Struvite Production from Dairy Processing Waste
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Struvite Precipitation
2.3. Analytical Methods
2.4. Process Optimisation and Statistical Analysis
3. Results
3.1. Optimisation of Struvite Recovery from AD Wastewaters
3.2. Improved Struvite Recovery with Mixed Dairy Processing Wastewaters
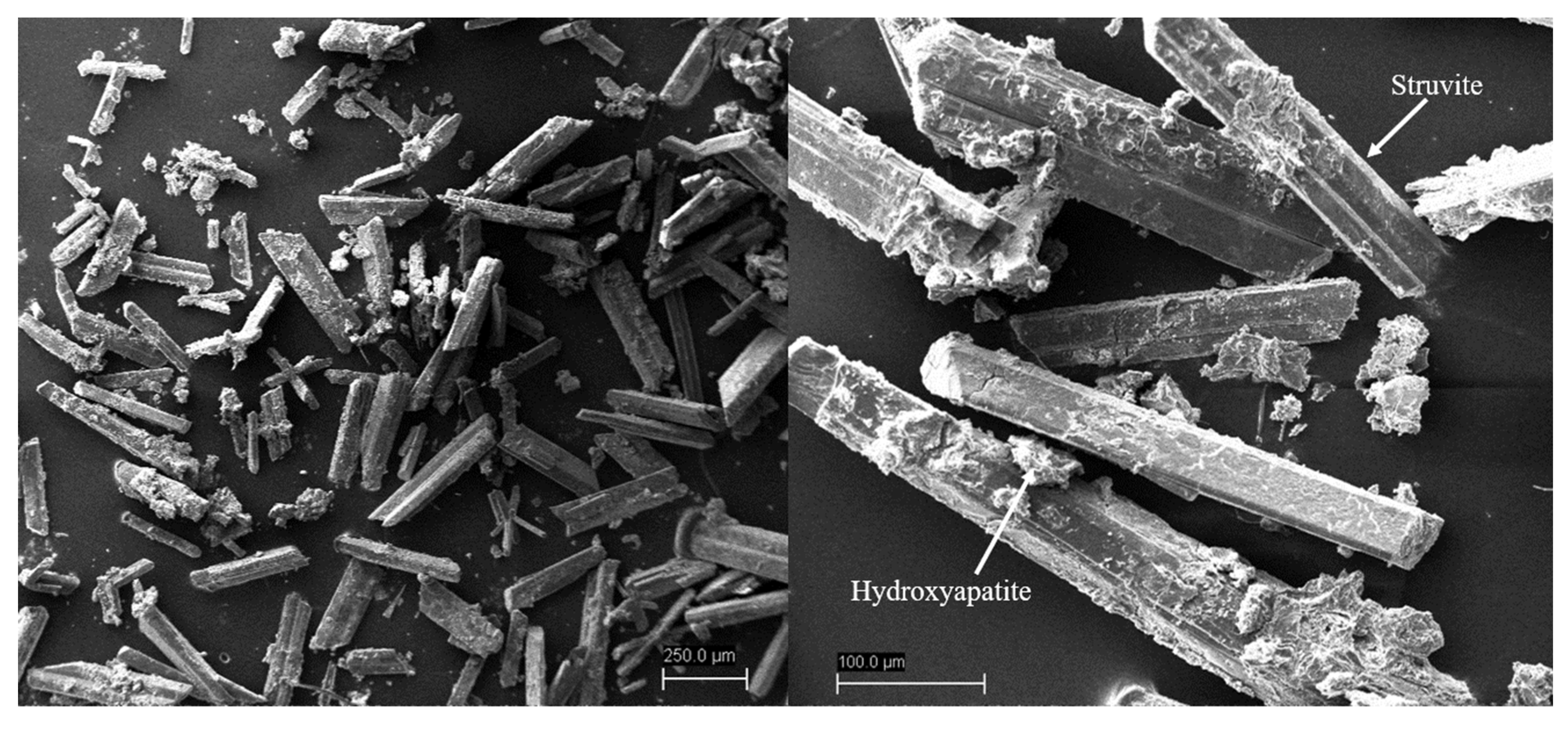
3.3. Characterization of Precipitates by XRD and SEM Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Childers, D.L.; Corman, J.; Edwards, M.; Elser, J. Sustainability Challenges of Phosphorus and Food: Solutions from Closing the Human Phosphorus Cycle. BioScience 2011, 61, 117–124. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Peak Phosphorus: Clarifying the Key Issues of a Vigorous Debate about Long-Term Phosphorus Security. Sustainability 2011, 3, 2027–2049. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Graedel, T.E. A half-century of global phosphorus flows, stocks, production, consumption, recycling, and environmental impacts. Glob. Environ. Chang. 2016, 36, 139–152. [Google Scholar] [CrossRef]
- Clabby, C. Does Peak Phosphorus Loom? Am. Sci. 2010, 98, 291–292. [Google Scholar]
- Rhodes, C.J. Peak Phosphorus—Peak Food? The Need to Close the Phosphorus Cycle. Sci. Prog. 2013, 96, 109–152. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; Jackson, M.; White, S. Phosphorus flows through the Australian food system: Identifying intervention points as a roadmap to phosphorus security. Environ. Sci. Policy 2013, 29, 87–102. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Doody, D.G.; Sylvester-Bradley, R. Achieving Sustainable Phosphorus Use in Food Systems through Circularisation. Sustainability 2018, 10, 1804. [Google Scholar] [CrossRef] [Green Version]
- Scholz, R.W.; Ulrich, A.E.; Eilittä, M.; Roy, A. Sustainable use of phosphorus: A finite resource. Sci. Total Environ. 2013, 461–462, 799–803. [Google Scholar] [CrossRef]
- Mehta, C.; Tuker, R.; Poad, G.; Davis, R.; McGahan, E.; Galloway, J. Nutrients in Australian agro-industrial residues: Production, characteristics and mapping. Australas. J. Environ. Manag. 2016, 23, 206–222. [Google Scholar] [CrossRef]
- Sarvajayakesavalu, S.; Lu, Y.; Whithers, P.; Pavinato, P.S.; Pan, G.; Chareonsudjai, P. Phosphorus recovery: A need for an integrated approach. Ecosyst. Health Sustain. 2018, 4, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, J.P.; Srivastava, V.C.; Mall, I.D. An Overview of Various Technologies for the Treatment of Dairy Wastewaters. Crit. Rev. Food Sci. Nutr. 2011, 51, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Graham, D.W.; Tamaki, H.; Zhang, T. Dominant and novel clades of Candidatus Accumulibacter phosphatis in 18 globally distributed full-scale wastewater treatment plants. Sci. Rep. 2015, 5, 11857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilk, M. A novel method of sewage sludge pre-treatment—HTC. In Proceedings of the 1st International Conference on the Sustainable Energy and Environment Development, Bangkok, Thailand, 20–21 March 2016. [Google Scholar]
- Rittmann, B.E.; Mayer, B.; Westerhoff, P.; Edwards, M. Capturing the lost phosphorus. Chemosphere 2011, 84, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J.A. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, Y.; Fujii, M. Three Years Experience of Operating and Selling Recovered Struvite from Full-Scale Plant. Environ. Technol. 2001, 22, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Latifian, M.; Liu, J.; Mattiasson, B. Struvite-based fertilizer and its physical and chemical properties. Environ. Technol. 2012, 33, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kwag, J.H.; Kim, J.H.; Ra, C. Recovery of nitrogen and phosphorus by struvite crystallization from swine wastewater. Desalination 2011, 277, 364–369. [Google Scholar] [CrossRef]
- Qian, F.; Song, Y. Phosphorus recovery from anaerobic-digested piggery wastewater by collecting reactor of map crystal. Huanjing Kexue Xuebao/Acta Sci. Circumstantiae 2014, 34, 2991–2997. [Google Scholar]
- Ryu, H.D.; Lee, S.L. Application of struvite precipitation as a pretreatment in treating swine wastewater. Process Bioch. 2010, 45, 563–572. [Google Scholar] [CrossRef]
- Yilmazel, Y.D.; Demirer, G.N. Removal and recovery of nutrients as struvite from anaerobic digestion residues of poultry manure. Environ. Technol. 2011, 32, 783–794. [Google Scholar] [CrossRef]
- Zeng, F.; Zhao, Q.; Jin, W.; Liu, Y.; Wang, K.; Lee, D.J. Struvite precipitation from anaerobic sludge supernatant and mixed fresh/stale human urine. J. Chem. Eng. 2018, 344, 254–261. [Google Scholar] [CrossRef]
- Butler, B. Struvite or Traditional Chemical Phosphorus Precipitation—What Option Rocks? Project Reports; Australian Meat Processor Corporation: Sydney, Australia, 2018. [Google Scholar]
- Uysal, A.; Kuru, B. The fertilizer effect of struvite recovered from dairy industry wastewater on the growth and nutrition of maize plant. Fresenius Environ. Bull. 2015, 24, 3155–3162. [Google Scholar]
- Slavov, A. Dairy Wastewaters—General Characteristics and Treatment Possibilities—A Review. Food Technol. Biotechnol. 2017, 55, 14–28. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. De Gruyter: Berlin, Germany, 2015. [Google Scholar]
- Babić-Ivančić, V.; Kontrec, J.; Kralj, D.; Brecevic, L. Precipitation diagrams of struvite and dissolution kinetics of different struvite morphologies. Croat. Chem. Acta 2002, 75, 89–106. [Google Scholar]
- Rouff, A.A. Temperature-dependent phosphorus precipitation and chromium removal from struvite-saturated solutions. J. Colloid Interface 2013, 392, 343–348. [Google Scholar] [CrossRef]
- Kim, D.; Min, K.J.; Lee, K.; Yu, M.S.; Park, K.Y. Effects of pH, molar ratios and pre-treatment on phosphorus recovery through struvite crystallization from effluent of anaerobically digested swine wastewater. Environ. Eng. Res. 2017, 22, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Dai, H.; Wu, Y.; Peng, Y.; Lu, X. A comprehensive review of phosphorus recovery from wastewater by crystallization processes. Chemosphere 2018, 197, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.I.H.; Mavinic, D.S.; Beckie, R.D. A solubility and thermodynamic study of struvite. Environ. Technol. 2007, 28, 1015–1026. [Google Scholar]
- Booker, N.A.; Priestley, A.J.; Fraser, I.H. Struvite Formation in Wastewater Treatment Plants: Opportunities for Nutrient Recovery. Environ. Technol. 1999, 20, 777–782. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, W.; Luo, X.; Xie, B.; Li, G.; Liang, H. Obtaining High-Purity Struvite from Anaerobically Digested Wastewater: Effects of pH, Mg/P, and Ca2+ Interactions. Environ. Eng. Sci. 2019, 36, 102–113. [Google Scholar] [CrossRef]
- Dodson, S.I. Introduction to Limnology, 1st ed.; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Muhmood, A.; Lu, J.; Dong, R.; Wu, S. Formation of struvite from agricultural wastewaters and its reuse on farmlands: Status and hindrances to closing the nutrient loop. J. Environ. Manag. 2019, 230, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Impact of calcium on struvite crystal size, shape and purity. J. Cryst. Growth 2005, 283, 514–522. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Burken, J.B.; Zhang, X.; Surampalli, R.Y. Engineered Struvite Precipitation: Impacts of Component-Ion Molar Ratios and pH. J. Environ. Eng. 2005, 131, 1433–1440. [Google Scholar] [CrossRef]
- Çelen, I.; Buchanan, J.R.; Burns, R.T.; Robinson, R.B.; Raman, D.R. Using a chemical equilibrium model to predict amendmets required to precipitate phosphorus as struvite in liquid swine manure. Water Res. 2007, 41, 1689–1696. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yoo, B.H.; Lim, S.J.; Kim, T.H.; Kim, S.K.; Kim, J.Y. Development and validation of an equilibrium model for struvite formation with calcium co-precipitation. J. Cryst. Growth 2013, 372, 129–137. [Google Scholar] [CrossRef]
- Siciliano, A.; Stillitano, M.A.; Limonti, C.; Marchio, F. Ammonium Removal from Landfill Leachate by Means of Multiple Recycling of Struvite Residues Obtained through Acid Decomposition. Appl. Sci. 2016, 6, 375. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Wu, Y. Improving the prediction of ammonium nitrogen removal through struvite precipitation. Environ. Sci. Pollut. Res. 2012, 19, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Fujii, S. Phosphate recovery by generating hydroxyapatite via reaction of calcium eluted from layered double hydroxides. Appl. Clay Sci. 2014, 99, 261–265. [Google Scholar] [CrossRef]
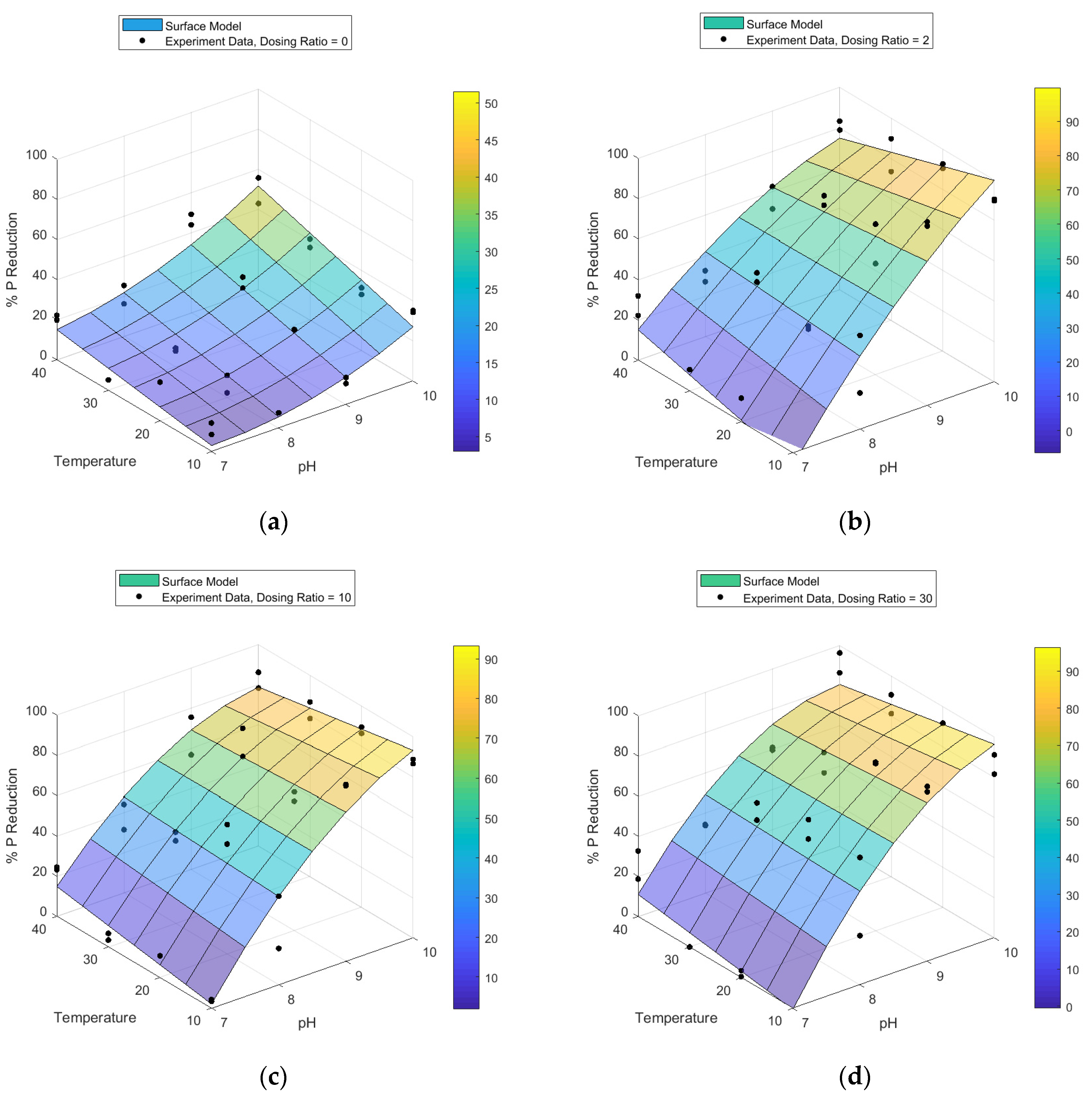
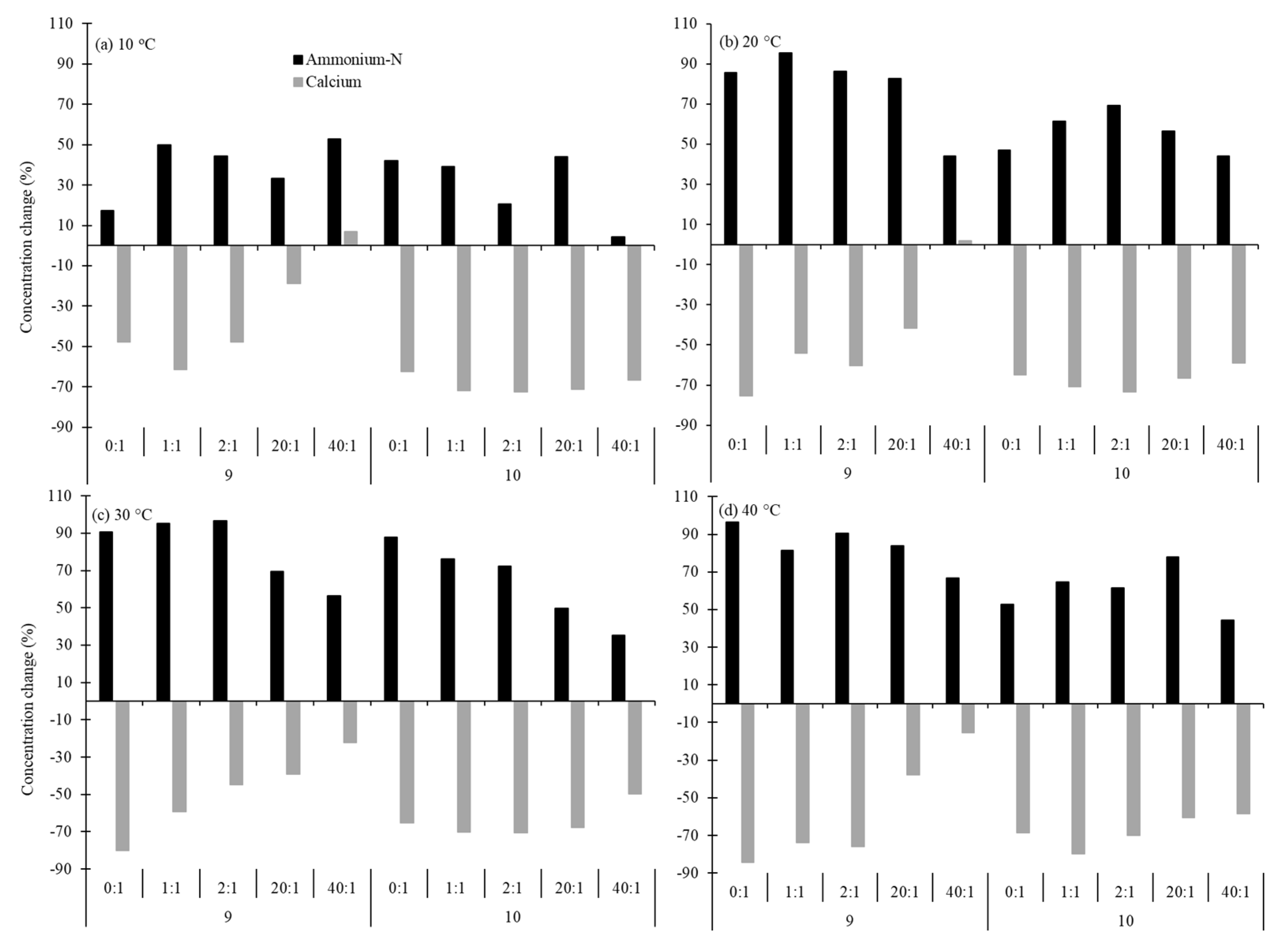
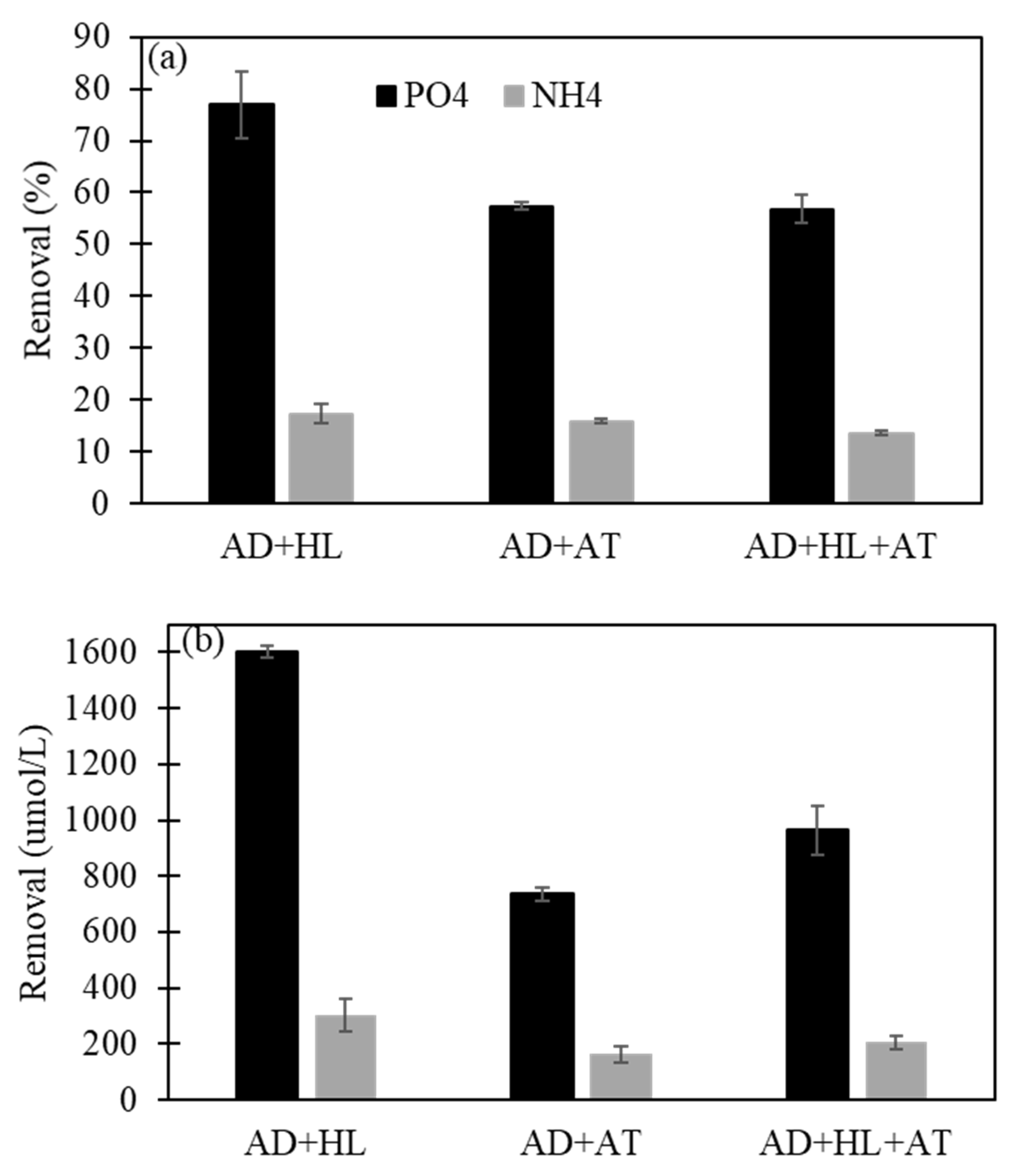
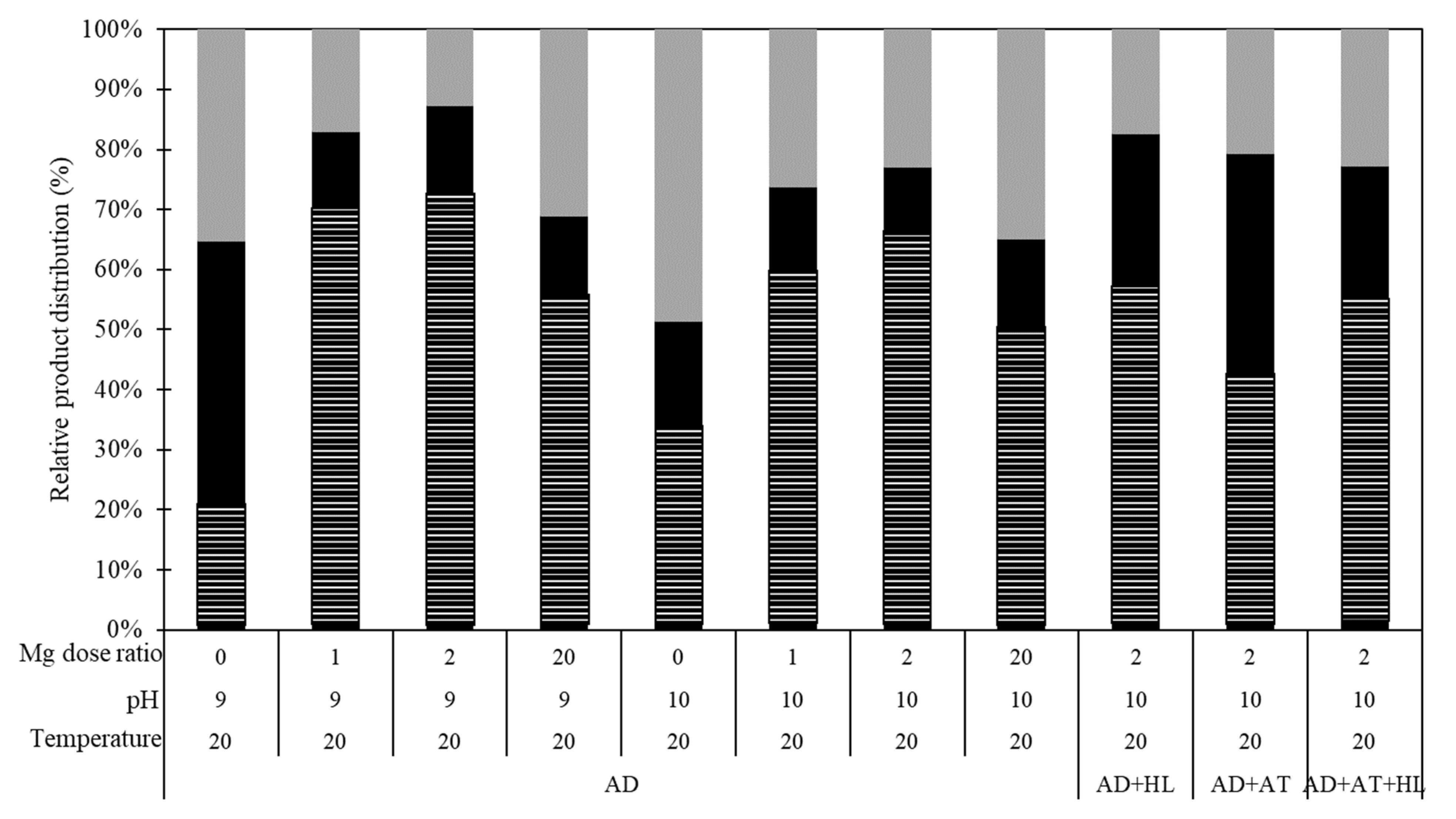
| Processing Wastewater | TP | PO43− | TN | NH4+ | Ca | Mg | pH |
|---|---|---|---|---|---|---|---|
| (mg/L) | (mg/L P) | (mg/L) | (mg/L N) | (mg/L) | (mg/L) | ||
| AD | 81.4 | 67.3 | 212.1 | 111.8 | 40.0 | 6.8 | 7.09 |
| HL | 248.2 | 210.8 | 51.9 | 5.5 | 98.1 | 9.0 | 3.85 |
| AT | 156.5 | 134.1 | 14.3 | 0.8 | 48.4 | 1.4 | 5.84 |
| Factor | Coefficient | 95% Confidence Interval | p-Value |
|---|---|---|---|
| ) | −220 | 30 | |
| ) | 2.1 | 1.2 | 0.00088 |
| ) | 29 | 4 | |
| ) | −0.23 | 0.15 | 0.0017 |
| ) | 0.066 | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McIntosh, S.; Hunt, L.; Thompson Brewster, E.; Rose, A.; Thornton, A.; Erler, D. Struvite Production from Dairy Processing Waste. Sustainability 2022, 14, 15807. https://doi.org/10.3390/su142315807
McIntosh S, Hunt L, Thompson Brewster E, Rose A, Thornton A, Erler D. Struvite Production from Dairy Processing Waste. Sustainability. 2022; 14(23):15807. https://doi.org/10.3390/su142315807
Chicago/Turabian StyleMcIntosh, Shane, Louise Hunt, Emma Thompson Brewster, Andrew Rose, Aaron Thornton, and Dirk Erler. 2022. "Struvite Production from Dairy Processing Waste" Sustainability 14, no. 23: 15807. https://doi.org/10.3390/su142315807





