Prediction of Banana Production Using Epidemiological Parameters of Black Sigatoka: An Application with Random Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Epidemiological Evaluation of Black Sigatoka in Host Plants
- a.
- The infection index (INC): Expresses the magnitude of the damage caused by the disease on a quantitative scale, whose ideal limit is 0.10. It was calculated using Equation (1). This index is a weighted average considering the severity of the BS attack in all the evaluated leaves. The INC gives us a single value indicating the magnitude of foliage damage, on a scale of 0 to 1. In plants in the acorn stage, a value of <0.10 is considered low, ≥ 0.10–0.15 as intermediate, and >0.15 as high [28,29].where INC = infection index, n = number of leaves in each grade, b = grade, N = number of grades used in the scale, and T = total number of leaves evaluated.
- b.
- State of the symptom (gross sum) (SIN): comes from the observations of the most developed state of the present symptom and the density of these symptoms in sheet No. 3 plants in acorn state. Through a spreadsheet, a numerical value (weight) was obtained for each state of the symptom to subsequently obtain the gross sum. The gross sum reflects the development of BS at an early stage and allows the development of new BS infections to be determined several weeks earlier than with all other parameters. It also allows us to evaluate if the last applied fungicide treatment had a significant effect on the control of BS, or if it is not controlling the disease. The gross sum can range between 0 and 140. A value below 50 is considered good disease control [28,29].
- c.
- d.
- Youngest leaf with symptoms (YLWS): number of the leaf-bearing symptoms. Leaves were numbered from the first (topmost) open leaf downward, according to the methodology proposed by Hernández et al. [31]. Youngest leaf with symptoms visible from the ground. These are usually stage 2 and 3 striae that can be identified from the ground.
- e.
- Youngest leaf spotted (YLS): number of the leaf-bearing spots with dry centers. A higher value of YLS indicates more healthy leaves on the plant. Leaves were numbered as for YLWS. It is the average of the youngest leaf with 10 or more spots with a grayish and dry center, which is the last stage of the development of BS. In the spot state with a grayish and dry center, the fungus produces ascospores [28,29].
2.3. Data Analysis
2.3.1. Exploratory Analysis
2.3.2. Random Forest Prediction
2.3.3. Model Performance Evaluation
3. Results
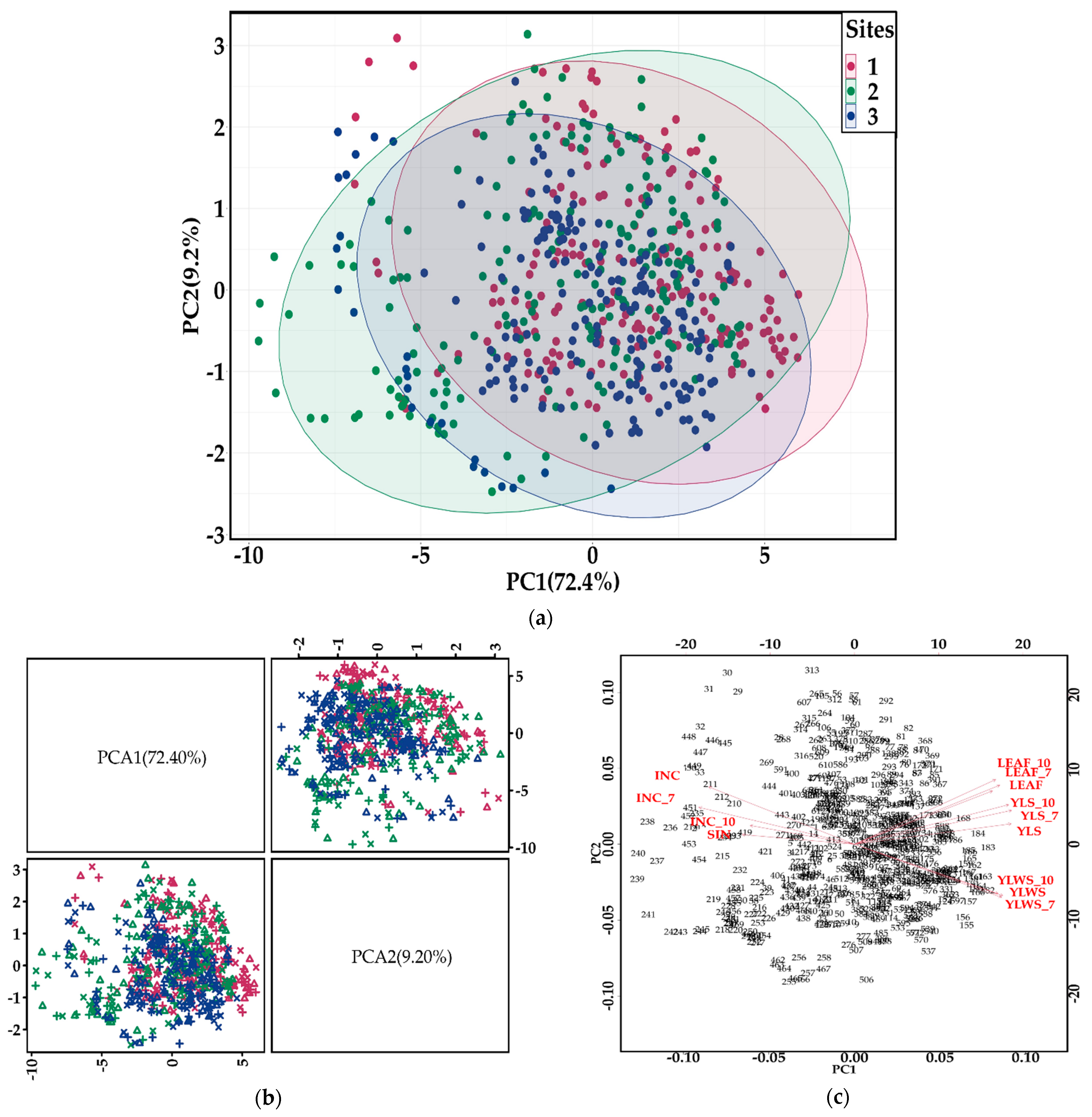
3.1. Descriptive Analysis
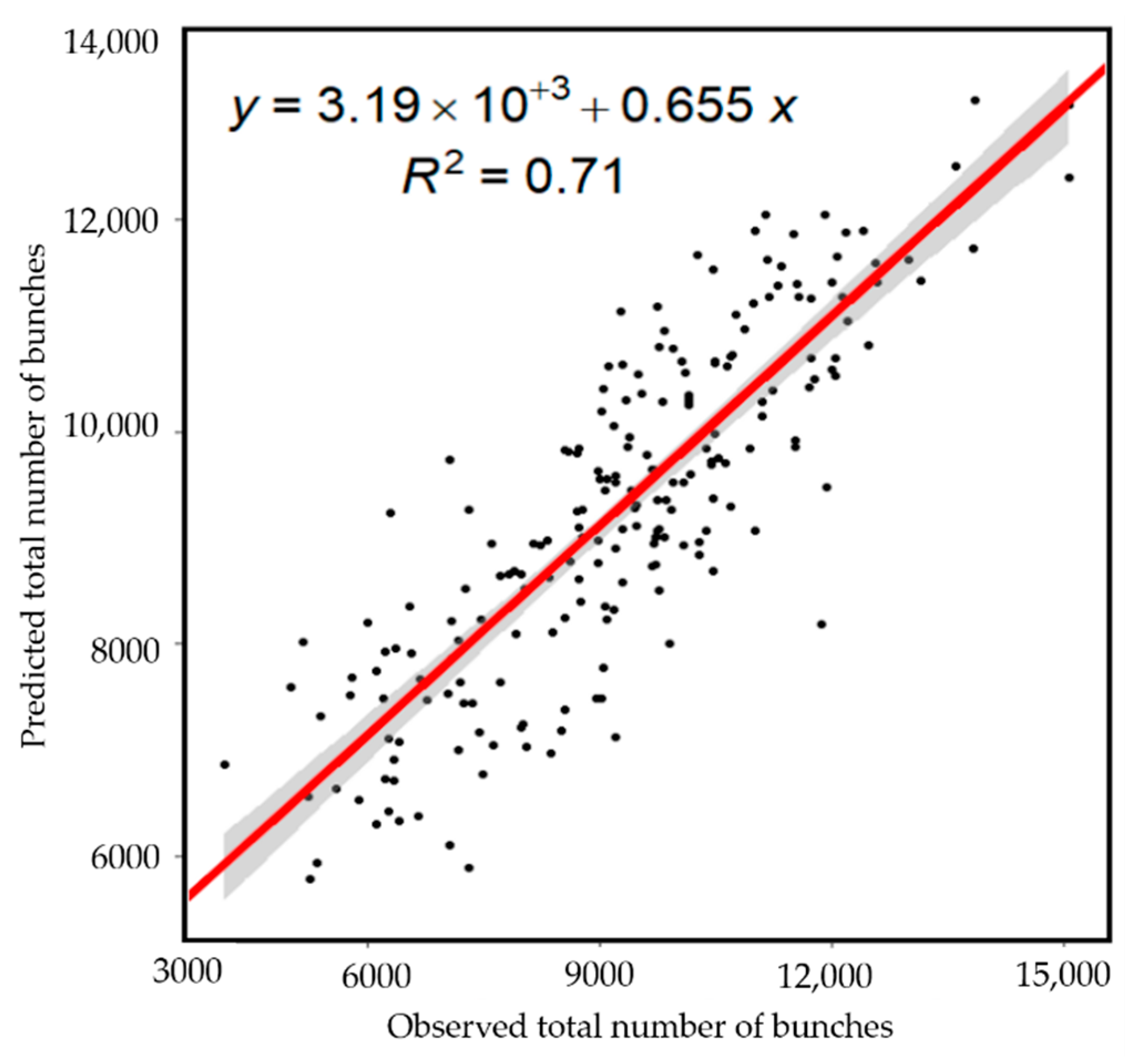
3.2. Global Banana Bunches Predictions
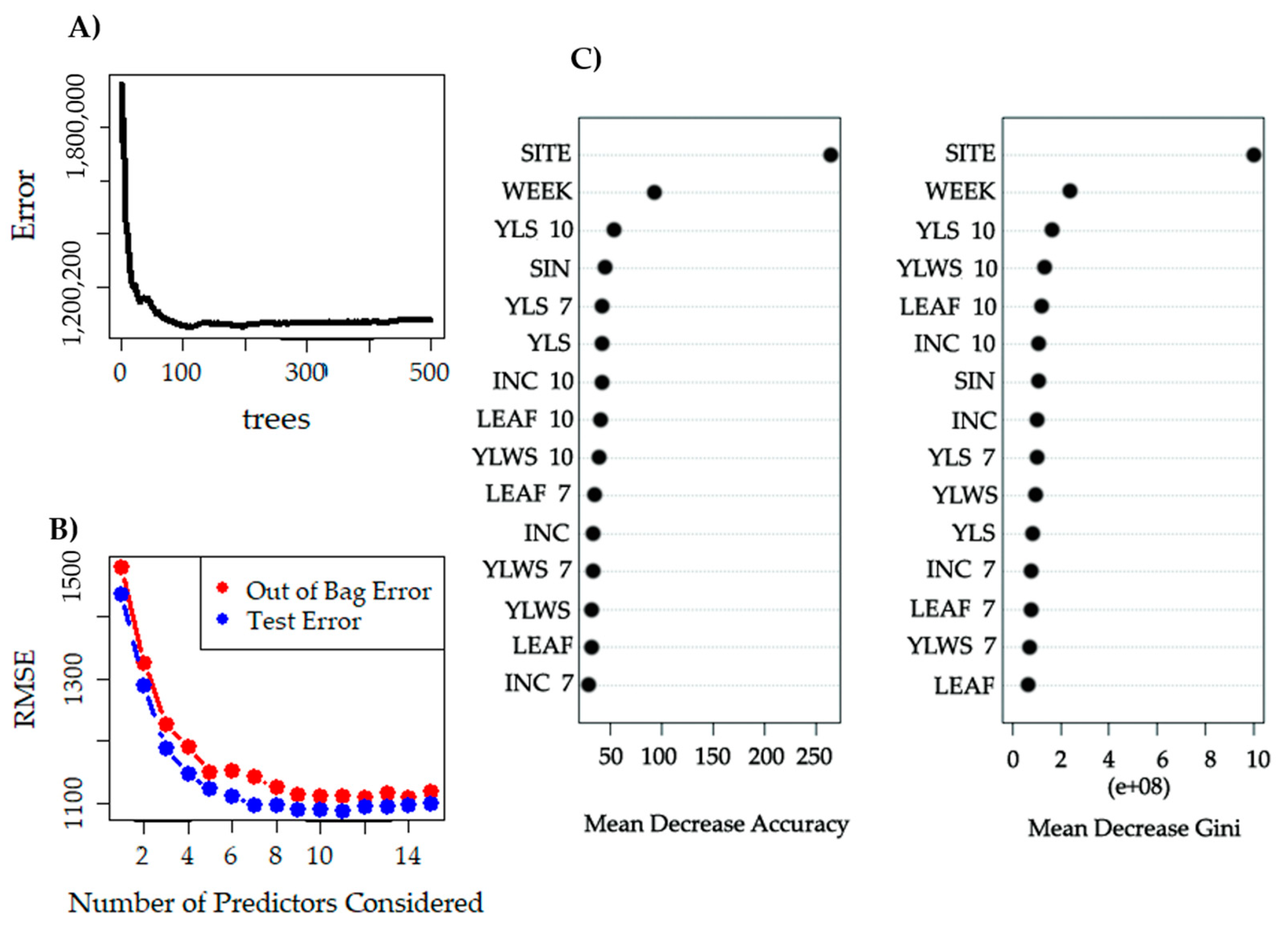
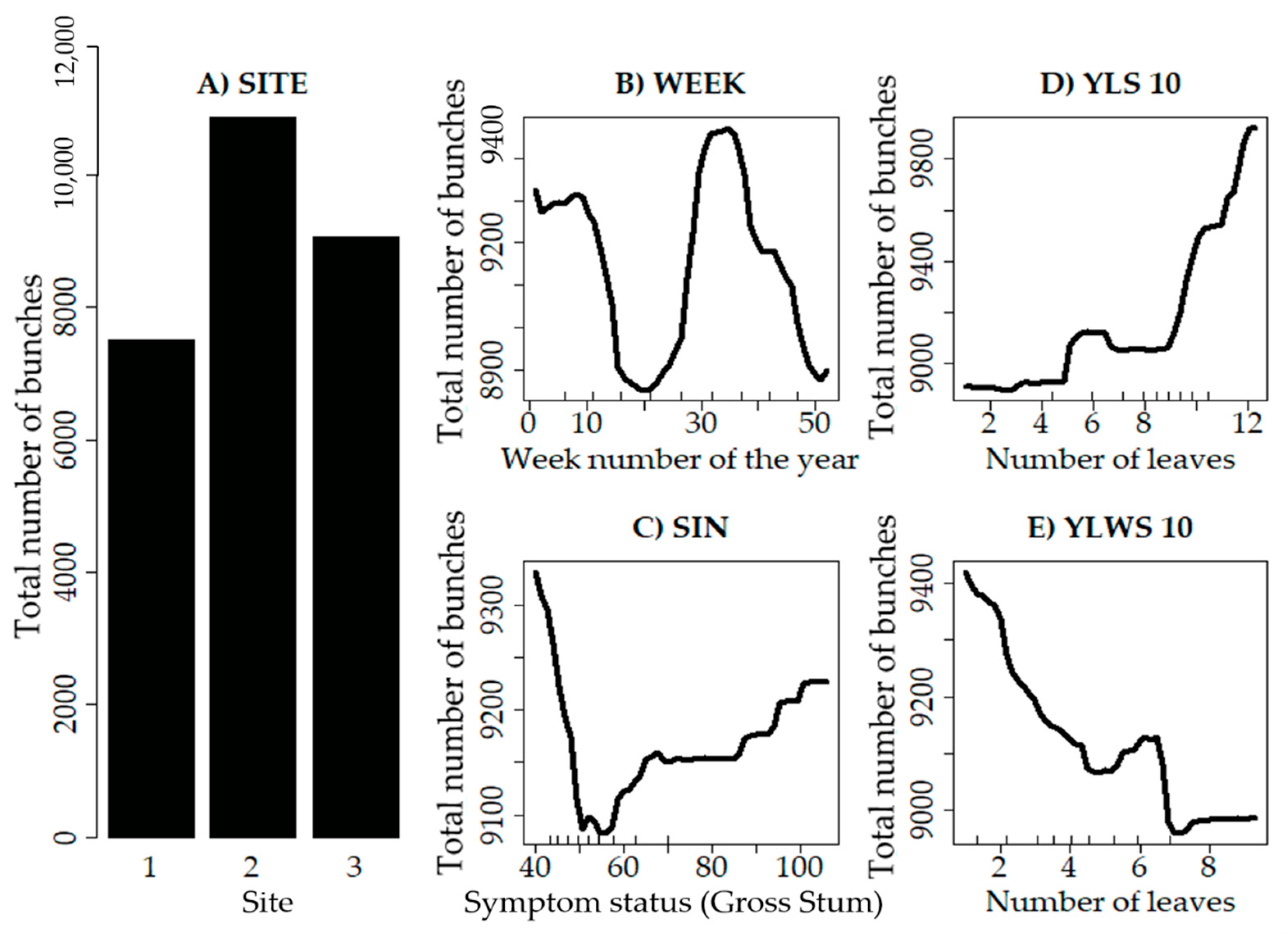
3.3. Identification of the Main Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Food and Agriculture Organization of the United Nation. Banana Market Review: Preliminary Results 2020. Available online: https://www.fao.org/3/cb5150en/cb5150en.pdf (accessed on 16 September 2022).
- Evans, E.A.; Ballen, F.H.; Siddiq, M. Banana production, global trade, consumption trends, postharvest handling and processing. In Handbook of Banana Production, Postharvest Science, Processing Technology and Nutrition; Siddiq, M., Ahmed, J., Lobo, M.G., Eds.; Wiley Online Library: New York, NY, USA, 2020; pp. 1–18. [Google Scholar] [CrossRef]
- Pitti, J.; Olivares, B.O.; Montenegro, E.; Miller, L.; Ñango, Y. The role of agriculture in the Changuinola district: A case of applied economics in Panama. Trop. Subtrop. Agroecosyst. 2021, 25, 017. [Google Scholar] [CrossRef]
- Martínez-Solórzano, G.E.; Rey-Brina, J.C. Bananos (Musa AAA): Importance, production, and trade in COVID-19 times. Agron. Mesoam. 2021, 32, 1034–1046. [Google Scholar] [CrossRef]
- Montenegro, E.J.; Pitti-Rodríguez, J.E.; Olivares-Campos, B.O. Identification of the main subsistence crops of Teribe: A case study based on multivariate techniques. Idesia 2021, 39, 83–94. [Google Scholar] [CrossRef]
- Rhodes, P.L. A new Banana disease in Fiji. Commonw. Phytopathol. News 1964, 10, 38–41. [Google Scholar]
- Marin, D.H.; Romero, R.A.; Guzman, M.; Sutton, T.B. Black Sigatoka: An increasing threat to banana cultivation. Plant Dis. 2003, 87, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, R.A. Sigatoka Leaf Diseases. In Compendium of Tropical Fruit Diseases; Ploetz, R.C., Zentmyer, G.A., Nishijinia, W.T., Rohrbach, K.G., Ohr, H.D., Eds.; American Phytopathological Society: St. Paul, MN, USA, 1994; pp. 12–14. [Google Scholar]
- Jacome, L.H.; Schuh, W.; Stevenson, R.E. Effect of temperature and relative humidity on germination and germ tube development of Mycosphaerella fijiensis var. difformis. Phytopathology 1991, 81, 1480–1485. [Google Scholar] [CrossRef]
- Fullerton, R.A.; Casonato, S.G. The infection of the fruit of ‘Cavendish’ banana by Pseudocercospora fijiensis. cause of black leaf streak (black Sigatoka). Eur. J. Plant Pathol. 2019, 155, 779–787. [Google Scholar] [CrossRef]
- Olivares, B.O.; Vega, A.; Calderón, M.A.R.; Rey, J.C.; Lobo, D.; Gómez, J.A.; Landa, B.B. Identification of Soil Properties Associated with the Incidence of Banana Wilt Using Supervised Methods. Plants 2022, 11, 2070. [Google Scholar] [CrossRef] [PubMed]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.; Staver, C.P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef]
- Olivares, B.O.; Paredes, F.; Rey, J.C.; Lobo, D.; Galvis-Causil, S. The relationship between the normalized difference vegetation index. rainfall. and potential evapotranspiration in a banana plantation of Venezuela. ST-JSSA 2021, 18, 58–64. [Google Scholar] [CrossRef]
- Churchill, A.C. Mycosphaerellafijiensis. the black leaf streak pathogen of banana: Progress towards understanding pathogen biology and detection. disease development. and the challenges of control. Mol. Plant Pathol. 2011, 12, 307–328. [Google Scholar] [CrossRef]
- Wang, Y.; Chee, M.C.; Edher, Z.; Hoang, M.D.; Fujimori, S.; Kathirgamanathan, S.; Bettencourt, J. Forecasting black sigatoka infection risks with latent neural odes. arXiv 2020, arXiv:2012.00752. [Google Scholar] [CrossRef]
- Ochoa, A.; Abaunza, F.; Rey, V. Forecasting black sigatoka in banana crops with stochastic models. In Proceedings of the VI International Banana Congress CORBANA, Miami, FL, USA, 19–20 April 2016. [Google Scholar]
- Ganry, J.; de Bellaire, L.D.L.; Mourichon, X. A biological forecasting system to control Sigatoka disease of bananas and plantains. Fruits 2008, 63, 381–387. [Google Scholar] [CrossRef][Green Version]
- de Hernández, R.M.A.G.; Rodríguez, V.; Caraballo, E.A.H. Predicción del rendimiento de un cultivo de plátano mediante redes neuronales artificiales de regresión generalizada. Publ. Cienc. Tecnol. 2012, 6, 31–40. [Google Scholar]
- Soares, J.D.R.; Pasqual, M.; Lacerda, W.S.; Silva, S.O.; Donato, S.L.R. Utilization of artificial neural networks in the prediction of the bunches’ weight in banana plants. Sci. Hortic. 2013, 155, 24–29. [Google Scholar] [CrossRef]
- Ye, H.C.; Huang, W.J.; Huang, S.Y.; Cui, B.; Dong, Y.Y.; Guo, A.T.; Ren, Y.; Jin, Y. Identification of banana fusarium wilt using supervised classification algorithms with UAV-based multi-spectral imagery. Int. J. Agric. Biol. 2020, 13, 136–142. [Google Scholar] [CrossRef]
- Selvaraj, M.G.; Vergara, A.; Montenegro, F.; Ruiz, H.A.; Safari, N.; Raymaekers, D.; Ocimati, W.; Ntamwira, J.; Tits, L.; Omondi, A.B.; et al. Detection of banana plants and their major diseases through aerial images and machine learning methods: A case study in DR Congo and Republic of Benin. ISPRS 2020, 169, 110–124. [Google Scholar] [CrossRef]
- Olivares, B.O.; Araya-Alman, M.; Acevedo-Opazo, C.; Rey, J.C.; Cañete-Salinas, P.; Kurina, F.G.; Balzarini, M.; Lobo, D.; Navas-Cortés, J.A.; Landa, B.B.; et al. Relationship between soil properties and banana productivity in the two main cultivation areas in Venezuela. J. Soil Sci. Plant Nutr. 2020, 20, 2512–2524. [Google Scholar] [CrossRef]
- Sangeetha, T.; Lavanya, G.; Jeyabharathi, D.; Rajesh Kumar, T.; Mythili, K. Detection of pest and disease in banana leaf using convolution Random Forest. Test Eng. Manag. 2020, 83, 3727–3735. [Google Scholar]
- Hou, J.C.; Hu, Y.H.; Hou, L.X.; Guo, K.Q.; Satake, T. Classification of ripening stages of bananas based on support vector machine. Int. J. Agric. Biol. Eng. 2015, 8, 99–103. [Google Scholar] [CrossRef]
- Sabilla, I.; Wahyuni, C.S.; Fatichah, C.; Herumurti, D. Determining banana types and ripeness from image using machine learning methods. In Proceedings of the 2019 International Conference of Artificial Intelligence and Information Technology (ICAIIT), Ouargla, Algeria, 13–15 March 2019; IEEE: New York, NY, USA, 2019; pp. 407–412. [Google Scholar]
- Montenegro, E.; Pitti, J. Analysis of climate risks in the indigenous food system The Teribe, Panama. Acta Nova 2020, 9, 713–736. [Google Scholar]
- Stover, R.H. A proposed international scale for estimating intensity of Banana leaf spot (Mycosphaerella musicola Leach). Trop. Agric. Trinidad Tobago 1971, 48, 185–196. [Google Scholar]
- Gauhl, F. Epidemiología y Ecología de la Sigatoka Negra (Mycosphaerella fijiensis, Morelet) en Plátano (Musa sp.), en Costa Rica; Trad; de Alemán, J.E., Ed.; UPEB: Panama City, Panama, 1990; p. 126. [Google Scholar]
- Gauhl, F.; Pasberg-Gauhl, C.; Jones, D.R. Disease cycle and epidemiology. In Diseases of Banana. Abacá and Enset; Jones, D.R., Ed.; CAB International: Wallingford, UK, 2000; pp. 56–62. [Google Scholar]
- Fouré, E. Black Leaf Streak Disease of Bananas and Plantains (Mycosphaerella fijiensis Morelet). Study of the symptoms and Stages of the Disease in Gabon; IRFA-CIRAD: Paris, France, 1985; p. 20. [Google Scholar]
- Hernández, L.; Hidalgo, W.; Linares, B.; Hernández, J.; Romero, N.; Fernández, S. Surveillance and forecast preliminary study for black sigatoka (Mycosphaerella fijiensis Morelet) disease in Musa AAB cv Hartón plantain crop in “Macagua-Jurimiquire” Yaracuy state. Rev. Fac. Agron. 2005, 22, 325–329. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Breiman, L.; Last, M.; Rice, J. Random forests: Finding quasars. In Statistical Challenges in Astronomy; Feigelson, E., Jogesh, G., Eds.; Springer: New York, NY, USA, 2003; pp. 243–254. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Strobl, C.; Boulesteix, A.L.; Zeileis, A.; Hothorn, T. Bias in random forest variable importance measures: Illustrations, sources, and a solution. BMC Bioinform. 2007, 8, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Guo, X.; Yu, H. Variable selection using mean decrease accuracy and mean decrease gini based on random forest. In Proceedings of the 2016 7th IEEE International Conference on Software Engineering and Service Science (ICSESS), Beijing, China, 26–28 August 2016; pp. 219–224. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification, and regression by random Forest. R News 2002, 2, 18–22. [Google Scholar]
- Jeong, J.H.; Resop, J.P.; Mueller, N.D.; Fleisher, D.H.; Yun, K.; Butler, E.E.; Timlin, D.J.; Shim, K.M.; Gerber, J.S.; Reddy, V.R.; et al. Random forests for global and regional crop yield predictions. PLoS ONE 2016, 11, e0156571. [Google Scholar] [CrossRef] [PubMed]
- Arcila, M.; Giraldo, G.; Duarte, J. Influencia de Las Condiciones Ambientales Sobre Las Propiedades Físicas y Químicas Durante La Maduración del Fruto de Plátano Dominico-Hartón (Musa AAB Simonds) en la Zona Cafetera Central; Cayón, G., Ed.; Poscosecha y Agroindustria del Plátano en el eje Cafetero de Colombia; Universidad del Quindío: Quindío, Colombia, 2000; pp. 101–124. [Google Scholar]
- Torres, N.; Hernández, J. Efecto del número de hojas en el desarrollo del racimo de plátano Hartón Musa AAB. Agroalim. Des. Sost. 2004, 5, 17–22. [Google Scholar]
- Barrera, J.; Cayón, G.; Robles, J. Influence of leaf and fruit epicarp exposition on development and quality of ’Hartón’ plantain (Musa AAB Simmonds) bunch. Agron. Colomb. 2009, 27, 73–79. [Google Scholar]
- Barrera, V.J.; Barraza, A.F.; Campo, A. Shadow effect on black sigatoka (Mycosphaerella fijiensis Morelet) in plantain cultivation cv harton (Musa AAB Simmonds). Rev. UDCA Actual. Divulg. Cient. 2016, 19, 317–323. [Google Scholar] [CrossRef]
- Belalcázar, S.; Merchán, V.; Mayorga, M. Control de Enfermedades. El Cultivo Del Plátano en el Trópico; Belalcázar, S., Ed.; Feriva: Bogota, Colombia, 1991; pp. 241–297. [Google Scholar]
- Cayón, G. Evolución de la fotosíntesis, transpiración y clorofila durante el desarrollo de la hoja de plátano (Musa AAB Simmonds). Infomusa 2001, 10, 12–15. [Google Scholar]
- Siles, P.; Bustamante, O.; Valdivia, E.; Burkhardt, J.; Staver, C. Photosynthetic performance of banana (Gross Michel, AAA) under a natural shade gradient. Acta Hortic. 2013, 986, 71–77. [Google Scholar] [CrossRef]
- Porras, A.; Hernández, A.; Pérez, L. Epidemiología de la Sigatoka Negra (Mycosphaerella fijiensis Morelet) en Cuba. II. Pronóstico Bio-Climático de los Tratamientos contra la Enfermedad en Plátanos (Musa spp. AAB). Rev. Mex. Fitopatol. 2000, 18, 27–35. [Google Scholar]
- Gauhl, F.; Pasberg-Gauhl, C. Epidemiology of black sigatoka disease on plantain in Nigeria. Phytopathology 1994, 84, 1080. [Google Scholar]
- Mobambo, K.N.; Gauhl, F.; Pasberg-Gauhl, C.; Zuofa, K. Season and plant age effect evaluation of plantain for response to black sigatoka disease. Crop Prot. 1996, 15, 609–614. [Google Scholar] [CrossRef]
- Nava, C.; Vera, J. Relation of leaves numbers a florewing time and wasted at reproductive cycle with bunch weight in plantain bunch plants under black Sigatoka attack. Rev. Fac. Agron. 2004, 21, 335–342. [Google Scholar]
- Rodríguez, P.; Cayón, G. Efecto de Mycosphaerella fijiensis sobre la fisiología de la hoja de banano. Agron. Colomb. 2008, 26, 256–265. [Google Scholar]
- Cedeño-Zambrano, J.R.; Díaz-Barrios, E.J.; de Jesús Conde-López, E.; Cervantes-Álava, A.R.; Avellán-Vásquez, L.E.; Zambrano-Mendoza, M.E.; Sánchez-Urdaneta, A.B. Evaluación de la severidad de Sigatoka negra (Mycosphaerella fijiensis Morelet) en platano “Barraganete” bajo fertilización con magnesio. Rev. Tec. 2021, 44, 4–12. [Google Scholar] [CrossRef]
- Villalta, R.; Guzmán, M. Capacidad de esporulación de Mycosphaerella fijiensis en tejido foliar de banano depositado en el suelo y efecto antiesporulante de la urea. Corbana 2005, 31, 41–43. [Google Scholar]
- Etebu, E.; Young, W. Control of black sigatoka disease: Challenges and prospects. Afr. J. Agric. Res. 2011, 6, 508–514. [Google Scholar] [CrossRef]
- Vázquez-Euán, R.; Chi-Manzanero, B.; Hernández-Velázquez, I.; Tzec-Simá, M.; Islas-Flores, I.; Martínez-Bolaños, L.; Garrido-Ramírez, E.R.; Canto-Canché, B. Identification of new hosts of Pseudocercospora fijiensis suggests innovative pest management programs for black sigatoka disease in banana plantations. Agronomy 2019, 9, 666. [Google Scholar] [CrossRef]
- Gómez-Correa, J.C.; Torres-Aponte, W.S.; Cayón-Salinas, D.G.; Hoyos-Carvajal, L.M.; Castañeda-Sánchez, D.A. Modelación espacial de la Sigatoka negra (Mycosphaerella fijiensis M. Morelet) en banano cv. Gran Enano. Rev. Ceres 2017, 64, 47–54. [Google Scholar] [CrossRef][Green Version]
- Bebber, D.P. Climate change effects on Black Sigatoka disease of banana. Philos. Trans. R. Soc. B 2019, 374, 20180269. [Google Scholar] [CrossRef]
- Grömping, U. Variable importance assessment in regression: Linear regression versus random forest. Am. Stat. 2009, 63, 308–319. [Google Scholar] [CrossRef]

| Site | Geographic Coordinates | Cultivated Area (ha) | Banana Lots (n) | |
|---|---|---|---|---|
| 1 | 9°25′43.0″ N | 82°32′57.1″ W | 160.75 | 56 |
| 2 | 9°28′09.2″ N | 82°31′27.9″ W | 212.91 | 77 |
| 3 | 9°30′05.5″ N | 82°35′42.2″ W | 184.51 | 47 |
| Site | Variable | Mean | S. D | S. E | CV | Min | Max | S | K | P (25) | P (50) | P (75) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 208) | Bunch (nº) | 7611.77 | 1648.12 | 114.28 | 21.65 | 4152.0 | 12,921.0 | 0.59 | 0.25 | 6404.00 | 7360.00 | 8620.00 |
| INC | 0.06 | 0.03 | 0.00 | 41.00 | 0.02 | 0.17 | 1.35 | 3.09 | 0.04 | 0.06 | 0.08 | |
| YLS (nº) | 14.08 | 1.42 | 0.10 | 10.12 | 9.35 | 16.60 | −1.08 | 1.09 | 13.25 | 14.40 | 15.16 | |
| YLWS (nº) | 11.06 | 1.41 | 0.10 | 12.71 | 7.45 | 14.28 | −0.08 | −0.43 | 10.10 | 11.00 | 11.96 | |
| SIN | 52.36 | 9.91 | 0.69 | 18.93 | 40.00 | 99.25 | 1.45 | 2.62 | 44.80 | 49.60 | 57.60 | |
| LEAF (nº) | 13.67 | 0.65 | 0.05 | 4.77 | 11.50 | 15.40 | −0.47 | 0.60 | 13.25 | 13.70 | 14.16 | |
| LEAF_7(nº) | 10.17 | 0.89 | 0.06 | 8.79 | 7.25 | 11.80 | −0.62 | 0.15 | 9.60 | 10.28 | 10.80 | |
| LEAF_10(nº) | 9.30 | 1.20 | 0.08 | 12.86 | 5.15 | 11.12 | −0.76 | 0.06 | 8.48 | 9.48 | 10.28 | |
| INC_7 | 0.13 | 0.05 | 0.00 | 42.37 | 0.03 | 0.31 | 1.06 | 2.02 | 0.09 | 0.12 | 0.16 | |
| YLS_7 (nº) | 10.08 | 1.93 | 0.13 | 19.12 | 4.75 | 13.20 | −0.71 | −0.07 | 8.88 | 10.48 | 11.48 | |
| YLWS_7 (nº) | 6.43 | 2.04 | 0.14 | 31.73 | 1.20 | 10.72 | −0.03 | −0.22 | 5.15 | 6.50 | 7.56 | |
| INC_10 | 0.17 | 0.06 | 0.00 | 37.41 | 0.05 | 0.39 | 0.72 | 0.74 | 0.13 | 0.17 | 0.21 | |
| YLS_10 (nº) | 8.66 | 2.24 | 0.16 | 25.86 | 2.55 | 12.32 | −0.65 | −0.27 | 7.25 | 9.04 | 10.24 | |
| YLWS_10 (nº) | 4.58 | 2.08 | 0.14 | 45.42 | 1.00 | 9.32 | 0.28 | −0.65 | 3.10 | 4.32 | 6.04 | |
| 2 (n = 208) | Bunch | 10,855.82 | 1575.60 | 109.25 | 14.51 | 6297.00 | 15,843.00 | 0.30 | 0.42 | 9899.00 | 10,640.00 | 11,775.00 |
| INC | 0.08 | 0.03 | 0.00 | 35.06 | 0.03 | 0.17 | 0.85 | 0.62 | 0.06 | 0.07 | 0.09 | |
| YLS (nº) | 13.24 | 1.93 | 0.13 | 14.60 | 7.95 | 16.04 | −0.78 | −0.40 | 11.96 | 13.84 | 14.56 | |
| YLWS (nº) | 10.13 | 1.40 | 0.10 | 13.84 | 6.50 | 13.36 | −0.05 | −0.36 | 9.00 | 10.28 | 11.00 | |
| SIN | 54.49 | 10.47 | 0.73 | 19.22 | 40.00 | 103.00 | 1.33 | 2.41 | 47.20 | 52.00 | 59.20 | |
| LEAF (nº) | 13.19 | 1.03 | 0.07 | 7.81 | 10.45 | 15.12 | −0.61 | −0.30 | 12.48 | 13.36 | 13.96 | |
| LEAF_7 (nº) | 9.47 | 1.30 | 0.09 | 13.73 | 5.85 | 11.56 | −0.67 | −0.53 | 8.48 | 9.84 | 10.52 | |
| LEAF_10(nº) | 8.42 | 1.60 | 0.11 | 18.99 | 4.73 | 10.92 | −0.55 | −0.97 | 7.08 | 9.00 | 9.80 | |
| INC_7 | 0.15 | 0.06 | 0.00 | 40.05 | 0.06 | 0.35 | 1.05 | 0.85 | 0.11 | 0.14 | 0.18 | |
| YLS_7 (nº) | 8.89 | 2.27 | 0.16 | 25.57 | 3.10 | 12.56 | −0.66 | −0.53 | 7.40 | 9.40 | 10.68 | |
| YLWS_7(nº) | 5.36 | 1.94 | 0.13 | 36.20 | 1.00 | 9.56 | −0.19 | −0.67 | 3.80 | 5.56 | 6.84 | |
| INC_10 | 0.21 | 0.08 | 0.01 | 38.67 | 0.09 | 0.45 | 0.90 | 0.20 | 0.14 | 0.19 | 0.26 | |
| YLS_10 (nº) | 7.31 | 2.51 | 0.17 | 34.34 | 1.00 | 11.68 | −0.75 | −0.42 | 5.85 | 7.92 | 9.28 | |
| YLWS_10(nº) | 3.53 | 1.88 | 0.13 | 53.35 | 1.00 | 7.40 | 0.22 | −1.20 | 1.70 | 3.50 | 5.32 | |
| 3 (n = 207) | Bunch (nº) | 8984.02 | 1479.62 | 102.84 | 16.47 | 5543.00 | 12,517.00 | −0.01 | −0.05 | 8137.00 | 8969.00 | 9915.00 |
| INC | 0.07 | 0.03 | 0.00 | 41.15 | 0.03 | 0.17 | 1.17 | 1.59 | 0.05 | 0.06 | 0.08 | |
| YLS (nº) | 13.04 | 1.49 | 0.10 | 11.45 | 8.45 | 15.85 | −0.94 | 0.63 | 12.30 | 13.36 | 14.04 | |
| YLWS (nº) | 10.53 | 1.22 | 0.08 | 11.59 | 7.45 | 13.30 | −0.09 | −0.40 | 9.60 | 10.56 | 11.35 | |
| SIN | 56.76 | 12.60 | 0.88 | 22.19 | 40.00 | 106.00 | 1.17 | 1.37 | 48.00 | 52.80 | 64.80 | |
| LEAF (nº) | 12.89 | 0.72 | 0.05 | 5.61 | 11.05 | 14.85 | −0.19 | −0.30 | 12.43 | 12.96 | 13.40 | |
| LEAF_7 (nº) | 9.62 | 0.75 | 0.05 | 7.84 | 7.45 | 11.45 | −0.66 | 0.77 | 9.28 | 9.70 | 10.12 | |
| LEAF_10 (nº) | 8.73 | 0.93 | 0.06 | 10.70 | 5.95 | 10.25 | −0.90 | 0.42 | 8.30 | 8.88 | 9.44 | |
| INC_7 | 0.14 | 0.05 | 0.00 | 40.45 | 0.05 | 0.32 | 1.17 | 1.65 | 0.09 | 0.13 | 0.16 | |
| YLS_7 (nº) | 9.27 | 1.78 | 0.12 | 19.21 | 3.78 | 12.65 | −0.81 | 0.13 | 8.18 | 9.72 | 10.52 | |
| YLWS_7 (nº) | 6.07 | 1.66 | 0.12 | 27.36 | 1.55 | 9.45 | −0.26 | −0.28 | 4.88 | 6.08 | 7.28 | |
| INC_10 | 0.19 | 0.17 | 0.01 | 88.06 | 0.05 | 2.43 | 10.90 | 137.99 | 0.13 | 0.17 | 0.22 | |
| YLS_10 (nº) | 7.85 | 2.00 | 0.14 | 25.48 | 2.85 | 11.15 | −0.84 | −0.23 | 6.30 | 8.40 | 9.36 | |
| YLWS_10 (nº) | 4.31 | 1.74 | 0.12 | 40.42 | 1.00 | 7.75 | 0.04 | −0.82 | 3.20 | 4.12 | 5.80 |
| K-Folds | Training Data | Testing Data | ||||
|---|---|---|---|---|---|---|
| RMSE (nº Bunches) | Variance Explained (%) | R2 | RMSE (nº Bunches) | Variance Explained (%) | R2 | |
| 1 | 1112.93 | 69.1 | 0.69 | 1132.31 | 72.0 | 0.72 |
| 2 | 1112.33 | 71.0 | 0.71 | 1101.23 | 71.0 | 0.71 |
| 3 | 1145.67 | 70.0 | 0.70 | 1090.26 | 69.0 | 0.69 |
| k-folds CI 95% * | 1076–1171 | 68.0–72.0 | 0.68–0.72 | 1054–1162 | 67.0–74.0 | 0.67–0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares, B.O.; Vega, A.; Rueda Calderón, M.A.; Montenegro-Gracia, E.; Araya-Almán, M.; Marys, E. Prediction of Banana Production Using Epidemiological Parameters of Black Sigatoka: An Application with Random Forest. Sustainability 2022, 14, 14123. https://doi.org/10.3390/su142114123
Olivares BO, Vega A, Rueda Calderón MA, Montenegro-Gracia E, Araya-Almán M, Marys E. Prediction of Banana Production Using Epidemiological Parameters of Black Sigatoka: An Application with Random Forest. Sustainability. 2022; 14(21):14123. https://doi.org/10.3390/su142114123
Chicago/Turabian StyleOlivares, Barlin O., Andrés Vega, María A. Rueda Calderón, Edilberto Montenegro-Gracia, Miguel Araya-Almán, and Edgloris Marys. 2022. "Prediction of Banana Production Using Epidemiological Parameters of Black Sigatoka: An Application with Random Forest" Sustainability 14, no. 21: 14123. https://doi.org/10.3390/su142114123
APA StyleOlivares, B. O., Vega, A., Rueda Calderón, M. A., Montenegro-Gracia, E., Araya-Almán, M., & Marys, E. (2022). Prediction of Banana Production Using Epidemiological Parameters of Black Sigatoka: An Application with Random Forest. Sustainability, 14(21), 14123. https://doi.org/10.3390/su142114123











