Comparative Study on the Adsorption Characteristics of Heavy Metal Ions by Activated Carbon and Selected Natural Adsorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Adsorption Experiments
2.3. Adsorption Kinetics
2.4. Adsorption Isotherm
2.5. Adsorption Thermodynamics
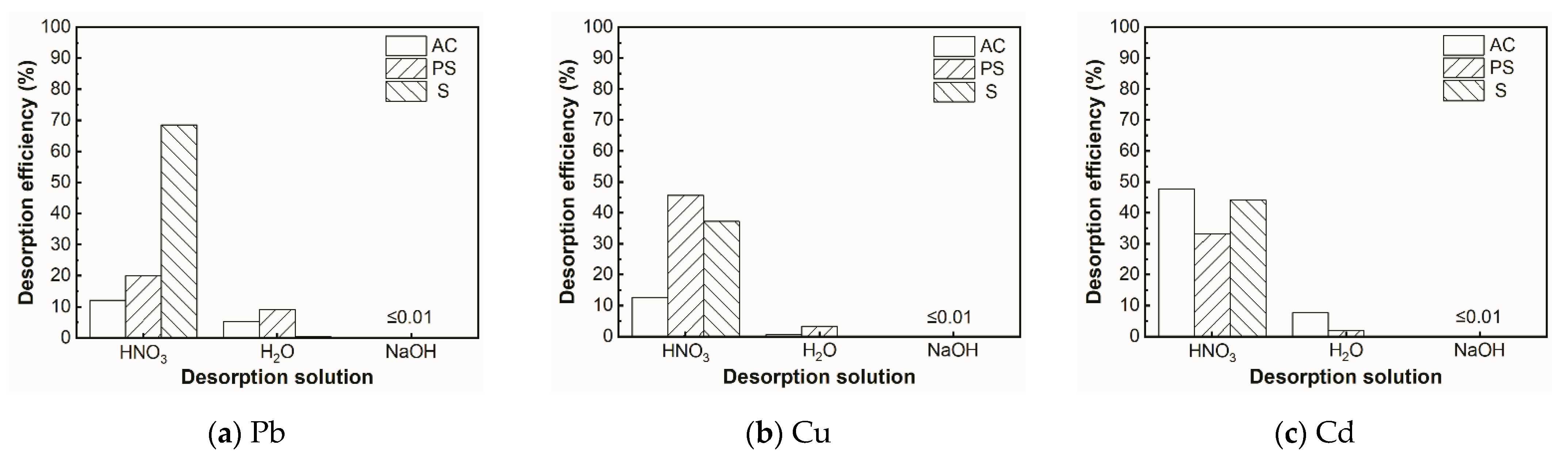
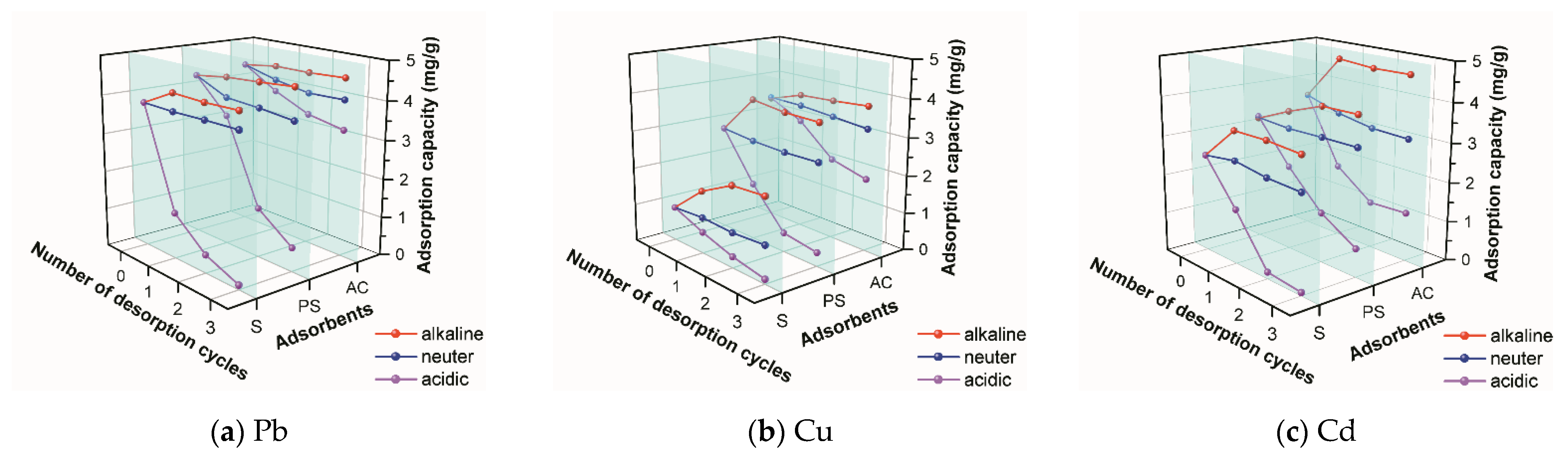
2.6. Desorption Experiment
3. Results and Discussion
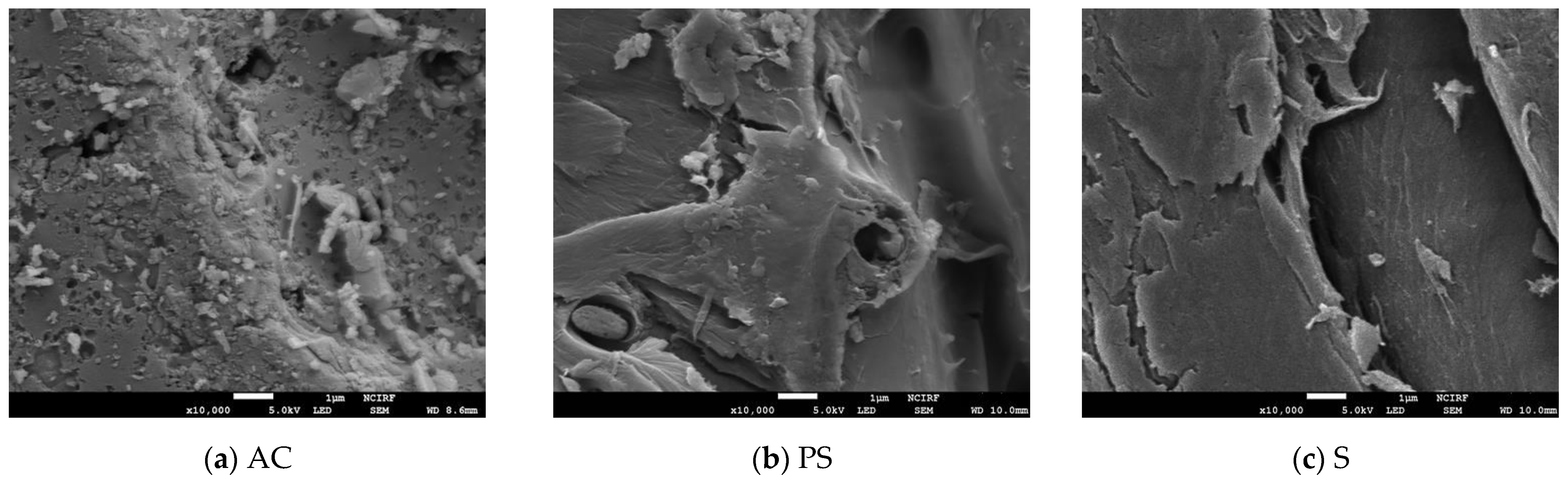
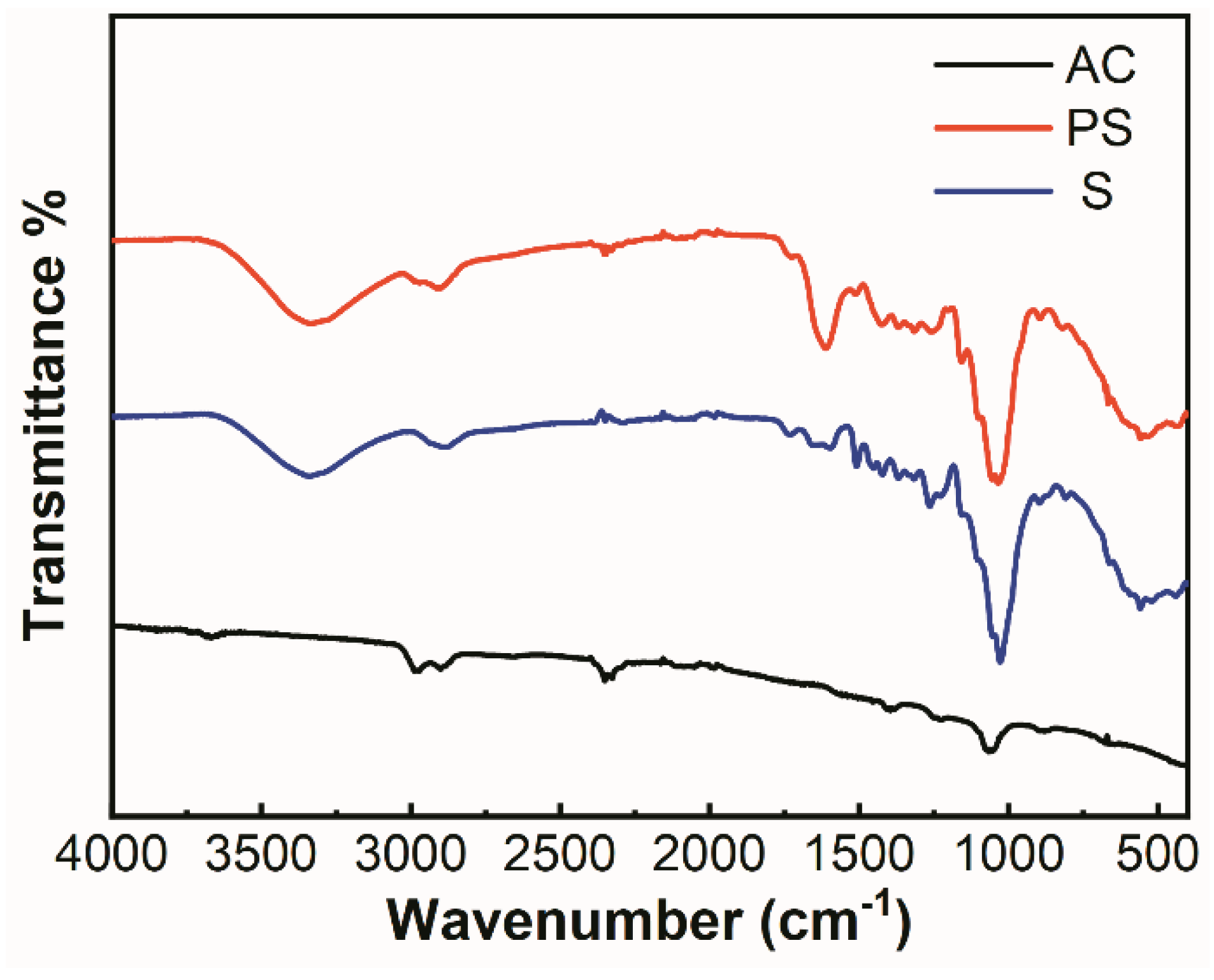
3.1. Adsorbent Characteristics
3.2. Factors Affecting Absorption
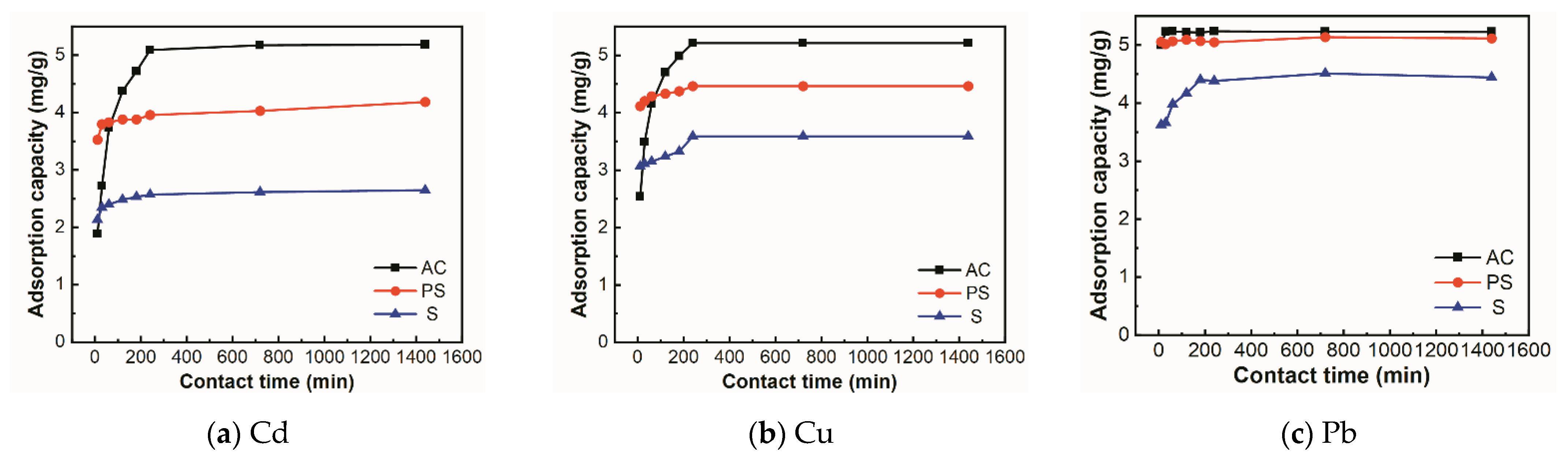
3.2.1. Effect of Contact Time
3.2.2. Effect of Temperature
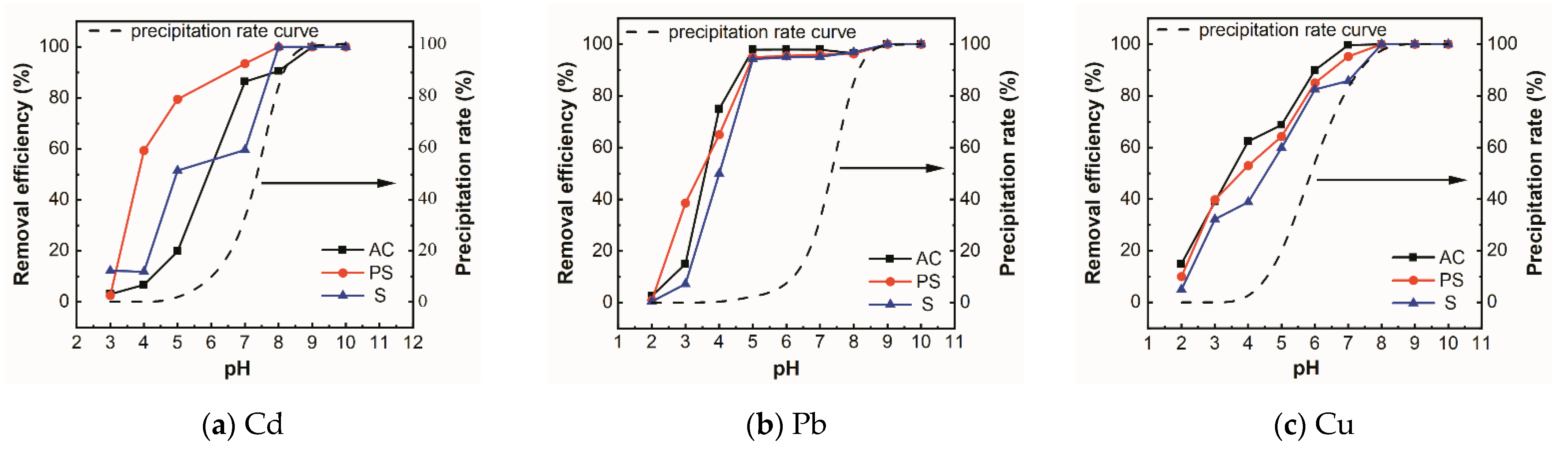
3.2.3. Effect of pH Value
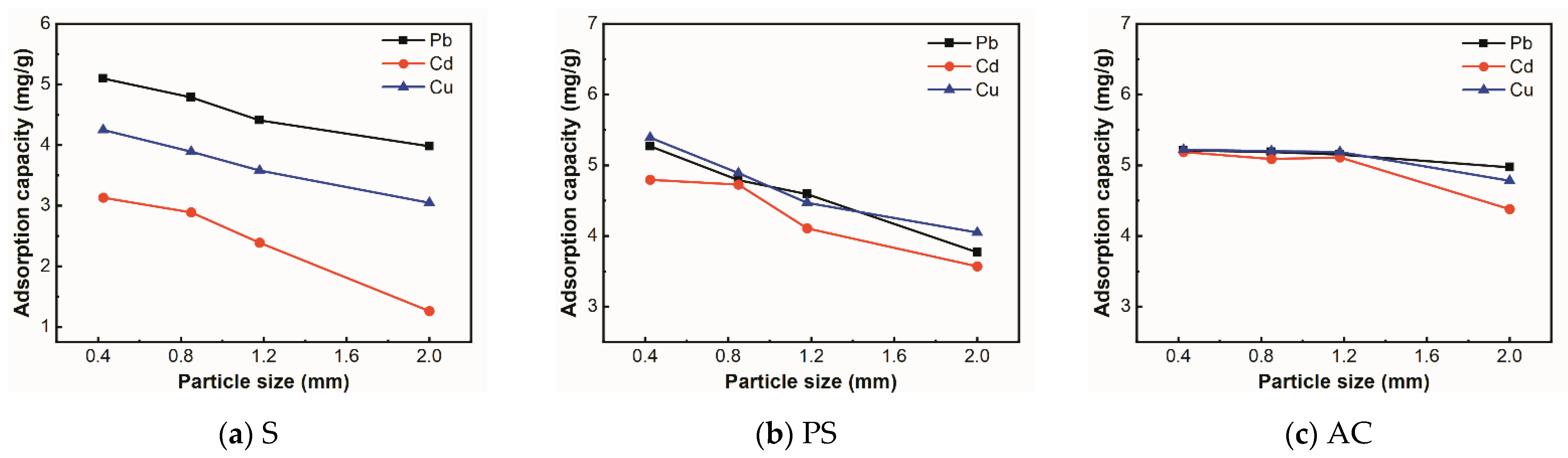
3.2.4. Effect of Adsorbent Particle Size
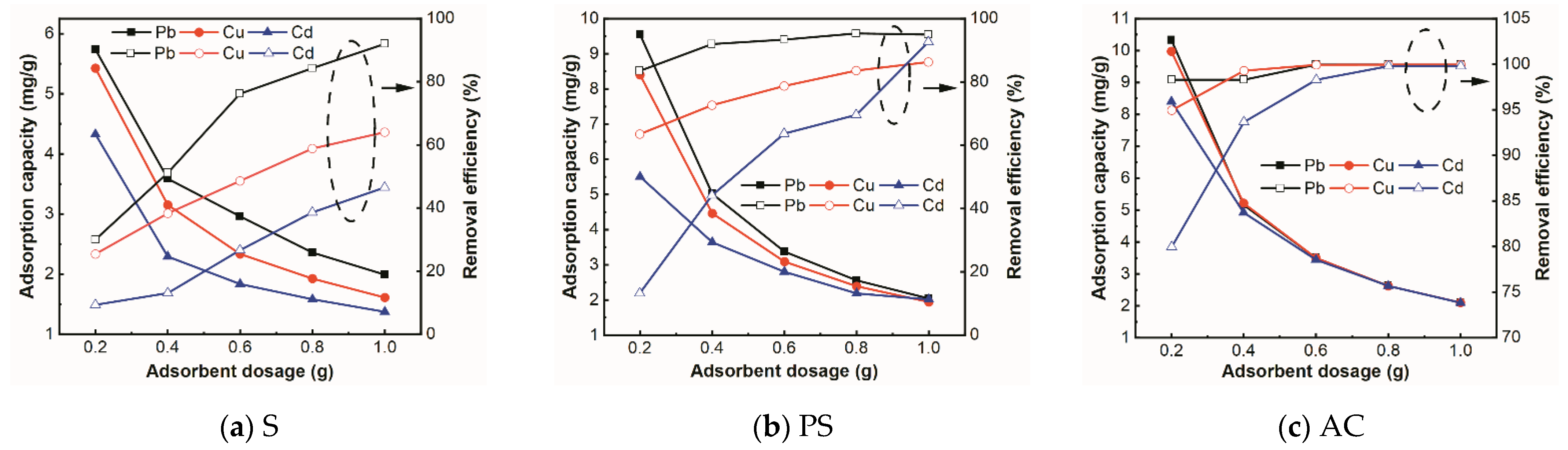
3.2.5. Effect of Adsorbent Dosage
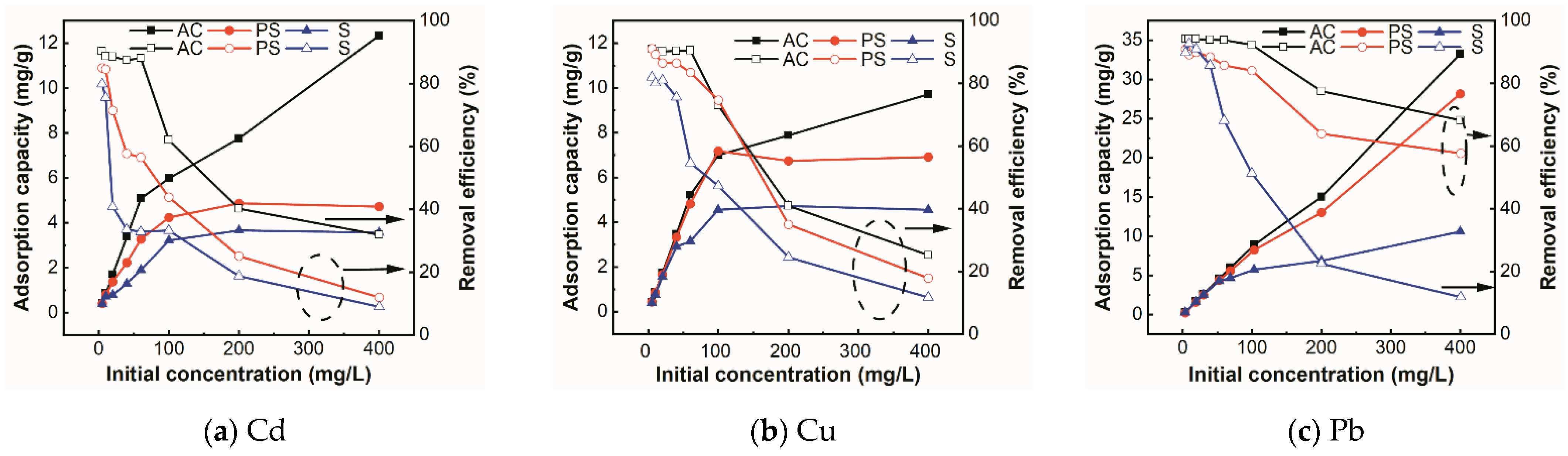
3.2.6. Effect of Initial Concentration
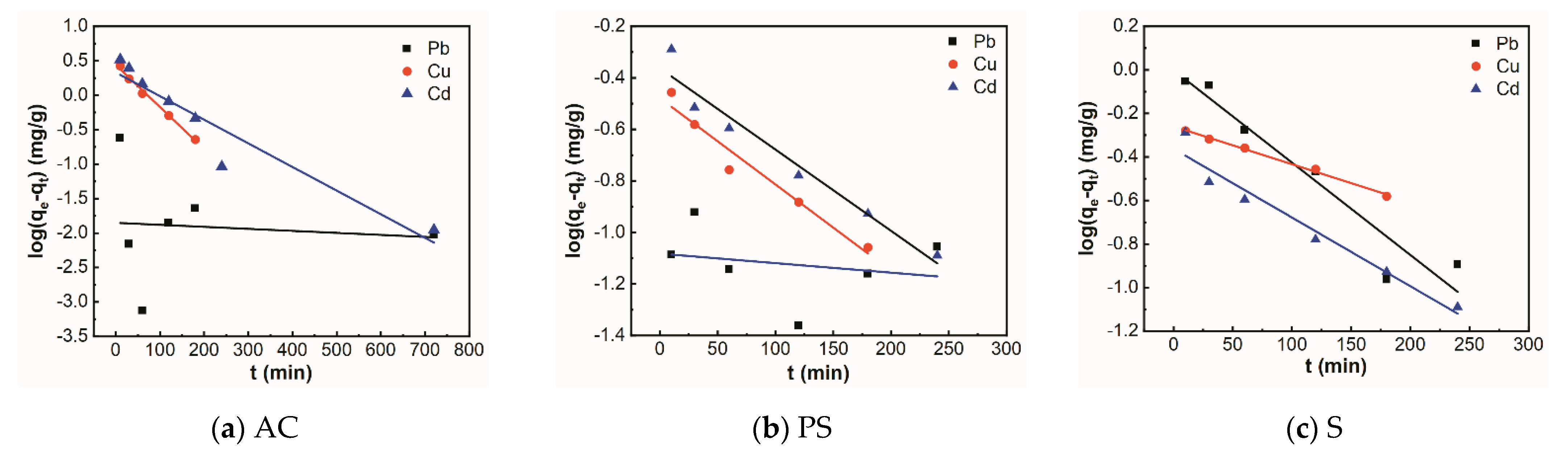
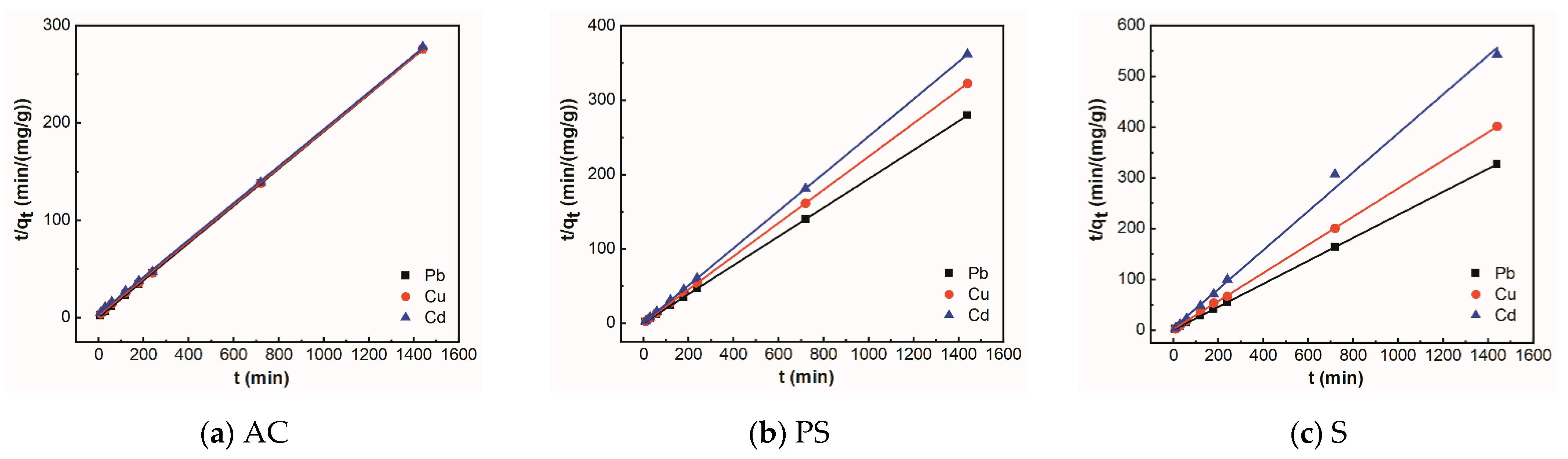
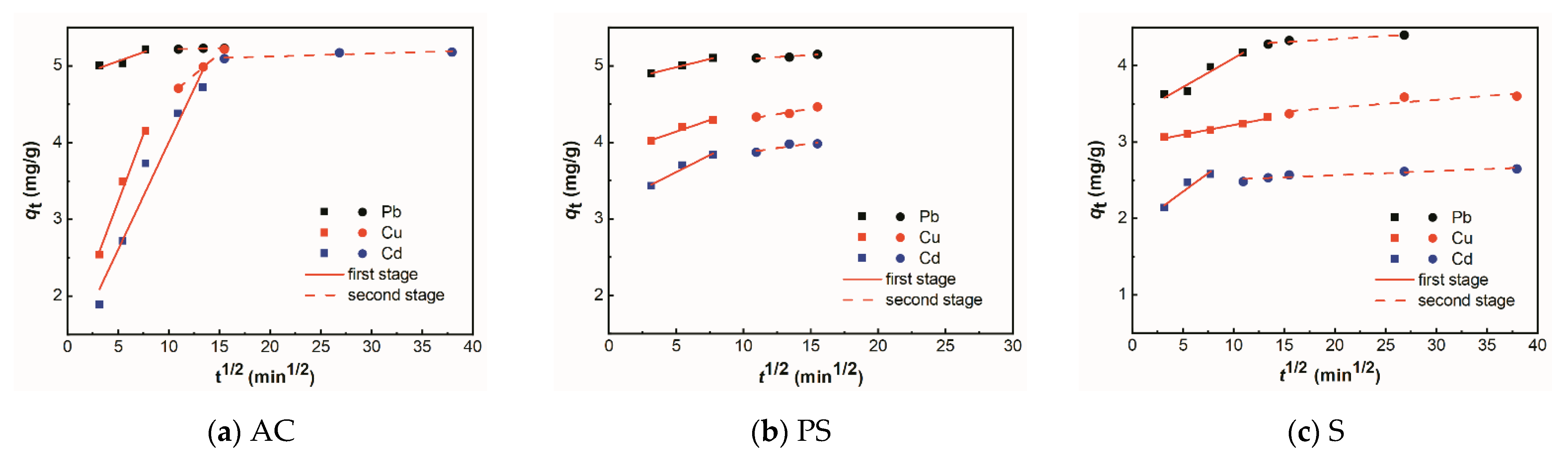
3.3. Adsorption Kinetics
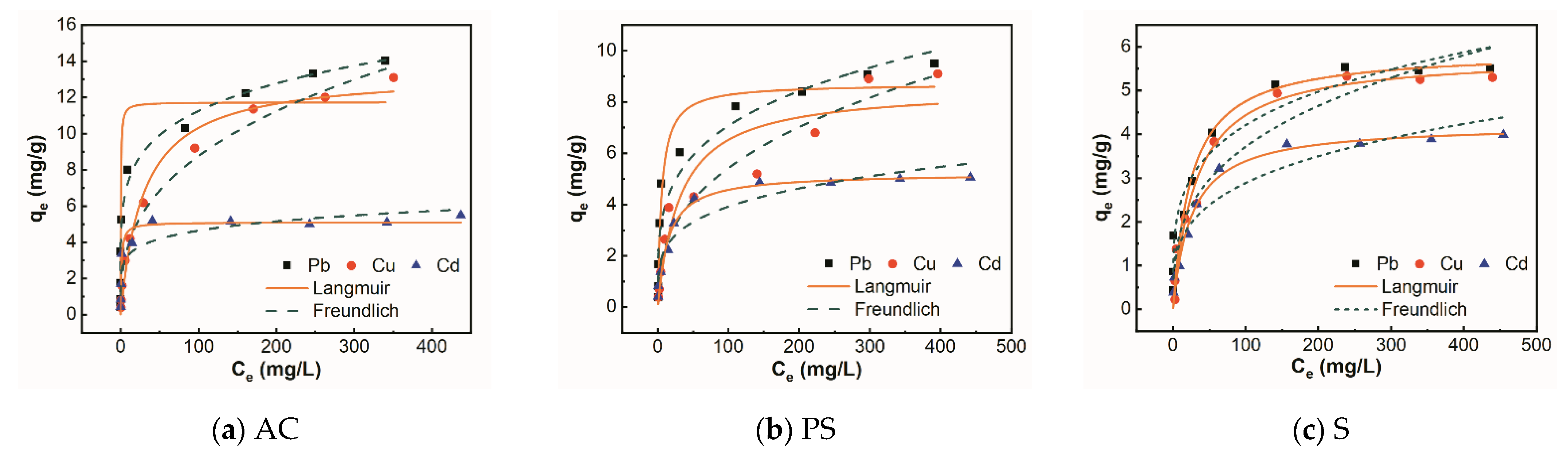
3.4. Adsorption Isotherm
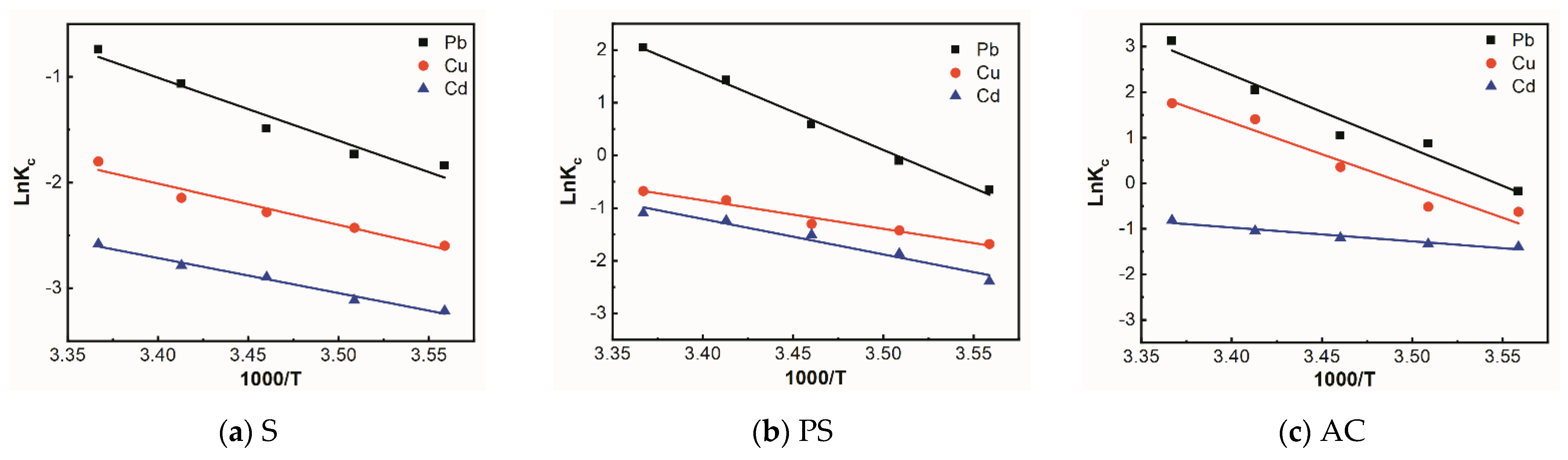
3.5. Adsorption Thermodynamics
3.6. Desorption Analysis
4. Conclusions
- (1)
- Peanut shell (PS), Sawdust (S), and commercial active carbon (AC) were compared for their adsorption and desorption of Pb(II), Cu(II), and Cd(II) ions from aqueous solutions. The results demonstrate that PS is a green adsorbent material that can replace the traditional adsorbent AC and has effective adsorption of Pb(II), Cu(II), and Cd(II) ions from the water.
- (2)
- For three adsorbents, the adsorption capacity increases with the increasing phase temperature, pH value, contact time, adsorbent dosage, and heavy metal ion concentration, but decreases with the increase of adsorbent particle size.
- (3)
- The adsorption kinetics were well described by the pseudo-second-order model, meanwhile, the adsorption isotherms were well described by the Langmuir or Freundlich models. The adsorption process is a spontaneous heat absorption process.
- (4)
- It was shown that the desorption rate of adsorbents is higher in acidic environments than in other environments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Chatterjee, A.; Abraham, J. Desorption of heavy metals from metal loaded sorbents and e-wastes: A review. Biotechnol. Lett. 2019, 41, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, M.; Duan, C.; Sun, J.; Xu, Y. Preparation and characterization of cellulose-based adsorbent and its application in heavy metal ions removal. Carbohydr. Polym. 2019, 206, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Tian, X.; Jiang, Z.; Cao, B.; Akindolie, M.S.; Hu, Q.; Feng, C.; Feng, Y.; Meng, X.; Zhang, Y. Multi-component adsorption of Pb(II), Cd(II) and Ni(II) onto microwave-functionalized cellulose: Kinetics, isotherms, thermodynamics, mechanisms and application for electroplating wastewater purification. J. Hazard. Mater. 2020, 387, 121718. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Amiri, M.J.; Mousavi, S. Kinetic, equilibrium and thermodynamic investigations of Ni (II), Cd (II), Cu (II) and Co (II) adsorption on barley straw ash. Water Resour. Ind. 2014, 6, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Parmar, M.; Thakur, L.S. Heavy metal Cu, Ni and Zn: Toxicity, health hazards and their removal techniques by low cost adsorbents: A short overview. Int. J. Plant Sci. 2013, 3, 143–157. [Google Scholar]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yava, I.; Unay, A.; Abdel-Daim, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of Floating Aquatic Plants in Phytoremediation of Heavy Metals Polluted Water: A Review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K.S.M. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery From Various Environments—A Review. Front. Environ. Sci. 2019, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Feng, X.; Long, R.; Wang, L.; Liu, C.; Bai, Z.; Liu, X. A review on heavy metal ions adsorption from water by layered double hydroxide and its composites. Sep. Purif. Technol. 2022, 284, 120099. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Q.; Nie, J.; Poon, C.S.; Yin, H.; Li, J.-s. Arsenate(V) removal from aqueous system by using modified incinerated sewage sludge ash (ISSA) as a novel adsorbent. Chemosphere 2021, 270, 129423. [Google Scholar] [CrossRef] [PubMed]
- Martin-Lara, M.A.; Calero, M.; Ronda, A.; Ianez-Rodriguez, I.; Escudero, C. Adsorptive Behavior of an Activated Carbon for Bisphenol A Removal in Single and Binary (Bisphenol A-Heavy Metal) Solutions. Water 2020, 12, 2150. [Google Scholar] [CrossRef]
- İnce, M.; Kaplan İnce, O. An Overview of Adsorption Technique for Heavy Metal Removal from Water/Wastewater: A Critical Review. Int. J. Pure Appl. Sci. 2017, 3, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Skoczko, I.; Gumiński, R.; Bos, E.; Zgłobicka, I. Impact of chemical activation on selected adsorption features of powdered activated carbon. Desalination Water Treat. 2021, 243, 165–179. [Google Scholar] [CrossRef]
- Szatyłowicz, E.; Skoczko, I. Studies on the efficiency of grundwater treatment process with adsorption on activated alumina. J. Ecol. Eng. 2017, 18, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Pardo, B.; Ferrer, N.; Sempere, J.; Gonzalez-Olmos, R. A key parameter on the adsorption of diluted aniline solutions with activated carbons: The surface oxygen content. Chemosphere 2016, 162, 181–188. [Google Scholar] [CrossRef]
- Zhu, W.; Du, W.; Shen, X.; Zhang, H.; Ding, Y. Comparative adsorption of Pb2+ and Cd2+ by cow manure and its vermicompost. Environ. Pollut. 2017, 227, 89–97. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Oh, M.; Park, J. Oyster Shell as a Low-Cost Adsorbent for Removing Heavy Metal Ions from Wastewater. Pol. J. Environ. Stud. 2019, 28, 2949–2959. [Google Scholar] [CrossRef] [PubMed]
- Veli, S.; Alyuez, B. Adsorption of copper and zinc from aqueous solutions by using natural clay. J. Hazard. Mater. 2007, 149, 226–233. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Kalkan, E.; Demir, N. Removal of copper from aqueous solution using red mud. Desalination 2010, 251, 90–95. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Kim, M.; Li, D.; Kim, H.; Huang, C.-p. The removal of phosphate by thermally treated red mud from water: The effect of surface chemistry on phosphate immobilization. Chemosphere 2020, 247, 125867. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, J.; Yu, J.X.; Wang, Y.; Chi, R.A. Effects of surface modification on heavy metal adsorption performance and stability of peanut shell and its extracts of cellulose, lignin, and hemicellulose. Environ. Sci. Pollut. Res. 2020, 27, 26502–26510. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, J. A general kinetic model for adsorption: Theoretical analysis and modeling. J. Mol. Liq. 2019, 288, 111100. [Google Scholar] [CrossRef]
- Akram, M.; Bhatti, H.N.; Iqbal, M.; Noreen, S.; Sadaf, S. Biocomposite efficiency for Cr(VI) adsorption: Kinetic, equilibrium and thermodynamics studies. J. Environ. Chem. Eng. 2017, 5, 400–411. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, J.; Hazra, S.; Basu, S. Adsorption of heavy metal ions by mesoporous ZnO and TiO2@ZnO monoliths: Adsorption and kinetic studies. Microchem. J. 2019, 145, 105–112. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Dong, X.; Park, J. Competitive Adsorption of Heavy Metal Ions from Aqueous Solutions onto Activated Carbon and Agricultural Waste Materials. Pol. J. Environ. Stud. 2020, 29, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.A.; Jellali, S.; Jedidi, N. Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour. Technol. 2010, 101, 5070–5075. [Google Scholar] [CrossRef]
- Taşar, Ş.; Kaya, F.; Özer, A. Biosorption of lead (II) ions from aqueous solution by peanut shells: Equilibrium, thermodynamic and kinetic studies. J. Environ. Chem. Eng. 2014, 2, 1018–1026. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Lima, E.C.; Royer, B.; Bach, M.V.; Dotto, G.L.; Pinto, L.A.A.; Calvete, T. Comparison of Spirulina platensis microalgae and commercial activated carbon as adsorbents for the removal of Reactive Red 120 dye from aqueous effluents. J. Hazard. Mater. 2012, 241, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ghazi, Z.A.; Saeed, M.; Ilyas, M.; Ahmad, R.; Khattak, A.M.; Iqbal, A. A comparative study of the removal of Cr(VI) from synthetic solution using natural biosorbents. N. J. Chem. 2017, 41, 10799–10807. [Google Scholar] [CrossRef]
- Kilic, M.; Kirbiyik, C.; Cepeliogullar, O.; Putun, A.E. Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl. Surf. Sci. 2013, 283, 856–862. [Google Scholar] [CrossRef]
- Chandarana, H.; Subburaj, S.; Kumar, P.S.; Kumar, M.A. Evaluation of phase transfer kinetics and thermodynamic equilibria of Reactive Orange 16 sorption onto chemically improved Arachis hypogaea pod powder. Chemosphere 2021, 276, 130136. [Google Scholar] [CrossRef] [PubMed]
- Skoczko, I.; Szatyłowicz, E. Removal of heavy metal ions by filtration on activated alumina-assisted magnetic field. Desalination Water Treat. 2018, 117, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.S.; Zohdy, M.H. Adsorption Kinetics of Toxic Heavy Metal Ions from Aqueous Solutions onto Grafted Jute Fibers with Acrylic Acid by Gamma Irradiation. J. Nat. Fibers 2018, 15, 506–516. [Google Scholar] [CrossRef]
- Barnie, S.; Zhang, J.; Wang, H.; Yin, H.; Chen, H. The influence of pH, co-existing ions, ionic strength, and temperature on the adsorption and reduction of hexavalent chromium by undissolved humic acid. Chemosphere 2018, 212, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Afroze, S.; Sen, T.K. A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water Air Soil Pollut. 2018, 229, 1–50. [Google Scholar] [CrossRef]
- Cherdchoo, W.; Nithettham, S.; Charoenpanich, J. Removal of Cr(VI) from synthetic wastewater by adsorption onto coffee ground and mixed waste tea. Chemosphere 2019, 221, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.; Xu, L.; Qiang, Z.; Dong, H.; Shi, H. Preparation of green biosorbent using rice hull to preconcentrate, remove and recover heavy metal and other metal elements from water. Chemosphere 2021, 262, 127940. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.B.; Singh, A.; Jha, J.M.; Srivastava, N.; Hashem, A.; Alakeel, M.A.; Abd Allah, E.F.; Gupta, V.K. Low-cost biochar adsorbents prepared from date and delonix regia seeds for heavy metal sorption. Bioresour. Technol. 2021, 339, 125606. [Google Scholar] [CrossRef] [PubMed]
- Al-Senani, G.M.; Al-Fawzan, F.F. Adsorption study of heavy metal ions from aqueous solution by nanoparticle of wild herbs. Egypt. J. Aquat. Res. 2018, 44, 187–194. [Google Scholar] [CrossRef]
- Elboughdiri, N.; Arellano-Garcia, H. The use of natural zeolite to remove heavy metals Cu (II), Pb (II) and Cd (II), from industrial wastewater. Cogent Eng. 2020, 7, 1782623. [Google Scholar] [CrossRef]
- Abd Elhafez, S.E.; Hamad, H.A.; Zaatout, A.A.; Malash, G.F. Management of agricultural waste for removal of heavy metals from aqueous solution: Adsorption behaviors, adsorption mechanisms, environmental protection, and techno-economic analysis. Environ. Sci. Pollut. Res. 2017, 24, 1397–1415. [Google Scholar] [CrossRef]
- Xiao, M.; Hu, J. Cellulose/chitosan composites prepared in ethylene diamine/potassium thiocyanate for adsorption of heavy metal ions. Cellulose 2017, 24, 2545–2557. [Google Scholar] [CrossRef]
- Guiza, S. Biosorption of heavy metal from aqueous solution using cellulosic waste orange peel. Ecol. Eng. 2017, 99, 134–140. [Google Scholar] [CrossRef]
- Islam, M.S.; Kwak, J.H.; Nzediegwu, C.; Wang, S.Y.; Palansuriya, K.; Kwon, E.E.; Naeth, M.A.; El-Din, M.G.; Ok, Y.S.; Chang, S.X. Biochar heavy metal removal in aqueous solution depends on feedstock type and pyrolysis purging gas. Environ. Pollut. 2021, 281, 117094. [Google Scholar] [CrossRef] [PubMed]
- Afroze, S.; Sen, T.K.; Ang, H.M. Adsorption removal of zinc (II) from aqueous phase by raw and base modified Eucalyptus sheathiana bark: Kinetics, mechanism and equilibrium study. Process Saf. Environ. Prot. 2016, 102, 336–352. [Google Scholar] [CrossRef]
- An, F.-Q.; Wu, R.-Y.; Li, M.; Hu, T.-P.; Gao, J.-F.; Yuan, Z.-G. Adsorption of heavy metal ions by iminodiacetic acid functionalized D301 resin: Kinetics, isotherms and thermodynamics. React. Funct. Polym. 2017, 118, 42–50. [Google Scholar] [CrossRef]
- Forghani, M.; Azizi, A.; Livani, M.J.; Kafshgari, L.A. Adsorption of lead(II) and chromium(VI) from aqueous environment onto metal-organic framework MIL-100(Fe): Synthesis, kinetics, equilibrium and thermodynamics. J. Solid State Chem. 2020, 291, 121636. [Google Scholar] [CrossRef]
- Priya, A.K.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.A.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of mechanism of heavy metals (Cr6+, Pb2+ & Zn2+) adsorption from aqueous medium using rice husk ash: Kinetic and thermodynamic approach. Chemosphere 2022, 286, 131796. [Google Scholar] [CrossRef]
- Reddy, K.J.; Wang, L.; Gloss, S.P. Solubility and mobility of copper, zinc and lead in acidic environments. Plant Soil 1995, 171, 53–58. [Google Scholar] [CrossRef]
- Smith, A.E. A study of the variation with pH of the solubility and stability of some metal ions at low concentrations in aqueous solution. Part I. Analyst 1973, 98, 65–68. [Google Scholar] [CrossRef]
- Duursma, E.K.; Sevenhuysen, W. Note on chelation and solubility of certain metals in sea water at different pH values. Neth. J. Sea Res. 1966, 3, 95–106. [Google Scholar] [CrossRef]
- Fang, T.; Guo, H.; Zeng, L.; Verma, V.; Nenes, A.; Weber, R.J. Highly acidic ambient particles, soluble metals, and oxidative potential: A link between sulfate and aerosol toxicity. Environ. Sci. Technol. 2017, 51, 2611–2620. [Google Scholar] [CrossRef]
- Lata, S.; Singh, P.K.; Samadder, S.R. Regeneration of adsorbents and recovery of heavy metals: A review. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478. [Google Scholar] [CrossRef] [Green Version]
- Chuan, M.C.; Shu, G.Y.; Liu, J.C. Solubility of heavy metals in a contaminated soil: Effects of redox potential and pH. Water Air Soil Pollut. 1996, 90, 543–556. [Google Scholar] [CrossRef]

| Adsorption Material | AC | PS | S |
|---|---|---|---|
| pH | 7.6 | 6.7 | 5.1 |
| Specific surface area (m2/g) | 532 | 3.6 | 1.25 |
| Fixed carbon (%) | 82.23 | 27.31 | 16.73 |
| Volatile substances (%) | 11.23 | 50.3 | 59.87 |
| Water content (%) | 3.76 | 8.96 | 9.1 |
| Absorbent | Element | Wt% | At% |
|---|---|---|---|
| AC | C | 92.21 | 94.03 |
| O | 7.91 | 5.97 | |
| PS | C | 50.09 | 57.89 |
| O | 47.39 | 41.11 | |
| Mg | 0.53 | 0.30 | |
| K | 1.05 | 0.37 | |
| Ca | 0.94 | 0.33 | |
| S | C | 55.97 | 62.87 |
| O | 44.03 | 37.13 |
| Adsorbent | Metal Ions | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| K1 | qe | R2 | K2 | qe | R2 | ||
| AC | Pb | 0.013 | 0.22 | 0.75 | 0.52 | 5.26 | 0.99 |
| Cu | 0.006 | 2.75 | 0.91 | 0.02 | 5.26 | 0.99 | |
| Cd | 0.004 | 2.25 | 0.92 | 0.05 | 5.26 | 0.99 | |
| PS | Pb | 0.028 | 0.11 | 0.98 | 0.26 | 5.26 | 0.99 |
| Cu | 0.003 | 0.32 | 0.94 | 0.10 | 4.55 | 0.99 | |
| Cd | 0.014 | 0.93 | 0.77 | 0.15 | 4.00 | 0.99 | |
| S | Pb | 0.005 | 0.92 | 0.96 | 0.06 | 4.35 | 0.99 |
| Cu | 0.002 | 0.55 | 0.98 | 0.04 | 3.57 | 0.99 | |
| Cd | 0.023 | 0.99 | 0.79 | 0.03 | 2.63 | 0.99 | |
| Adsorbent | Metal Ions | Diffusion Coefficient | |||||
|---|---|---|---|---|---|---|---|
| First Stage | Second Stage | ||||||
| Kd | D | R2 | Kd | D | R2 | ||
| AC | Pb | 0.05 | 4.83 | 0.73 | 0.01 | 5.19 | 0.87 |
| Cu | 0.35 | 1.47 | 0.98 | 0.11 | 3.48 | 0.99 | |
| Cd | 0.28 | 1.21 | 0.94 | 0.01 | 5.04 | 0.68 | |
| PS | Pb | 0.43 | 4.76 | 0.99 | 0.01 | 1.97 | 0.86 |
| Cu | 0.06 | 3.84 | 0.92 | 0.03 | 4.01 | 0.89 | |
| Cd | 0.09 | 3.17 | 0.93 | 0.03 | 3.61 | 0.76 | |
| S | Pb | 0.08 | 3.34 | 0.9 | 0.01 | 4.19 | 0.84 |
| Cu | 0.03 | 2.97 | 0.98 | 0.01 | 3.24 | 0.74 | |
| Cd | 0.10 | 1.87 | 0.85 | 0.01 | 2.46 | 0.83 | |
| Adsorbent | Metal Ions | Freundlich Isotherm | Langmuir Isotherm | ||||
|---|---|---|---|---|---|---|---|
| KF | n | R2 | KL | b | R2 | ||
| AC | Pb | 4.86 | 5.48 | 0.98 | 3.14 | 11.73 | 0.91 |
| Cu | 1.70 | 2.81 | 0.99 | 0.03 | 13.33 | 0.98 | |
| Cd | 2.33 | 6.66 | 0.87 | 0.96 | 5.11 | 0.96 | |
| PS | Pb | 2.19 | 3.94 | 0.93 | 0.19 | 8.70 | 0.96 |
| Cu | 0.97 | 2.68 | 0.96 | 0.03 | 8.54 | 0.90 | |
| Cd | 1.30 | 4.17 | 0.92 | 0.07 | 5.23 | 0.98 | |
| S | Pb | 1.39 | 4.16 | 0.96 | 0.04 | 5.91 | 0.92 |
| Cu | 0.85 | 3.11 | 0.92 | 0.03 | 5.81 | 0.97 | |
| Cd | 0.82 | 3.64 | 0.92 | 0.04 | 4.23 | 0.98 | |
| Adsorbent | Metal Ions | ΔS | ΔH | ΔG | R2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 281 K | 285 K | 289 K | 293 K | 297 K | |||||
| PS | Pb | 421.99 | 0.12 | −11.86 | −12.03 | −12.20 | −12.36 | −12.53 | 0.99 |
| Cu | 145.71 | 0.045 | −40.94 | −41.53 | −42.11 | −42.69 | −43.28 | 0.96 | |
| Cd | 179.98 | 0.056 | −50.57 | −51.29 | −52.01 | −52.73 | −53.45 | 0.94 | |
| S | Pb | 160.00 | 0.049 | −44.96 | −45.60 | −46.24 | −46.88 | −47.52 | 0.94 |
| Cu | 93.79 | 0.033 | −26.35 | −26.73 | −27.10 | −27.48 | −27.85 | 0.94 | |
| Cd | 71.24 | 0.028 | −20.02 | −20.30 | −20.59 | −20.87 | −21.16 | 0.98 | |
| AC | Pb | 478.43 | 0.13 | −13.44 | −13.64 | −13.83 | −14.02 | −14.21 | 0.95 |
| Cu | 405.28 | 0.12 | −11.39 | −11.55 | −11.71 | −11.87 | −12.04 | 0.93 | |
| Cd | 77.41 | 0.025 | −21.75 | −22.06 | −22.37 | −22.68 | −22.99 | 0.93 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Dong, X.; Liu, X.; Xu, X.; Duan, W.; Park, J.; Gao, L.; Lu, Y. Comparative Study on the Adsorption Characteristics of Heavy Metal Ions by Activated Carbon and Selected Natural Adsorbents. Sustainability 2022, 14, 15579. https://doi.org/10.3390/su142315579
Li J, Dong X, Liu X, Xu X, Duan W, Park J, Gao L, Lu Y. Comparative Study on the Adsorption Characteristics of Heavy Metal Ions by Activated Carbon and Selected Natural Adsorbents. Sustainability. 2022; 14(23):15579. https://doi.org/10.3390/su142315579
Chicago/Turabian StyleLi, Jiashi, Xiaoqiang Dong, Xiaofeng Liu, Xin Xu, Wei Duan, Junboum Park, Lei Gao, and Yisi Lu. 2022. "Comparative Study on the Adsorption Characteristics of Heavy Metal Ions by Activated Carbon and Selected Natural Adsorbents" Sustainability 14, no. 23: 15579. https://doi.org/10.3390/su142315579





