 167–180. [Google Scholar] [CrossRef]
- Pereira, R.; Valente, L.M.; Sousa-Pinto, I.; Rema, P. Apparent nutrient digestibility of seaweeds by rainbow trout (Oncorhynchus mykiss) and Nile tilapia (Oreochromis niloticus). Algal Res. 2012, 1, 77–82. [Google Scholar] [CrossRef]
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Bobin-Dubigeon, C.; Hoebler, C.; Lognone, V.; Dagorn-Scaviner, C.; Mabeau, S. Chemical composition, physico-chemical properties, enzymatic inhibition and fermentative characteristics of dietary fibres from edible seaweeds. Sci. Aliment. 1997, 17, 619–639. [Google Scholar]
- Wong, K.H.; Cheung, P.C. Nutritional evaluation of some subtropical red and green seaweeds Part II. In vitro protein digestibility and amino acid profiles of protein concentrates. Food Chem. 2001, 72, 11–17. [Google Scholar] [CrossRef]
- Horie, Y.; Sugase, K.; Horie, K. Physiological differences of soluble and insoluble dietary fibre fractions of brown algae and mushrooms in pepsin activity in vitro and protein digestibility. Asia Pac. J. Clin. Nutr. 1995, 4, 251–255. [Google Scholar]
- Marrion, O.; Fleurence, J.; Schwertz, A.; Guéant, J.-L.; Mamelouk, L.; Ksouri, J.; Villaume, C. Evaluation of protein in vitro digestibility of Palmaria palmata and Gracilaria verrucosa. J. Appl. Phycol. 2005, 17, 99–102. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Mann, J.; Dumas, A. Apparent digestibility of nutrients, energy, essential amino acids and fatty acids of juvenile Atlantic salmon (Salmo salar L.) diets containing whole-cell or cell-ruptured Chlorella vulgaris meals at five dietary inclusion levels. Aquaculture 2017, 481, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Devi, M.A.; Subbulakshmi, G.; Devi, K.M.; Venkataraman, L.V. Studies on the proteins of mass-cultivated, blue-green alga (Spirulina platensis). J. Agric. Food Chem. 1981, 29, 522–525. [Google Scholar] [CrossRef]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. The lipids. In Fish Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 181–257. [Google Scholar]
- Cho, C.; Slinger, S.; Bayley, H. Bioenergetics of salmonid fishes: Energy intake, expenditure and productivity. Comp. Biochem. Physiol. 1982, 73B, 25–41. [Google Scholar] [CrossRef]
- Sigurgisladottir, S.; Lall, S.P.; Parrish, C.C.; Ackman, R.G. Cholestane as a digestibility marker in the absorption of polyunsaturated fatty acid ethyl esters in Atlantic salmon. Lipids 1992, 27, 418–424. [Google Scholar] [CrossRef]
- Austreng, E.; Skrede, A.; Eldegard, Å. Digestibility of fat and fatty acids in rainbow trout and mink. Aquaculture 1980, 19, 93–95. [Google Scholar] [CrossRef]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Merican, Z.O.; Shim, K. Apparent digestibility of lipid and fatty acids in residual lipids of meals by adult Penaeus monodon. Aquaculture 1995, 133, 275–286. [Google Scholar] [CrossRef]
- Allen, K.M.; Habte-Tsion, H.-M.; Thompson, K.R.; Filer, K.; Tidwell, J.H.; Kumar, V. Freshwater microalgae (Schizochytrium sp.) as a substitute to fish oil for shrimp feed. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Johnsen, R.; Grahl-Nielsen, O.; Roem, A. Relative absorption of fatty acids by Atlantic salmon Salmo salar from different diets, as evaluated by multivariate statistics. Aquac. Nutr. 2000, 6, 255–261. [Google Scholar] [CrossRef]
- Ng, W.-K.; Codabaccus, B.M.; Carter, C.G.; Nichols, P.D. Replacing dietary fish oil with palm fatty acid distillate improves fatty acid digestibility in rainbow trout, Oncorhynchus mykiss, maintained at optimal or elevated water temperature. Aquaculture 2010, 309, 165–172. [Google Scholar] [CrossRef]
- Ng, W.K.; Campbell, P.J.; Dick, J.R.; Bell, J.G. Interactive effects of dietary palm oil concentration and water temperature on lipid digestibility in rainbow trout, Oncorhynchus mykiss. Lipids 2003, 38, 1031–1038. [Google Scholar] [CrossRef]
- Caballero, M.; Obach, A.; Rosenlund, G.; Montero, D.; Gisvold, M.; Izquierdo, M. Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture 2002, 214, 253–271. [Google Scholar] [CrossRef]
- Hansen, J.Ø.; Berge, G.M.; Hillestad, M.; Krogdahl, Å.; Galloway, T.F.; Holm, H.; Holm, J.; Ruyter, B. Apparent digestion and apparent retention of lipid and fatty acids in Atlantic cod (Gadus morhua) fed increasing dietary lipid levels. Aquaculture 2008, 284, 159–166. [Google Scholar] [CrossRef]
- Lech, G.P.; Reigh, R.C. Plant products affect growth and digestive efficiency of cultured Florida pompano (Trachinotus carolinus) fed compounded diets. PLoS ONE 2012, 7, e34981. [Google Scholar] [CrossRef]
- Roy, S.S.; Pal, R. Microalgae in aquaculture: A review with special references to nutritional value and fish dietetics. In Proceedings of the Zoological Society; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–8. [Google Scholar]
- Moheimani, N.R.; Vadiveloo, A.; Ayre, J.M.; Pluske, J.R. Nutritional profile and in vitro digestibility of microalgae grown in anaerobically digested piggery effluent. Algal Res. 2018, 35, 362–369. [Google Scholar] [CrossRef]
- Thodesen, J.; Storebakken, T.; Shearer, K.D.; Rye, M.; Bjerkeng, B.; Gjerde, B. Genetic variation in mineral absorption of large Atlantic salmon (Salmo salar) reared in seawater. Aquaculture 2001, 194, 263–271. [Google Scholar] [CrossRef]
- Erpel, F.; Restovic, F.; Arce-Johnson, P. Development of phytase-expressing Chlamydomonas reinhardtii for monogastric animal nutrition. BMC Biotechnol. 2016, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.; Yúfera, M.; Valente, L.M.; Rema, P. Feed transit and apparent protein, phosphorus and energy digestibility of practical feed ingredients by Senegalese sole (Solea senegalensis). Aquaculture 2010, 302, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, S. Phosphorus requirements and intake in fish. INRA Prod. Anim. 2005, 18, 203–208. [Google Scholar] [CrossRef]
- Oliva-Teles, A. Apparent digestibility coefficients of feedstuffs in seabass (Dicentrarchus labrax) juveniles. Aquat. Living Resour. 1998, 11, 187–191. [Google Scholar]
- Pereira, H.; Sardinha, M.; Santos, T.; Gouveia, L.; Barreira, L.; Dias, J.; Varela, J. Incorporation of defatted microalgal biomass (Tetraselmis sp. CTP4) at the expense of soybean meal as a feed ingredient for juvenile gilthead seabream (Sparus aurata). Algal Res. 2020, 47, 101869. [Google Scholar] [CrossRef]
- Feng, W.; Zhu, Y.; Wu, F.; He, Z.; Zhang, C.; Giesy, J.P. Forms and lability of phosphorus in algae and aquatic macrophytes characterized by solution 31 P NMR coupled with enzymatic hydrolysis. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Mukherjee, C.; Chowdhury, R.; Ray, K. Phosphorus recycling from an unexplored source by polyphosphate accumulating microalgae and cyanobacteria—A step to phosphorus security in agriculture. Front. Microbiol. 2015, 6, 1421. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, S.; Coves, D.; Dutto, G.; Blanc, D. Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture 2004, 230, 391–404. [Google Scholar] [CrossRef]
- Bureau, D.; Harris, A.; Cho, C. Apparent digestibility of rendered animal protein ingredients for rainbow trout (Oncorhynchus mykiss). Aquaculture 1999, 180, 345–358. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Shields, R.; Lupatsch, I. 5 Algae for Aquaculture and Animal Feeds. In Microalgal Biotechnology: Integration and Economy; De Gruyter: Berlin, Germany, 2012; pp. 79–100. [Google Scholar]
| Microalgae | Biomass Processing/ Pre-Treatment | Aqua Species | Pellet | Dry Atter (%) | Protein (%) | Lipid (%) | Carbohydrate (%) | Energy (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Chlorella sp. | _ | Nile tilapia (Oreochromis niloticus) | Meat grinder | 73.4 | 80 | 94.4 | _ | 83.9 | [6] |
| Chlorella vulgaris | _ | African catfish (Clarias gariepinus) | Extruded into sinking pellet | 70.7 | 80.7 | 78.1 | 84.6 | 71.6 | [17] |
| Chlorella vulgaris | _ | Atlantic salmon (Salmo salar L.) | Steam pelleted | _ | 79.5 | 69.9 | 45 | 59.6 | [83] |
| Chlorella vulgaris | High pressure homogenization | Atlantic salmon (Salmo salar L.) | Steam pelleted | _ | 85.4 | 82.1 | 82.7 | 76.5 | [83] |
| Chlorella vulgaris | _ | European seabass (Dicentrarchus labrax) | Twin-screw extruder (Clextral BC 45) | 41.2 | 85.5 | 84.9 | _ | 81.5 | [34] |
| Chlorella vulgaris | Bead milling | European seabass (Dicentrarchus labrax) | Twin-screw extruder (Clextral BC 45) | 63.4 | 88.6 | 81.2 | _ | 90.4 | [34] |
| Chlorella vulgaris | Enzymatic processing | European seabass (Dicentrarchus labrax) | Twin-screw extruder (Clextral BC 45) | 63.4 | 87.6 | 78.4 | _ | 90.6 | [34] |
| Chlorella vulgaris | _ | Nile tilapia (Oreochromis niloticus) | Extruded into sinking pellet | 73.7 | 80.9 | 84.3 | 70.4 | 73.9 | [17] |
| Desmodesmus sp. | Defatting | Atlantic salmon (Salmo salar) | Cold pelleted | 31.8 | 54.1 | _ | _ | _ | [21] |
| Desmodesmus sp. | Defatting | Atlantic salmon (Salmo salar) | Twin-screw cooking extruder | 46.9 | 67.1 | _ | _ | 50.9 | [21] |
| Isochrysis sp. | _ | Rainbow trout (Oncorhynchus mykiss) | Steam-pelleted | 77.1 | 86.5 | 62.8 | _ | 72.6 | [35] |
| Nannochloropsis gaditana | _ | African catfish (Clarias gariepinus) | Extruded into sinking pellet | 61.1 | 72.4 | 65.1 | 46.9 | 59.5 | [17] |
| Nannochloropsis gaditana | _ | African catfish (Clarias gariepinus) | Extruded into sinking pellet | 48.3 | 59.3 | 40.3 | 31.7 | 46.6 | [68] |
| Nannochloropsis gaditana | Bead milling | African catfish (Clarias gariepinus) | Extruded into sinking pellet | 63.7 | 75.6 | 76.8 | 34.9 | 63.5 | [68] |
| Nannochloropsis gaditana | Commercial processing (Nutrispring® Liquid 40) | African catfish (Clarias gariepinus) | Extruded into sinking pellet | 60.3 | 67.7 | 47.2 | 45.4 | 53 | [68] |
| Nannochloropsis gaditana | Freeze-drying | African catfish (Clarias gariepinus) | Extruded into sinking pellet | 47 | 59.8 | 49.9 | 43.3 | 46.7 | [68] |
| Nannochloropsis gaditana | Frozen thawing | African catfish (Clarias gariepinus) | Extruded into sinking pellet | 50.2 | 65.2 | 41.2 | 28.1 | 48.8 | [68] |
| Nannochloropsis gaditana | Pasteurization | African catfish (Clarias gariepinus) | Extruded into sinking pellet | 45.2 | 55.5 | 44 | 48.7 | 43.7 | [68] |
| Nannochloropsis gaditana | _ | Nile tilapia (Oreochromis niloticus) | Extruded into sinking pellet | 66.9 | 74.7 | 74.5 | 21.6 | 65.1 | [17] |
| Nannochloropsis gaditana | _ | Nile tilapia (Oreochromis niloticus) | Twin-screw extruder (Clextral) into sinking pellets | 48.4 | 61.5 | 50.4 | 34.9 | 51 | [18] |
| Nannochloropsis gaditana | Bead-milling | Nile tilapia (Oreochromis niloticus) | Twin-screw extruder (Clextral) into sinking pellets | 66.3 | 78 | 82 | 56.7 | 69.2 | [18] |
| Nannochloropsis gaditana | Commercially processed (nutrispring® Liquid 40) | Nile tilapia (Oreochromis niloticus) | Twin-screw extruder (Clextral) into sinking pellets | 61.2 | 72.9 | 66.4 | 46.6 | 60.6 | [18] |
| Nannochloropsis gaditana | Freeze-dried | Nile tilapia (Oreochromis niloticus) | Twin-screw extruder (Clextral) into sinking pellets | 50.6 | 60.6 | 57.8 | 38.5 | 53.1 | [18] |
| Nannochloropsis gaditana | Frozen thawed | Nile tilapia (Oreochromis niloticus) | Twin-screw extruder (Clextral) into sinking pellets | 55.2 | 66.2 | 53 | 40.5 | 57.1 | [18] |
| Nannochloropsis gaditana | Pasteurized | Nile tilapia (Oreochromis niloticus) | Twin-screw extruder (Clextral) into sinking pellets | 50.2 | 60.7 | 56.1 | 38 | 53.1 | [18] |
| Nannochloropsis oceanica | _ | European seabass (Dicentrarchus labrax) | Twin-screw extruder (Clextral BC 45) | 32 | 81.6 | 63.1 | _ | 76.2 | [34] |
| Nannochloropsis oceanica | Bead milling | European seabass (Dicentrarchus labrax) | Twin-screw extruder (Clextral BC 45) | 53.6 | 81 | 56.1 | _ | 76.6 | [34] |
| Nannochloropsis oceanica | Enzymatic processing | European seabass (Dicentrarchus labrax) | Twin-screw extruder (Clextral BC 45) | 59.4 | 87.9 | 63.8 | _ | 87 | [34] |
| Nannochloropsis oculata | Defatting | Nile tilapia (Oreochromis niloticus) | Meat grinder | _ | 73.5 | 60.6 | _ | 72.8 | [38] |
| Nannochloropsis oculata | _ | Nile tilapia (Oreochromis niloticus) | Meat grinder | _ | 81.1 | 64.2 | _ | 80 | [38] |
| Nannochloropsis sp. | Defatting | Atlantic salmon (Salmo salar) | Twin-screw cooking extruder | 63.1 | 72.4 | _ | _ | 60.5 | [21] |
| Nannochloropsis sp. | Defatting | Atlantic salmon (Salmo salar) | Cold pelleted | 47.9 | 72.9 | _ | _ | _ | [21] |
| Nannochloropsis sp. | Defatting | European seabass (Dicentrarchus labrax) | Dry pelleted at 50°C using pellet press | _ | 85.4 | _ | _ | 68 | [32] |
| Nannochloropsis sp. | _ | Rainbow trout (Oncorhynchus mykiss) | Steam-pelleted | 56.7 | 69.3 | 60.1 | _ | 62.1 | [35] |
| Scenedesmus dimorphus | _ | African catfish (Clarias gariepinus) | Extruded into sinking pellet | 58.2 | 68.3 | 68.3 | 62.3 | 61.4 | [17] |
| Scenedesmus dimorphus | _ | Nile tilapia (Oreochromis niloticus) | Extruded into sinking pellet | 55.8 | 67 | 65.1 | 56.9 | 58.5 | [17] |
| Schizochytrium sp. | _ | Nile tilapia (Oreochromis niloticus) | Meat grinder | 81.8 | 81.7 | 97.9 | _ | 86.5 | [6] |
| Schizochytrium sp. | _ | Rainbow trout (Oncorhynchus mykiss) | California Pellet Mill (model CPM CL-5) | 90.8 | 90.8 | 85.9 | _ | 84.3 | [65] |
| Schizochytrium sp. | _ | Rainbow trout (Oncorhynchus mykiss) | California Pellet Mill (model CPM CL-5) | 97.8 | 88.2 | 85.8 | _ | 81.9 | [65] |
| Spirulina maxima | _ | African catfish (Clarias gariepinus) | Extruded into sinking pellet | 73.1 | 81.4 | 89.1 | 66.3 | 75.3 | [17] |
| Spirulina maxima | _ | Nile tilapia (Oreochromis niloticus) | Extruded into sinking pellet | 74.7 | 82.5 | 82.4 | 68.2 | 75.8 | [17] |
| Spirulina sp. | _ | Nile tilapia (Oreochromis niloticus) | Meat grinder | 79.7 | 86.1 | 94.5 | _ | 86.3 | [6] |
| Tetraselmis sp. | _ | European seabass (Dicentrarchus labrax) | Twin-screw extruder (Clextral BC 45) | −19.1 | 69.7 | −92.4 | _ | 48.9 | [34] |
| Tetraselmis sp. | Bead milling | European seabass (Dicentrarchus labrax) | Twin-screw extruder (Clextral BC 45) | 51.4 | 83.6 | −101.2 | _ | 81.1 | [34] |
| Tetraselmis sp. | Enzymatic processed | European seabass (Dicentrarchus labrax) | Twin-screw extruder (Clextral BC 45) | 12.5 | 73.7 | −795.0 | _ | 68.3 | [34] |
| Microalgae | Biomass Processing/Pre-Treatment | In Vitro Conditions | Dry Matter (%) | Protein (%) | Carbohydrate (%) | Reference |
|---|---|---|---|---|---|---|
| Chlorella pyrenoidosa | _ | In vitro (pepsin-pancreatic system) | _ | 75.3 | _ | [61] |
| Chlorella sorokiniana F&M-M49 | _ | In vitro (pepsin-pancreatic system) | 55 | 50 | 60 | [24] |
| Chlorella sorokiniana IAM C-212 | _ | In vitro (pepsin-pancreatic system) | 72 | 70 | 72 | [24] |
| Chlorella vulgaris Allma | _ | In vitro (pepsin-pancreatic system) | 70 | 75 | 70 | [24] |
| Klamath | _ | In vitro (pepsin-pancreatic system) | 68 | 70 | 70 | [24] |
| Nannochloropsis granulata | _ | In vitro pH-Stat using pyloric caeca enzyme extract of rainbow trout | _ | 79.1 | _ | [67] |
| Nannochloropsis granulata | Super Critical Fluid 70 °C extracted cell | In vitro pH-Stat using pyloric caeca enzyme extract of rainbow trout | _ | 86.2 | _ | [67] |
| Nannochloropsis granulata | Super Critical Fluid 90 °C extracted cell | In vitro pH-Stat using pyloric caeca enzyme extract of rainbow trout | _ | 87.9 | _ | [67] |
| Nannochloropsis oceanica F&M-M24 | _ | In vitro (pepsin-pancreatic system) | 55 | 50 | 60 | [24] |
| Nannochloropsis sphaeroides F&M-C117 | _ | In vitro (pepsin-pancreatic system) | 65 | 80 | 68 | [24] |
| Porphyridium purpureum F&M-M46 | _ | In vitro (pepsin-pancreatic system) | 48 | 70 | 50 | [24] |
| Phaeodactylum tricornutum F&M-M40 | _ | In vitro (pepsin-pancreatic system) | 50 | 70 | 55 | [24] |
| Spirulina pacifica | _ | In vitro (pepsin-pancreatic system) | _ | 85.6 | _ | [61] |
| Spirulina platensis | _ | In vitro (pepsin-pancreatic system) | _ | 85 | _ | [84] |
| Spirulina platensis | _ | In vitro (pepsin-pancreatic system) | _ | 94.3 | _ | [61] |
| Spirulina platensis F&M-C256 | _ | In vitro (pepsin-pancreatic system) | 78 | 80 | 80 | [24] |
| Tisochrysis lutea F&M-M36 | _ | In vitro (pepsin-pancreatic system) | 65 | 60 | 65 | [24] |
| Tetraselmis suecica F&M-M33 | _ | In vitro (pepsin-pancreatic system) | 50 | 65 | 55 | [24] |
| Tetraselmis suecica F&M-M33 | Nutrient starvation of cell | In vitro (pepsin-pancreatic system) | 55 | 70 | 58 | [24] |
| Microalgae | Biomass ProCessing/Pre-Treatment | Aqua Species | Arginine (%) | Histidine (%) | Lysine (%) | Threonine (%) | Isoleucine (%) | Leucine (%) | Valine (%) | Methionine (%) | Phenylalanine (%) | Tryptophan (%) | Refrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorella vulgaris | _ | Atlantic salmon (Salmo salar L.) | 83.3 | 77.9 | 97.3 | 73.3 | 79.4 | 79.2 | 78 | 83.4 | 81.8 | 90 | [83] |
| Chlorella vulgaris | High pressure homogenization | Atlantic salmon (Salmo salar L.) | 94.6 | 93.1 | 92.7 | 91.5 | 90.5 | 92.4 | 92.2 | 89.6 | 89.2 | 68.8 | [83] |
| Chlorella vulgaris | _ | European seabass (Dicentrarchus labrax) | 93.2 | 74.4 | 73.8 | 90.3 | 83.4 | 89.1 | 86.7 | 97.1 | 88.7 | _ | [34] |
| Chlorella vulgaris | Bead milling | European seabass (Dicentrarchus labrax) | 92.2 | 88.2 | 71.7 | 92.3 | 91.9 | 92.6 | 92.7 | 95.8 | 92.3 | _ | [34] |
| Chlorella vulgaris | Enzymatic processed | European seabass (Dicentrarchus labrax) | 92.8 | 50.3 | 73.8 | 89 | 89.7 | 89.2 | 90.5 | 67.3 | 88.3 | _ | [34] |
| Chlorella sp. | _ | Nile tilapia (Oreochromis niloticus) | 96.7 | 94.1 | 68.9 | 90.5 | 86.5 | 93.4 | 91.5 | 93.9 | 92.3 | 95.5 | [6] |
| Isochrysis sp. | _ | Rainbow trout (Oncorhynchus mykiss) | 99.2 | 93.2 | 101.4 | 94 | 92.1 | 94.5 | 98.5 | 94.9 | 94.4 | 84.4 | [35] |
| Nannochloropsis oceanica | _ | European seabass (Dicentrarchus labrax) | 86.1 | 92 | 87.5 | 86.2 | 90.5 | 88.5 | 91.1 | 61.4 | 87.4 | _ | [34] |
| Nannochloropsis oceanica | Bead milling | European seabass (Dicentrarchus labrax) | 94.6 | 83.6 | 90.5 | 90.5 | 88.4 | 88.1 | 88.6 | _ | 88.4 | _ | [34] |
| Nannochloropsis oceanica | Enzymatic processed | European seabass (Dicentrarchus labrax) | 90.6 | 78.8 | 86.4 | 88.7 | 86.7 | 86 | 87.5 | 91.2 | 89.9 | _ | [34] |
| Nannochloropsis oculata | _ | Nile tilapia (Oreochromis niloticus) | 71.4 | 74 | 75.8 | 66.6 | 79.8 | 78.3 | 77.7 | 88.1 | 72.5 | 86.5 | [38] |
| Nannochloropsis sp. | _ | Rainbow trout (Oncorhynchus mykiss) | 74.5 | 74.1 | 72.6 | 67.4 | 63.1 | 71.8 | 58.9 | 69.8 | 64.8 | 11.8 | [35] |
| Schizochytrium sp. | _ | Nile tilapia (Oreochromis niloticus) | 100 | 93.1 | 90.9 | 93.3 | 91.9 | 100 | 99 | 100 | 100 | 89.6 | [6] |
| Spirulina sp. | _ | Nile tilapia (Oreochromis niloticus) | 94 | 100 | 100 | 95.3 | 94.9 | 99.7 | 93.2 | 100 | 100 | 96.2 | [6] |
| Spirulina sp. | Defatted | Nile tilapia (Oreochromis niloticus) | 83 | 76.7 | 81.5 | 60.5 | 73.6 | 81.3 | 73.4 | 64.1 | 74 | 56.1 | [38] |
| Tetraselmis sp. | _ | European seabass (Dicentrarchus labrax) | 81.4 | 59.4 | 84.2 | 74.8 | 76.5 | 71.1 | 69.1 | 73.9 | 74.4 | _ | [24] |
| Tetraselmis sp. | Bead milling | European seabass (Dicentrarchus labrax) | 90.9 | 78.7 | 76.2 | 87.8 | 93.9 | 85.6 | 93.3 | 89.9 | 85 | _ | [24] |
| Tetraselmis sp. | Enzymatic processed | European seabass (Dicentrarchus labrax) | 95 | 49.3 | 76.8 | 87.5 | 78 | 77.4 | 86.1 | 86.5 | 83.7 | _ | [24] |
| Microalgae | Biomass Processing/Pre-treatment | Aqua Species | Pellet | Total SFA (%) | Total MUFA (%) | 20:5n3 EPA (%) | 22:6n3 DHA (%) | Total PUFA (%) | Refrence |
|---|---|---|---|---|---|---|---|---|---|
| Chlorella sp. | _ | Nile tilapia (Oreochromis niloticus) | Meat grinder | 74.7 | 69.6 | _ | _ | 90.9 | [6] |
| Isochrysis sp. | _ | Rainbow trout (Oncorhynchus mykiss) | Steam-pelleted | 58.9 | 72.2 | 87.7 | 91 | 91.7 | [35] |
| Nannochloropsis oculata | _ | Nile tilapia (Oreochromis niloticus) | Meat grinder | 39.6 | 57.1 | 94 | _ | 74.1 | [38] |
| Nannochloropsis oculata | Defatted | Nile tilapia (Oreochromis niloticus) | Meat grinder | 82.2 | 54.8 | 96.9 | _ | 58.1 | [38] |
| Nannochloropsis sp. | _ | Rainbow trout (Oncorhynchus mykiss) | Steam-pelleted | 55.9 | 44.7 | 69.4 | _ | 61.8 | [35] |
| Schizochytrium sp. | _ | Nile tilapia (Oreochromis niloticus) | Meat grinder | 52 | 84.8 | _ | _ | 97.5 | [6] |
| Schizochytrium sp. | _ | Rainbow trout (Oncorhynchus mykiss) | California Pellet Mill (model CPM CL-5) | 70.6 | 92.1 | 98.7 | 98.5 | 98.5 | [65] |
| Schizochytrium sp. | _ | Rainbow trout (Oncorhynchus mykiss) | California Pellet Mill (model CPM CL-5) | 77.4 | 87.5 | 98.4 | 99.1 | 98.7 | [65] |
| Spirulina sp. | _ | Nile tilapia (Oreochromis niloticus) | Meat grinder | 75.5 | 76.1 | _ | _ | 79.1 | [6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annamalai, S.N.; Das, P.; Thaher, M.I.A.; Abdul Quadir, M.; Khan, S.; Mahata, C.; Al Jabri, H. Nutrients and Energy Digestibility of Microalgal Biomass for Fish Feed Applications. Sustainability 2021, 13, 13211. https://doi.org/10.3390/su132313211
Annamalai SN, Das P, Thaher MIA, Abdul Quadir M, Khan S, Mahata C, Al Jabri H. Nutrients and Energy Digestibility of Microalgal Biomass for Fish Feed Applications. Sustainability. 2021; 13(23):13211. https://doi.org/10.3390/su132313211
Chicago/Turabian StyleAnnamalai, Senthil Nagappan, Probir Das, Mahmoud I. A. Thaher, Mohammad Abdul Quadir, Shoyeb Khan, Chandan Mahata, and Hareb Al Jabri. 2021. "Nutrients and Energy Digestibility of Microalgal Biomass for Fish Feed Applications" Sustainability 13, no. 23: 13211. https://doi.org/10.3390/su132313211
APA StyleAnnamalai, S. N., Das, P., Thaher, M. I. A., Abdul Quadir, M., Khan, S., Mahata, C., & Al Jabri, H. (2021). Nutrients and Energy Digestibility of Microalgal Biomass for Fish Feed Applications. Sustainability, 13(23), 13211. https://doi.org/10.3390/su132313211