Characteristics of Phytoplankton Production in Wet and Dry Seasons in Hyper-Eutrophic Lake Taihu, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sampling and Laboratory Analysis
2.2. Primary Productivity Estimation Based on Vertically Generalized Production Model (VGPM)
2.3. Trophic Level Index (TLI) Method
2.4. Data Processing and Analysis
3. Results and Discussion
3.1. Variations of Environmental Factors in Wet and Dry Seasons
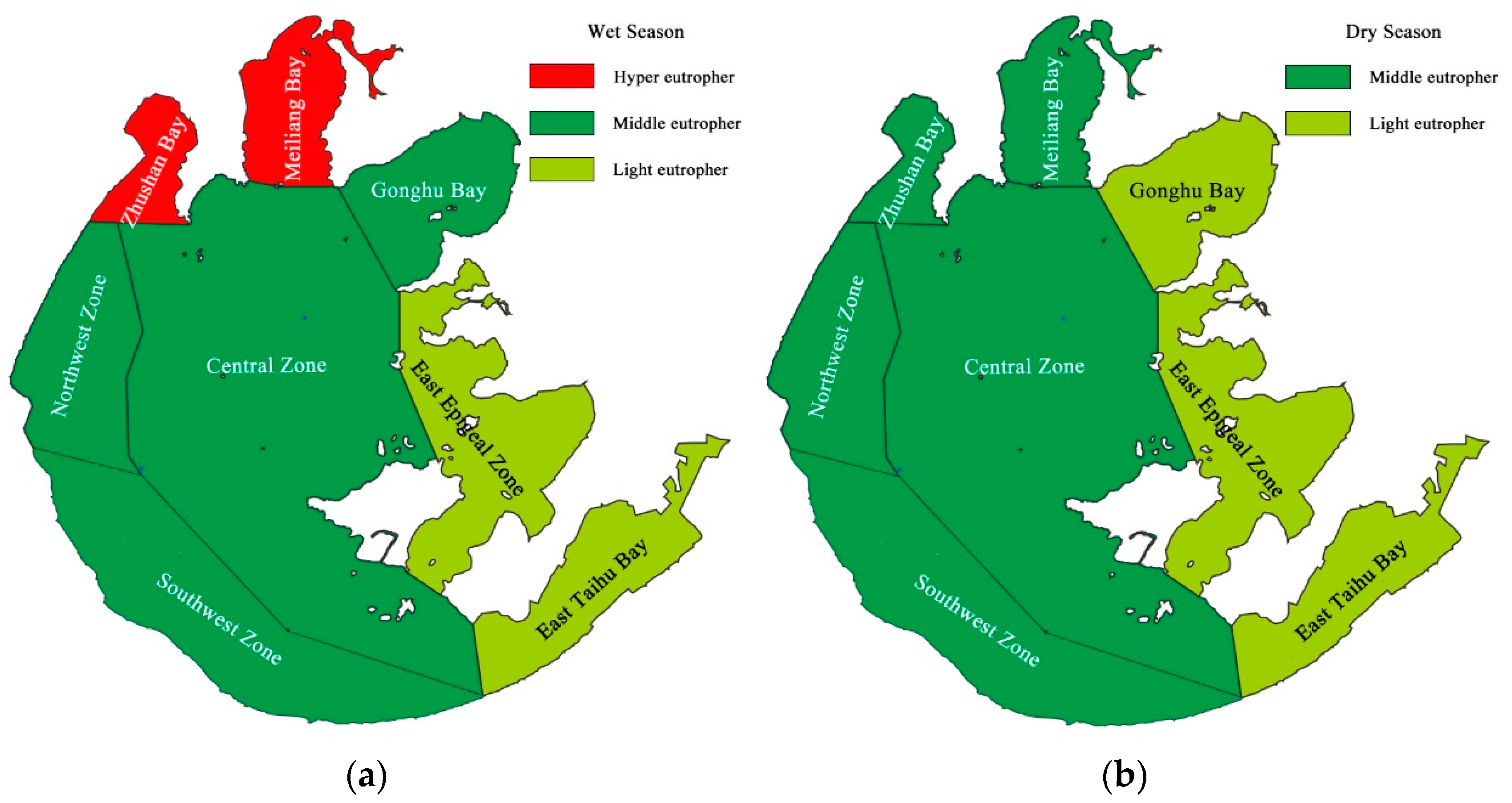
3.2. Trophic Status of Lake Taihu in Wet and Dry Seasons
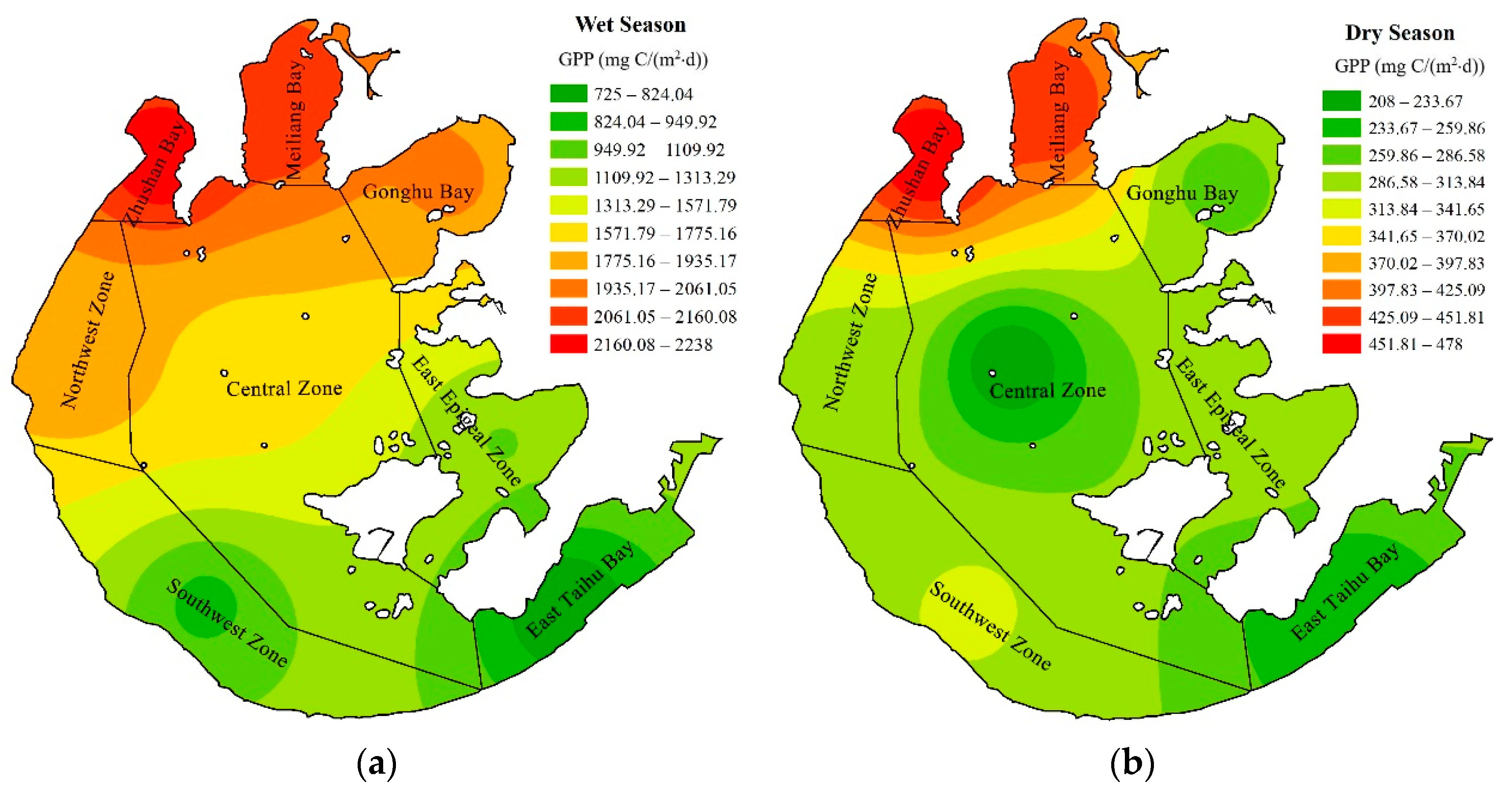
3.3. Spatial Distribution of Primary Productivity in Wet and Dry Seasons
3.4. Influencing Factors of Primary Productivity and Its Correlation with Environmental Factors
4. Conclusions
- (1)
- Phytoplankton primary productivity and physicochemical factors in Lake Taihu showed obvious seasonal differences. D, SD, pH, T, Chl-a, TP, and PPeu were significantly higher in the wet season than in the dry season, while DO, TN, and TSS were higher in the dry season than in the wet season.
- (2)
- Most of the lake areas in Taihu are in the middle eutrophic state regardless of the season; there are only three areas (Zhushan Bay, Meiliang Bay, and Gonghu Bay) where the trophic status changes with the seasons. The northwest region has the heaviest degree of eutrophication due to the dense ports, prevailing summer wind, and long water-retention time. The southeast region has the lightest degree of eutrophication, as it is a typical grass-type area with strong self-purification ability and less blooms.
- (3)
- The primary productivity in the wet season is about five times larger than that in the dry season. Zhushan Bay and Meiliang Bay have the largest PPeu values, which are significantly higher than those of other lake areas regardless of the season, while East Epigeal Zone and East Taihu Bay always have the lowest PPeu values. The spatial distribution of primary productivity is obviously inhomogeneous in the wet season, while the distribution of PPeu in the dry season is uniform in different lake areas and does not show significant differences.
- (4)
- Chl-a, T, and SD can be used to estimate phytoplankton primary productivity in Lake Taihu in different seasons, and the regression equation of PPeu in the wet season is: PPeu = 0.658Chl-a + 0.201SD + 0.187T (R2 = 0.993). The equation in the dry season is: PPeu = 0.163T + 0.681Chl-a + 0.536SD (R2 = 0.999). The main factors influencing primary productivity are Chl-a, TP, TN, and SD/TSS in summer and T, Chl-a, and SD/TSS in winter.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, B. Lake eutrophication: Control countermeasures and recycling exploitation. Ecol. Eng. 2009, 35, 1569–1573. [Google Scholar] [CrossRef]
- Bronmark, C.; Hansson, L.A. Environmental issues in lakes and ponds: Current state and perspectives. Environ. Conserv. 2002, 29, 290–307. [Google Scholar] [CrossRef]
- Kim, T.H.; Chae, C.U. Environmental impact analysis of acidification and eutrophication due to emissions from the production of concrete. Sustainability 2016, 8, 578. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Yin, Y.; Wang, M.; Qin, B. Wind and submerged aquatic vegetation influence bio-optical properties in large shallow Lake Taihu, China. J. Geophys. Res. 2013, 118, 713–727. [Google Scholar] [CrossRef]
- Lürling, M.; van Oosterhout, F. Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Res. 2013, 47, 6527–6537. [Google Scholar] [CrossRef] [PubMed]
- Stone, R. China aims to turn tide against toxic lake pollution. Science 2011, 333, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Passy, P.; Le Gendre, R.; Garnier, J.; Cugier, P.; Callens, J.; Paris, F.; Billen, G.; Riou, P.; Romero, E. Eutrophication modelling chain for improved management strategies to prevent algal blooms in the Bay of Seine. Mar. Ecol. Prog. Ser. 2016, 543, 107–125. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; O’Malley, R.T.; Siegel, D.A.; McClain, C.R.; Sarmiento, J.L.; Feldman, G.C.; Milligan, A.J.; Falkowski, P.G.; Letelier, R.M.; Boss, E.S. Climate-driven trends in contemporary ocean productivity. Nature 2006, 444, 752–755. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y.B.; Park, C.H.; Baek, S.H. Characterization of seasonal phytoplankton pigments and functional types around offshore island in the East/Japan Sea, based on HPLC pigment analysis. Sustainability 2022, 14, 5306. [Google Scholar] [CrossRef]
- Peng, G.; Li, X.X.; Hao, C.; Wang, M.H. The determination of the primary production of phytoplankton in summer in Gehu lake. Fish. Econ. Res. 2007, 20, 46–48. (In Chinese) [Google Scholar]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- Kolding, J.; van Zwieten, P.A.M. Relative lake level fluctuations and their influence on productivity and resilience in tropical lakes and reservoirs. Fish. Res. 2012, 115, 99–109. [Google Scholar] [CrossRef]
- Kennedy, J.T.; Whalen, S.C. Seasonality and controls of phytoplankton productivity in the middle Cape Fear River, USA. Hydrobiologia 2008, 598, 203–217. [Google Scholar] [CrossRef]
- Boulion, V.V. Contribution of major groups of autotrophic organisms to primary production of water bodies. Water Resour. 2004, 31, 92–102. [Google Scholar] [CrossRef]
- Qin, B.; Zhu, G.; Zhang, L.; Luo, L.; Gao, G.; Gu, B. Estimation of internal nutrient release in large shallow Lake Taihu, China. Sci. China Ser. D 2006, 49, 38–50. [Google Scholar] [CrossRef]
- Qin, B.; Xu, P.; Wu, Q.; Luo, L.; Zhang, Y. Environmental issues of Lake Taihu, China. Hydrobiologia 2007, 581, 3–14. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.F. Blooms bite the hand that feeds them. Science 2013, 342, 433–434. [Google Scholar] [CrossRef]
- Dai, X.L.; Qian, P.Q.; Ye, L.; Song, T. Changes in nitrogen and phosphorus concentrations in Lake Taihu, 1985–2015. J. Lake Sci. 2016, 28, 935–943. (In Chinese) [Google Scholar]
- Qian, H.Z. The Influence of Wind Field to the Spatial Distribution of Chlorophyll-A Concentration. M.D.; Nanjing University of Information Science & Technology: Nanjing, China, 2012. (In Chinese) [Google Scholar]
- State Environmental Protection Administration of China. Water and Wastewater Monitoring and Analysis Method, 4th ed.; China Environmental Science Press: Beijing, China, 2002; pp. 243–257. (In Chinese) [Google Scholar]
- Talling, J.F. The phytoplankton population as a compound photosynthetic system. New Phytol. 1957, 56, 133–149. [Google Scholar] [CrossRef]
- Cadée, G.C. Primary production of the Guyana coast. Neth. J. Sea Res. 1975, 9, 128–143. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Falkowski, P.G. Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol. Oceanogr. 1997, 42, 1–20. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Li, D. Progress and prospect of remote sensing on phytoplankton primary productivity estimation. Remote Sens. Inform. 2017, 32, 1–9. (In Chinese) [Google Scholar]
- Zhang, Y. Progress and prospect in lake optics: A review. J. Lake Sci. 2011, 23, 483–497. (In Chinese) [Google Scholar]
- Wang, J.L.; Fu, Z.S.; Qiao, H.X.; Liu, F.X. Assessment of eutrophication and water quality in the estuarine area of Lake Wuli, Lake Taihu, China. Sci. Total Environ. 2019, 650, 1392–1402. [Google Scholar] [CrossRef]
- Jin, X.C.; Liu, S.K.; Zhang, Z.S.; Tu, Q.Y.; Xu, N.N. Lake Environment in China; Ocean Press: Beijing, China, 1995. (In Chinese) [Google Scholar]
- Yang, X.E.; Wu, X.; Hao, H.L.; He, Z.L. Mechanisms and assessment of water eutrophication. J. Zhejiang Univ. Sci. B 2008, 9, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Y.; Zhu, G.W.; Zhao, L.L.; Yao, X.; Zhang, Y.L.; Gao, G.; Qin, B.Q. Influence of algal bloom degradation on nutrient release at the sediment-water interface in Lake Taihu, China. Environ. Sci. Pollut. Res. 2013, 20, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Wei, J.; Gao, X.M.; Chen, D.; Weng, S.L.; Du, W.; Wang, W.C.; Wang, J.W.; Tang, C.Y.; Zhang, S.S. Turbulent bursting and sediment resuspension in hyper-eutrophic Lake Taihu, China. J. Hydrol. 2018, 565, 581–588. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y.P.; Chen, D.; Nwankwegu, A.S.; Tang, C.Y.; Bu, M.S.; Zhang, S.S. The influence of ship wave on turbulent structures and sediment exchange in large shallow Lake Taihu, China. J. Hydrol. 2020, 586, 124853. [Google Scholar] [CrossRef]
- Xu, H.; Paerl, H.W.; Qin, B.Q.; Zhu, G.W.; Gao, G. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnol. Oceanogr. 2010, 55, 420–432. [Google Scholar] [CrossRef]
- Chen, Y.W.; Qin, B.Q.; Teubner, K.; Dokulil, M.T. Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. J. Plankton Res. 2003, 25, 445–453. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Qin, B.Q.; Hu, W.P.; Wang, S.; Chen, Y.W.; Chen, W.M. Temporal-spatial variations of euphotic depth of typical lake regions in Lake Taihu and its ecological environmental significance. Sci. China Ser. D 2006, 49, 431–442. [Google Scholar] [CrossRef]
- Chen, R.; Ju, M.T.; Chu, C.L.; Jing, W.Q.; Wang, Y.Q. Identification and quantification of physicochemical parameters influencing chlorophyll-a concentrations through combined principal component analysis and factor analysis: A case study of the Yuqiao Reservoir in China. Sustainability 2018, 10, 936. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.H.; Wang, J.; Wang, J.J.; Wang, J.X.L. Seasonal dependency of controlling factors on the phytoplankton production in Taihu Lake, China. J. Environ. Sci. 2019, 76, 278–288. [Google Scholar] [CrossRef]
- Schallenberg, M.; Burns, C.W. Effects of sediment resuspension on phytoplankton production: Teasing apart the influences of light, nutrients and algal entrainment. Freshw. Biol. 2004, 49, 143–159. [Google Scholar] [CrossRef]
- Harrison, P.J.; Khan, N.; Yin, K.; Saleem, M.; Bano, N.; Nisa, M.; Ahmed, S.I.; Rizvi, N.; Azam, F. Nutrient and phytoplankton dynamics in two mangrove tidal creeks of the Indus River delta, Pakistan. Mar. Ecol. Prog. Ser. 1997, 157, 13–19. [Google Scholar] [CrossRef] [Green Version]

| Parameters | Chl-a | TP | TN |
|---|---|---|---|
| rij | 1 | 0.84 | 0.82 |
| rij2 | 1 | 0.7056 | 0.6724 |
| Parameters | Wet Season | Dry Season |
|---|---|---|
| D/m | 2.55 ± 0.26 | 1.94 ± 0.23 |
| SD/m | 0.63 ± 0.04 | 0.44 ± 0.03 |
| pH | 9.40 ± 0.18 | 7.86 ± 0.19 |
| T/°C | 30.58 ± 2.54 | 9.35 ± 0.67 |
| DO/(mg/L) | 6.09 ± 0.25 | 11.36 ± 0.55 |
| Chl-a/(mg/L) | 45.57 ± 3.11 | 15.81 ± 1.28 |
| TP/(mg/L) | 0.14 ± 0.02 | 0.10 ± 0.02 |
| TN/(mg/L) | 1.83 ± 0.26 | 2.75 ± 0.42 |
| TSS/(mg/L) | 43.14 ± 5.29 | 51.47 ± 4.37 |
| PPeu/(mg C/(m2·d)) | 1586.83 ± 542.01 | 320.82 ± 110.34 |
| TLI | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 |
|---|---|---|---|---|---|---|---|---|
| Wet season | 71.73 | 70.10 | 67.53 | 56.02 | 54.59 | 60.79 | 65.88 | 63.64 |
| Dry season | 66.67 | 60.78 | 57.12 | 56.00 | 54.78 | 60.40 | 63.17 | 60.94 |
| PPeu | SD | pH | T | DO | Chl-a | TP | TN | TSS | |
|---|---|---|---|---|---|---|---|---|---|
| PPeu | 1 | ||||||||
| SD | −0.799 * | 1 | |||||||
| pH | 0.435 | −0.734 * | 1 | ||||||
| T | 0.583 | −0.816 * | 0.474 | 1 | |||||
| DO | −0.520 | 0.689 | −0.850 ** | −0.266 | 1 | ||||
| Chl-a | 0.975 ** | −0.831 * | 0.456 | 0.607 | −0.580 | 1 | |||
| TP | 0.953 ** | −0.775 * | 0.392 | 0.574 | −0.545 | 0.985 ** | 1 | ||
| TN | 0.895 ** | −0.698 | 0.331 | 0.637 | −0.386 | 0.927 ** | 0.959 ** | 1 | |
| TSS | 0.776 * | −0.949 ** | 0.756 * | 0.704 | −0.765 * | 0.830 * | 0.817 * | 0.746 * | 1 |
| PPeu | SD | pH | T | DO | Chl-a | TP | TN | TSS | |
|---|---|---|---|---|---|---|---|---|---|
| PPeu | 1 | ||||||||
| SD | 0.676 | 1 | |||||||
| pH | 0.643 | 0.151 | 1 | ||||||
| T | 0.829 * | 0.661 | 0.618 | 1 | |||||
| DO | −0.511 | −0.244 | −0.890 ** | −0.757 * | 1 | ||||
| Chl-a | 0.732 * | 0.001 | 0.644 | 0.474 | −0.702 | 1 | |||
| TP | 0.375 | −0.039 | 0.569 | 0.553 | −00.549 | 0.517 | 1 | ||
| TN | 0.585 | 0.079 | 0.685 | 0.573 | −00.548 | 0.734 * | 0.902 ** | 1 | |
| TSS | −0.712 * | −0.914 ** | −0.356 | −0.767 * | 0.463 | −0.130 | −0.204 | −0.296 | 1 |
| Period | Multiple Stepwise Regression Equations | R2 | F | p |
|---|---|---|---|---|
| Wet season | PPeu = 0.975Chl-a | 0.943 | 116.931 | <0.001 |
| PPeu = 0.831Chl-a + 0.233SD | 0.972 | 104.470 | <0.001 | |
| PPeu = 0.658Chl-a + 0.201SD + 0.187T | 0.993 | 83.960 | <0.001 | |
| Dry season | PPeu = 0.840T | 0.656 | 14.377 | 0.009 |
| PPeu = 0.619T + 0.466Chl-a | 0.823 | 17.281 | 0.006 | |
| PPeu = 0.163T + 0.681Chl-a + 0.536SD | 0.999 | 1563.688 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Ji, X.; Hu, W. Characteristics of Phytoplankton Production in Wet and Dry Seasons in Hyper-Eutrophic Lake Taihu, China. Sustainability 2022, 14, 11216. https://doi.org/10.3390/su141811216
Wei J, Ji X, Hu W. Characteristics of Phytoplankton Production in Wet and Dry Seasons in Hyper-Eutrophic Lake Taihu, China. Sustainability. 2022; 14(18):11216. https://doi.org/10.3390/su141811216
Chicago/Turabian StyleWei, Jin, Xiaonan Ji, and Wei Hu. 2022. "Characteristics of Phytoplankton Production in Wet and Dry Seasons in Hyper-Eutrophic Lake Taihu, China" Sustainability 14, no. 18: 11216. https://doi.org/10.3390/su141811216






