Pretreatment of Slaughterhouse Effluent Treatment Plant Sludge Using Electro-Fenton Process for Anaerobic Digestion
Abstract
:1. Introduction
2. Materials and Methods
3. Results
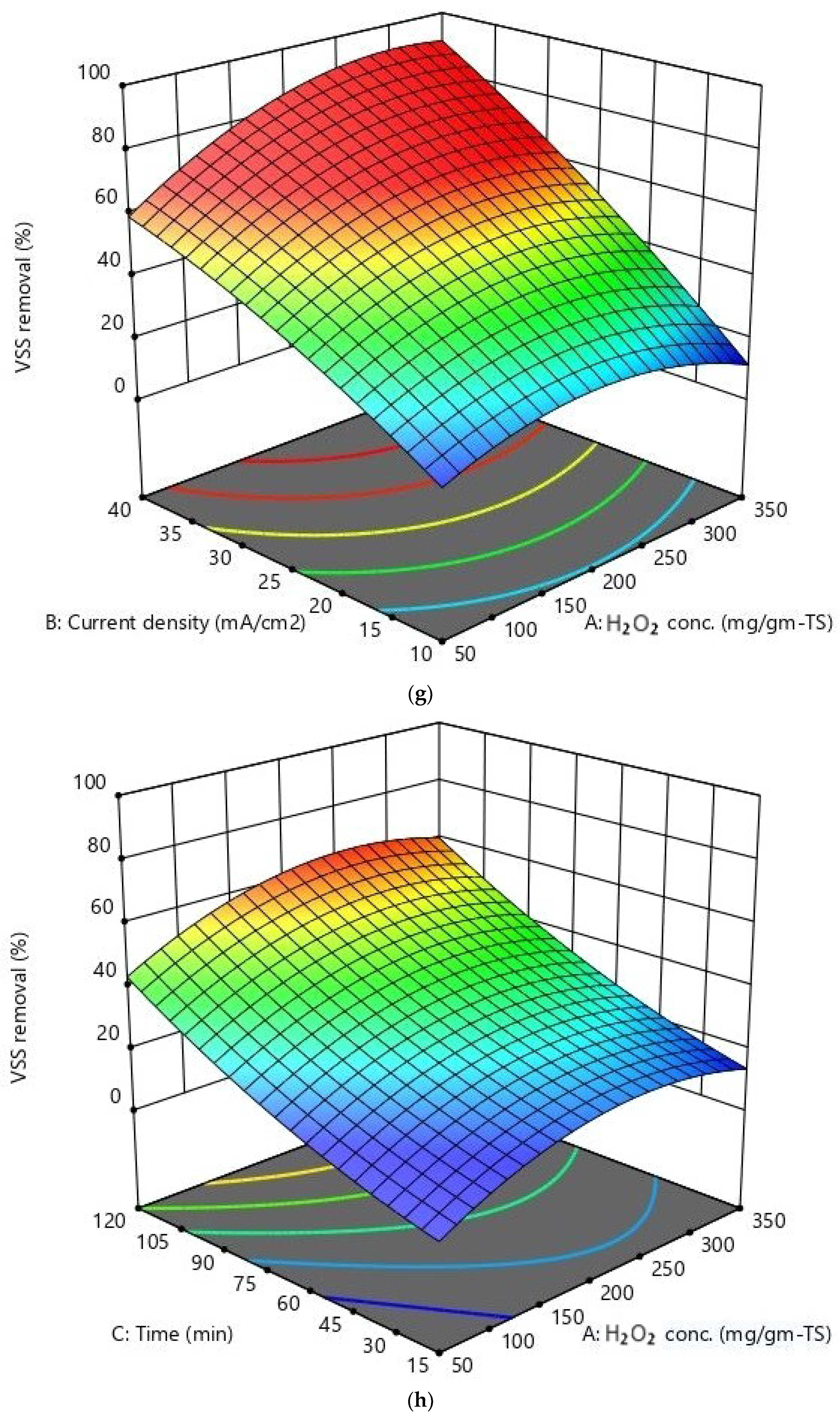
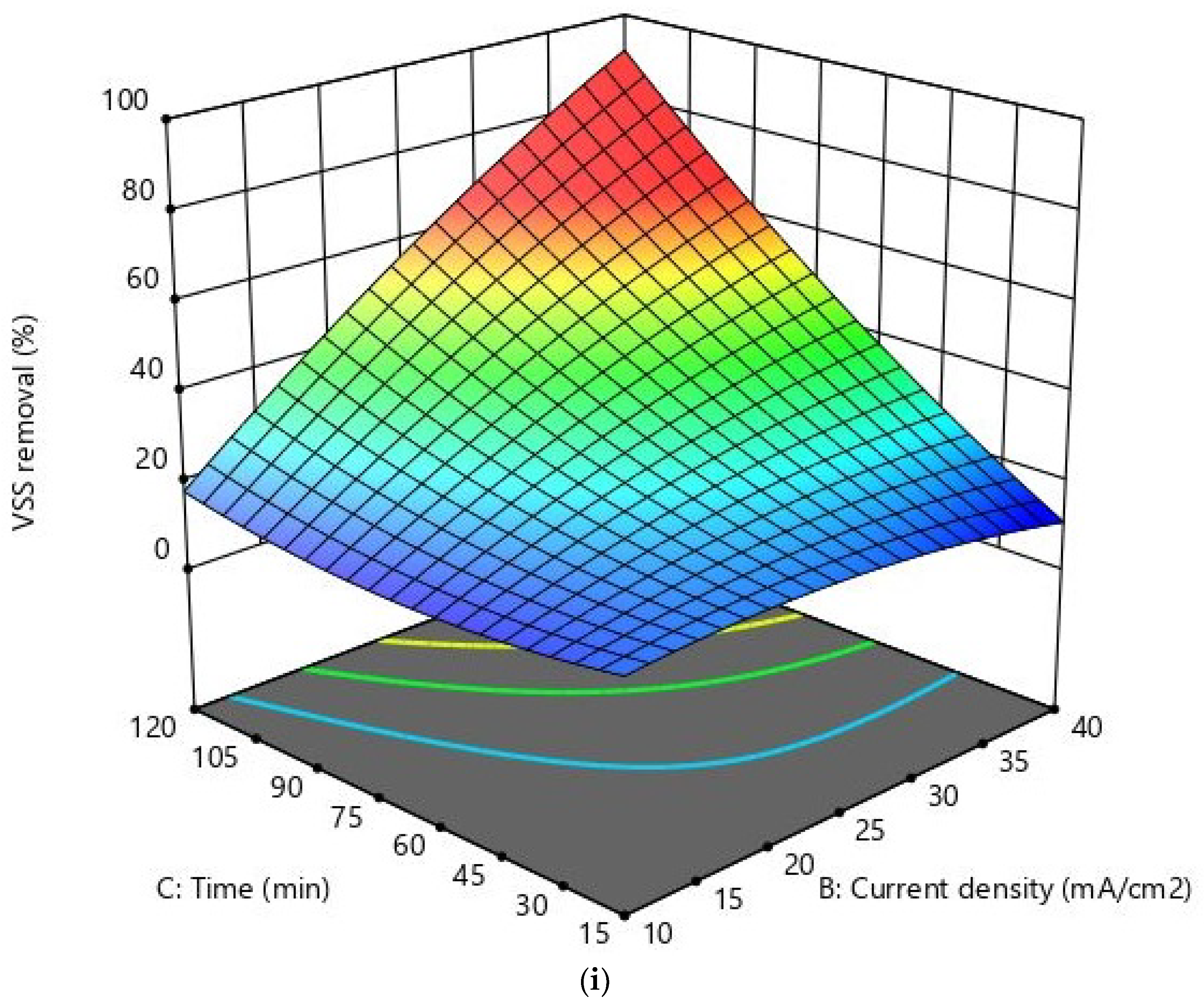
Response Surface Methodology (RSM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vítězová, M.; Kohoutová, A.; Vítěz, T.; Hanišáková, N.; Kushkevych, I. Methanogenic microorganisms in industrial wastewater anaerobic treatment. Processes 2020, 8, 1546. [Google Scholar] [CrossRef]
- Christensen, M.L.; Keiding, K.; Nielsen, P.H.; Jørgensen, M.K. Dewatering in biological wastewater treatment: A review. Water Res. 2015, 82, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jiang, G.; Wang, Q.; Yuan, Z. A review on sludge conditioning by sludge pre-treatment with a focus on advanced oxidation. RSC Adv. 2014, 4, 50644–50652. [Google Scholar] [CrossRef]
- Wu, C.; Jin, L.; Zhang, P.; Zhang, G. Effects of potassium ferrate oxidation on sludge disintegration, dewaterability and anaerobic biodegradation. Int. Biodeterior. Biodegrad. 2015, 102, 137–142. [Google Scholar] [CrossRef]
- Heng, G.C.; Isa, M.H.; Lock, S.S.M.; Ng, C.A. Process optimization of waste activated sludge in anaerobic digestion and biogas production by electrochemical pre-treatment using ruthenium oxide coated titanium electrodes. Sustainability 2021, 13, 4874. [Google Scholar] [CrossRef]
- Ennouri, H.; Miladi, B.; Diaz, S.Z.; Güelfo, L.A.F.; Solera, R.; Hamdi, M.; Bouallagui, H. Effect of thermal pretreatment on the biogas production and microbial communities balance during anaerobic digestion of urban and industrial waste activated sludge. Bioresour. Technol. 2016, 214, 184–191. [Google Scholar] [CrossRef]
- Jin, B.; Wang, S.; Xing, L.; Li, B.; Peng, Y. Long term effect of alkali types on waste activated sludge hydrolytic acidification and microbial community at low temperature. Bioresour. Technol. 2016, 200, 587–597. [Google Scholar] [CrossRef]
- Rumky, J.; Ncibi, M.C.; Burgos-Castillo, R.C.; Deb, A.; Sillanpää, M. Optimization of integrated ultrasonic-Fenton system for metal removal and dewatering of anaerobically digested sludge by Box-Behnken design. Sci. Total Environ. 2018, 645, 573–584. [Google Scholar] [CrossRef]
- Heng, G.C.; Isa, M.H.; Lim, J.-W.; Ho, Y.-C.; Zinatizadeh, A.A.L. Enhancement of anaerobic digestibility of waste activated sludge using photo-Fenton pretreatment. Environ. Sci. Pollut. Res. 2017, 24, 27113–27124. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Chang, S.W. Effects of Sludge Concentration and Disintegration/Solubilization Pretreatment Methods on Increasing Anaerobic Biodegradation Efficiency and Biogas Production. Sustainability 2021, 13, 12887. [Google Scholar] [CrossRef]
- Zawieja, I.; Worwąg, M. Biogas Production from excess sludge oxidized with peracetic acid (PAA). Energies 2021, 14, 3434. [Google Scholar] [CrossRef]
- Luo, K.; Yang, Q.; Li, X.M.; Yang, G.J.; Liu, Y.; Wang, D.B.; Zheng, W.; Zeng, G.M. Hydrolysis kinetics in anaerobic digestion of waste activated sludge enhanced by α-amylase. Biochem. Eng. J. 2012, 62, 17–21. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Q.B.; Laurens, L.L.; Jarvis, E.E.; Nagle, N.J.; Chen, S.; Frear, C.S. Mechanism, kinetics and microbiology of inhibition caused by long-chain fatty acids in anaerobic digestion of algal biomass. Biotechnol. Biofuels 2015, 8, 141. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Q.; Wang, D.; Yang, Q.; Wu, Y.; Li, Y.; Fu, Q.; Yang, F.; Liu, Y.; Ni, B.J.; et al. Thermal-alkaline pretreatment of polyacrylamide flocculated waste activated sludge: Process optimization and effects on anaerobic digestion and polyacrylamide degradation. Bioresour. Technol. 2019, 281, 158–167. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N.; Dai, X.; Tao, W.; Jenkinson, I.R.; Li, Z. Enhanced dewaterability of sludge during anaerobic digestion with thermal hydrolysis pretreatment: New insights through structure evolution. Water Res. 2018, 131, 177–185. [Google Scholar] [CrossRef]
- Kavitha, S.; Banu, J.R.; IvinShaju, C.D.; Kaliappan, S.; Yeom, I.T. Fenton mediated ultrasonic disintegration of sludge biomass: Biodegradability studies, energetic assessment, and its economic viability. Bioresour. Technol. 2016, 221, 1–8. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, B.; Cheng, P.; Zhu, N.; Yin, C.; Ying, L. Pilot-scale study of enhanced anaerobic digestion of waste activated sludge by electrochemical and sodium hypochlorite combination pretreatment. Int. Biodeterior. Biodegrad. 2016, 110, 227–234. [Google Scholar] [CrossRef]
- Cosgun, S.; Semerci, N. Combined and individual applications of ozonation and microwave treatment for waste activated sludge solubilization and nutrient release. J. Environ. Manag. 2019, 241, 76–83. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, W.; Liu, H.; Wang, G.; Liu, H. Evaluation of volatile fatty acids production and dewaterability of waste activated sludge with different thermo-chemical pretreatments. Int. Biodeterior. Biodegrad. 2018, 129, 170–178. [Google Scholar] [CrossRef]
- Feki, E.; Battimelli, A.; Sayadi, S.; Dhouib, A.; Khoufi, S. High-rate anaerobic digestion of waste activated sludge by integration of electro-Fenton process. Molecules 2020, 25, 626. [Google Scholar] [CrossRef] [PubMed]
- Pilli, S.; More, T.T.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Fenton pre-treatment of secondary sludge to enhance anaerobic digestion: Energy balance and greenhouse gas emissions. Chem. Eng. J. 2016, 283, 285–292. [Google Scholar] [CrossRef]
- Şahinkaya, S.; Kalıpcı, E.; Aras, S. Disintegration of waste activated sludge by different applications of Fenton process. Process Saf. Environ. Prot. 2015, 93, 274–281. [Google Scholar] [CrossRef]
- Yang, H.G.; Chun, H.Y.; Pak, D. Improvement of sludge anaerobic degradability by combined electro-flotation and electro-oxidation treatment. Biochem. Eng. J. 2014, 90, 44–48. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, M.; Ren, G.; Yang, W.; Liang, L. A highly energy-efficient flow-through electro-Fenton process for organic pollutants degradation. Electrochim. Acta 2016, 200, 222–230. [Google Scholar] [CrossRef]
- Mohajeri, S.; Aziz, H.A.; Isa, M.H.; Zahed, M.A.; Adlan, M.N. Statistical optimization of process parameters for landfill leachate treatment using electro-Fenton technique. J. Hazard. Mater. 2010, 176, 749–758. [Google Scholar] [CrossRef]
- Mohajeri, S.; Hamidi, A.A.; Isa, M.H.; Zahed, M.A. Landfill Leachate Treatment through Electro-Fenton Oxidation. Pollution 2019, 5, 199–209. [Google Scholar]
- He, D.Q.; Wang, L.F.; Jiang, H.; Yu, H.Q. A Fenton-like process for the enhanced activated sludge dewatering. Chem. Eng. J. 2015, 272, 128–134. [Google Scholar] [CrossRef]
- Canizares, P.; Sáez, C.; Lobato, J.; Rodrigo, M.A. Electrochemical oxidation of polyhydroxybenzenes on boron-doped diamond anodes. Ind. Eng. Chem. Res. 2004, 43, 6629–6637. [Google Scholar] [CrossRef]
- Astals, S.; Koch, K.; Weinrich, S.; Hafner, S.D.; Tait, S.; Peces, M. Impact of storage conditions on the methanogenic activity of anaerobic digestion inocula. Water 2020, 12, 1321. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E. Electro-Fenton process: Fundamentals and reactivity. Electro-Fent. Process 2017, 1–28. [Google Scholar] [CrossRef]
- Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2017.
- Anderson, G.K.; Yang, G. Determination of bicarbonate and total volatile acid concentration in anaerobic digesters using a simple titration. Water Environ. Res. 1992, 64, 53–59. [Google Scholar] [CrossRef]
- Lowry, O.H. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Khoufi, S.; Louhichi, A.; Sayadi, S. Optimization of anaerobic co-digestion of olive mill wastewater and liquid poultry manure in batch condition and semi-continuous jet-loop reactor. Bioresour. Technol. 2015, 182, 67–74. [Google Scholar] [CrossRef]
- Feki, E.; Sayadi, S.; Loukil, S.; Dhouib, A.; Khoufi, S. Comparison between thermo-alkaline and electro-fenton disintegration effect on waste activated sludge anaerobic digestion. BioMed Res. Int. 2019, 2019, 2496905. [Google Scholar] [CrossRef]
- Olvera-Vargas, H.; Zheng, X.; Garcia-Rodriguez, O.; Lefebvre, O. Sequential “electrochemical peroxidation–Electro-Fenton” process for anaerobic sludge treatment. Water Res. 2019, 154, 277–286. [Google Scholar] [CrossRef]
- Zhang, H.; Fei, C.; Zhang, D.; Tang, F. Degradation of 4-nitrophenol in aqueous medium by electro-Fenton method. J. Hazard. Mater. 2007, 145, 227–232. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Noordin, M.Y.; Venkatesh, V.C.; Sharif, S.; Elting, S.; Abdullah, A. Application of response surface methodology in describing the performance of coated carbide tools when turning AISI 1045 steel. J. Mater. Process. Technol. 2004, 145, 46–58. [Google Scholar] [CrossRef]
- Elibol, M. Response surface methodological approach for inclusion of perfluorocarbon in actinorhodin fermentation medium. Process Biochem. 2002, 38, 667–673. [Google Scholar] [CrossRef]
- Wong, L.-P.; Isa, M.H.; Bashir, M.J.K. Disintegration of palm oil mill effluent organic solids by ultrasonication: Optimization by response surface methodology. Process Saf. Environ. Prot. 2018, 114, 123–132. [Google Scholar] [CrossRef]
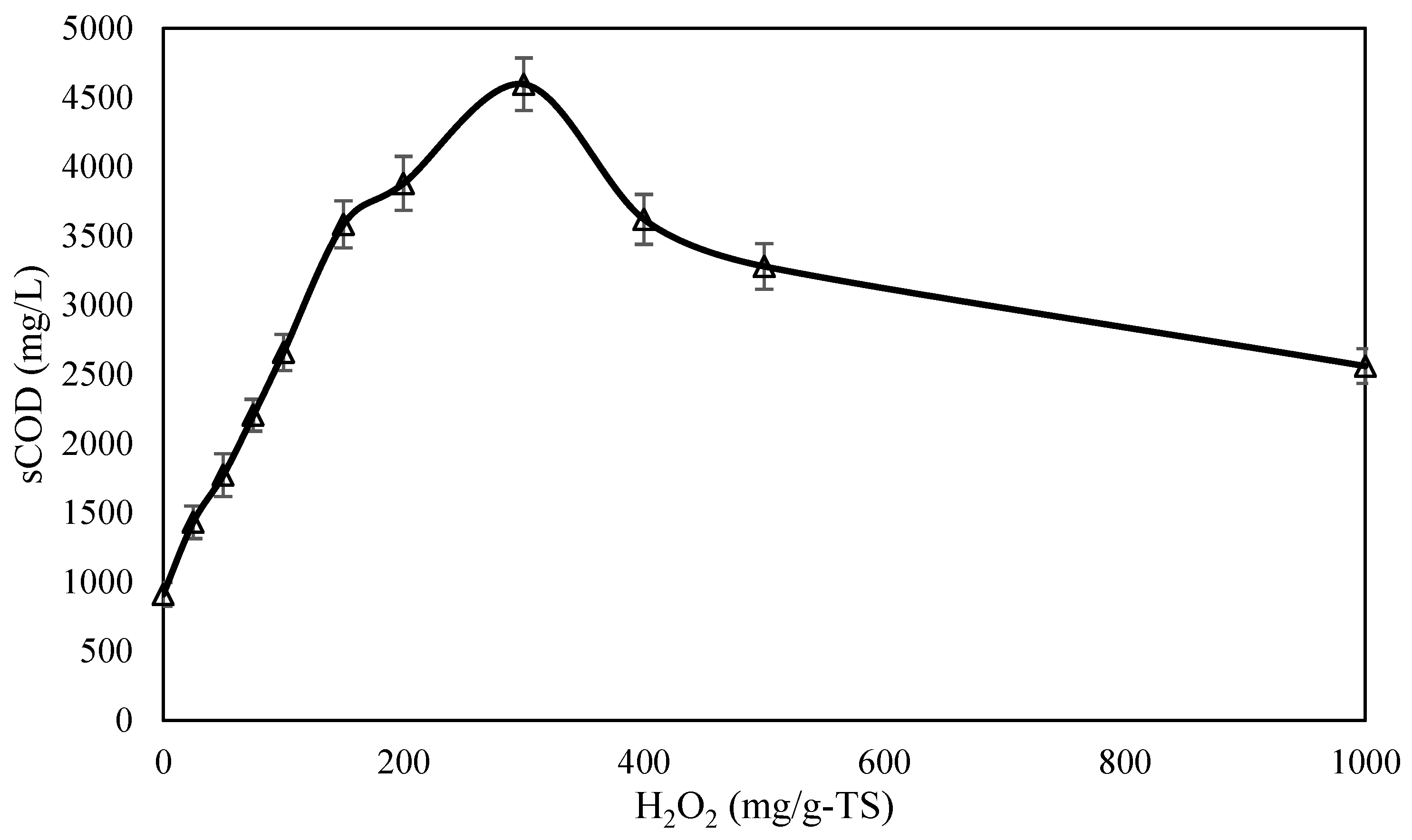




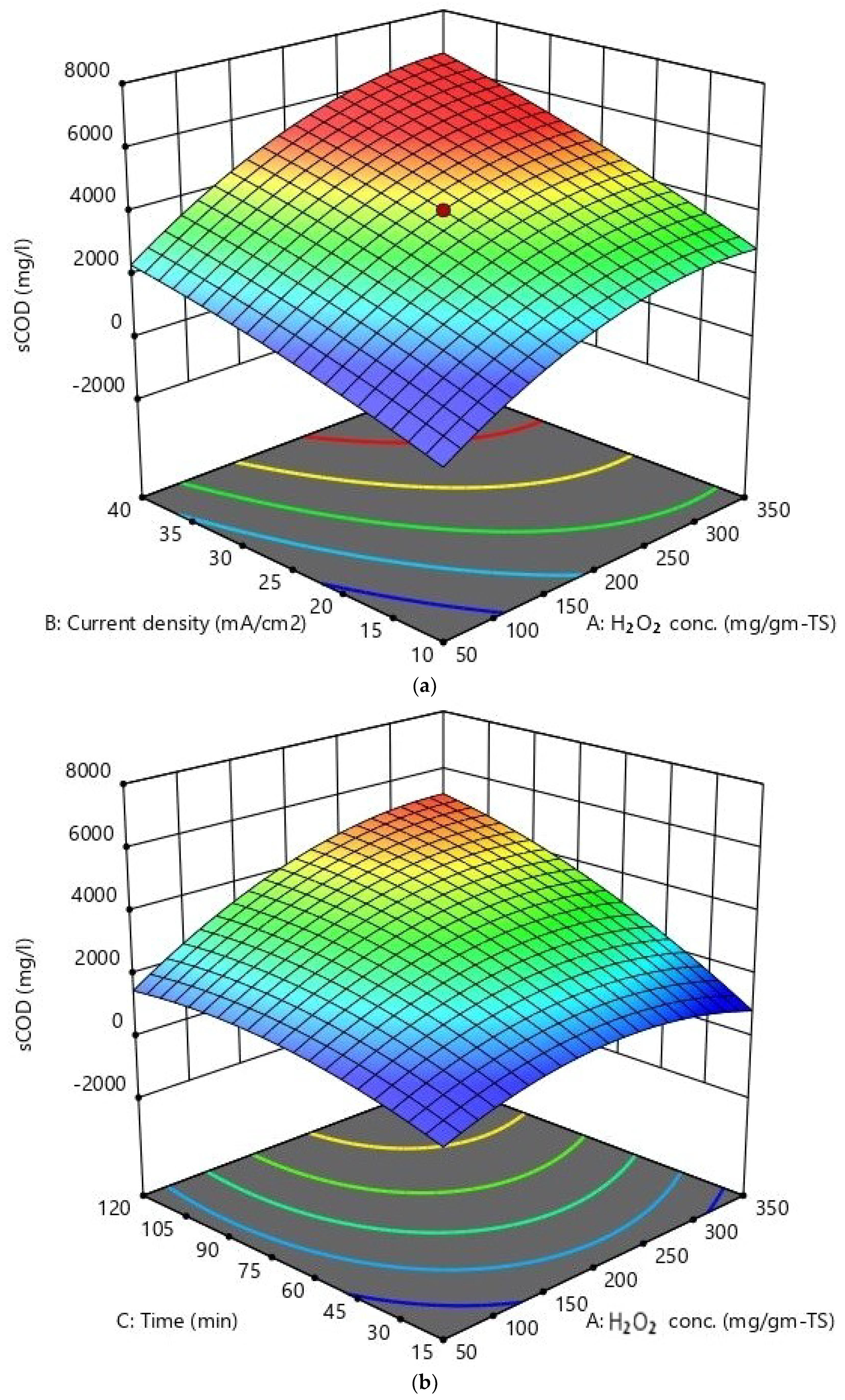
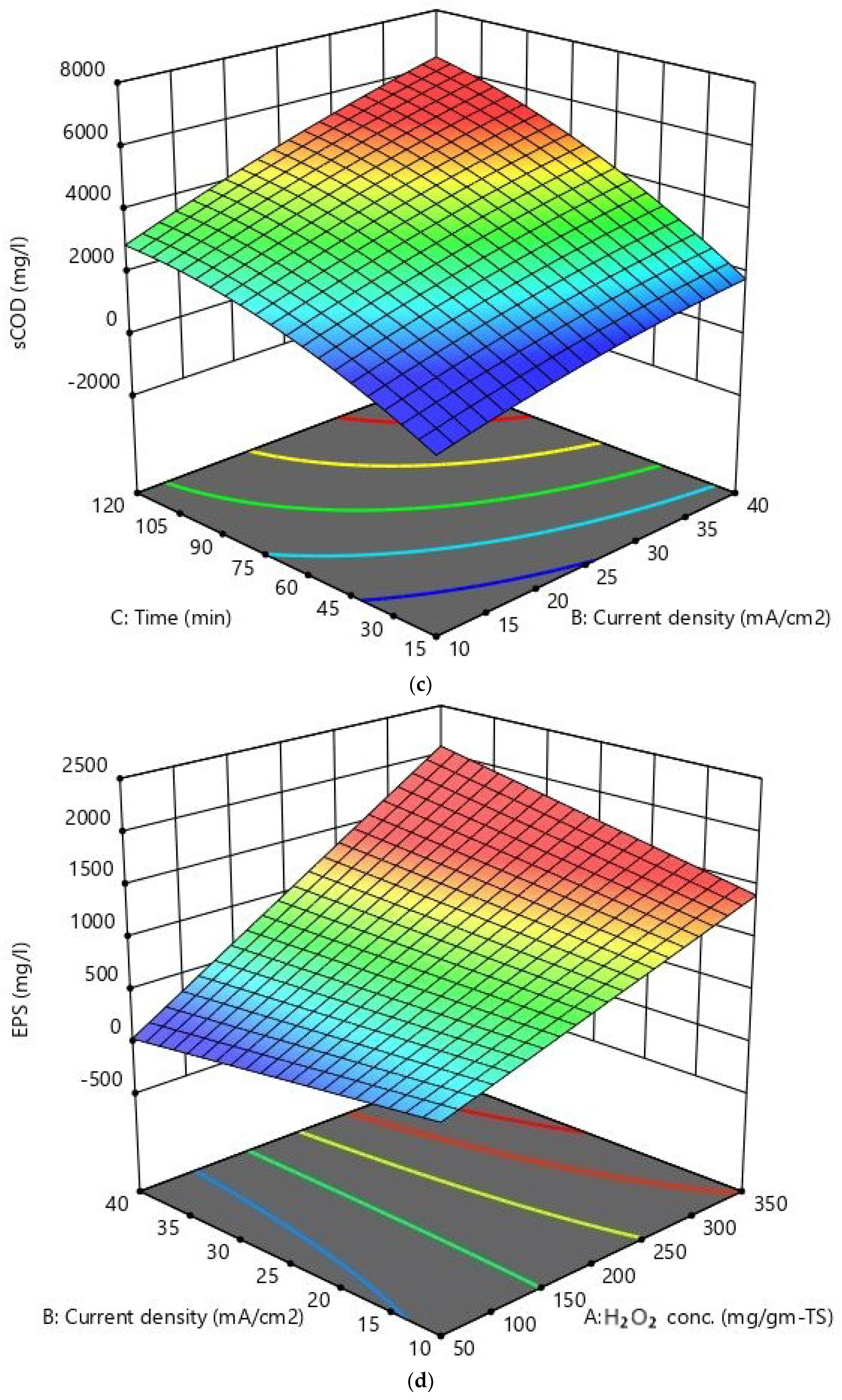
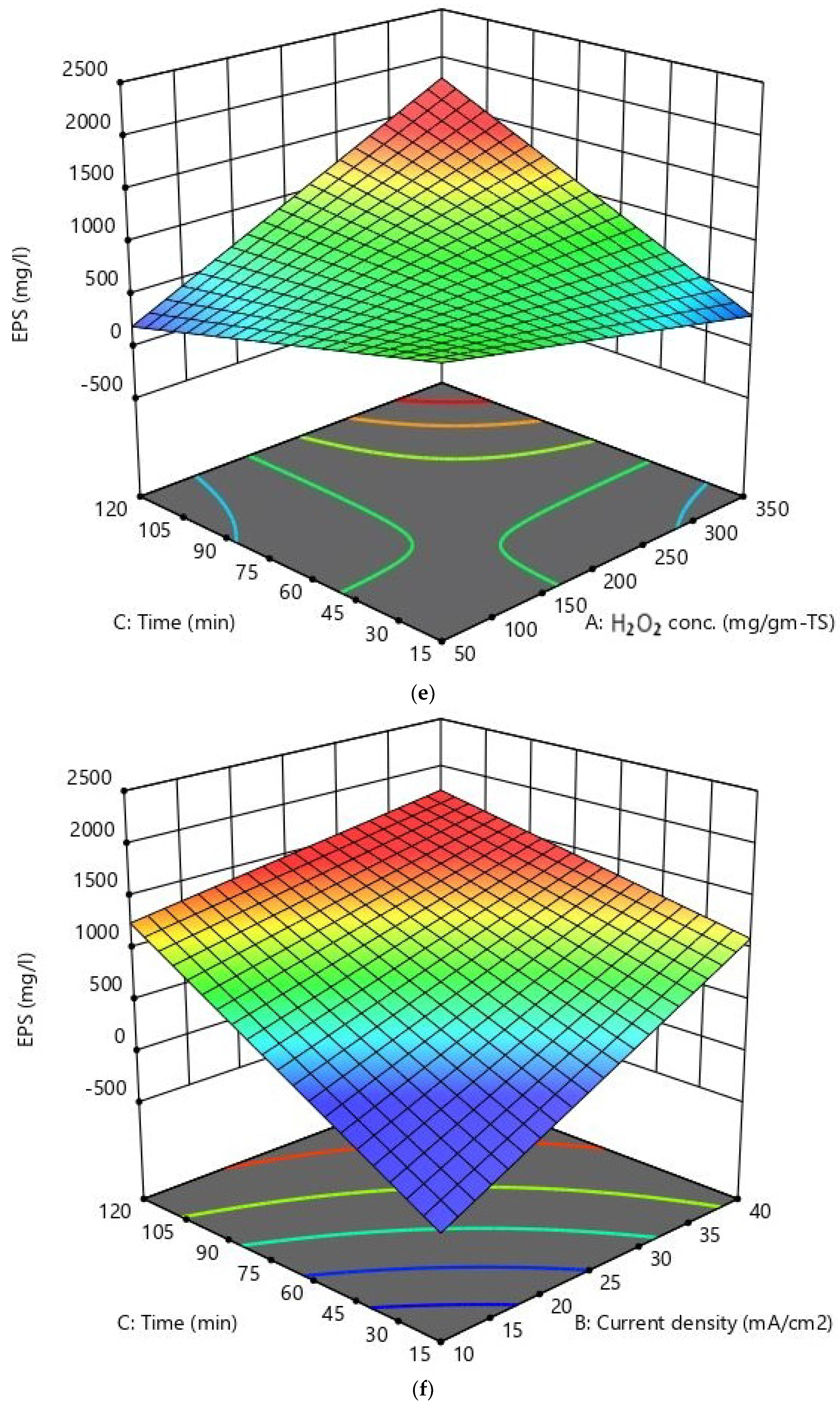

| Parameter (Unit) | Mean Value ± SD |
|---|---|
| pH | 7.3 ± 0.1 |
| EC (mS/cm) | 7.2 ± 1.1 |
| TDS (mg/L) | 3470 ± 710 |
| TS (mg/L) | 30,140 ± 4070 |
| VS (mg/L) | 21,000 ± 4960 |
| SS (mg/L) | 26,420 ± 3270 |
| VSS (mg/L) | 19,970 ± 4590 |
| sBOD5 (mg/L) | 454 ± 63 |
| sCOD (mg/L) | 1087 ± 130 |
| tCOD (mg/L) | 35,082 ± 9118 |
| VFA (mg/L) | 52 ± 11 |
| Alkalinity (mg/L as CaCO3) | 1242 ± 141 |
| sEPS (mg/L) | 196 ± 23 |
| PO43− (mg/L) | 154 ± 44 |
| TKN (mg/L) | 185 ± 97 |
| NO2-N (mg/L) | 2.93 ± 0.56 |
| NO3-N (mg/L) | 28.75 ± 4.99 |
| Parameters | Method/Reference |
|---|---|
| TDS | Probe CDC-401, Hach |
| EC | Probe CDC-401, Hach |
| pH | Probe pH-201, Hach |
| COD | USEPA reactor digestion method 8000, Hach |
| PO43- | Molybdovanadate method 8114, Hach |
| TKN | Nessler method 8075, Hach |
| NO2-N | Ferrous Sulphate method 8153, Hach |
| NO3-N | Cadmium-reduction method 8039, Hach |
| BOD5 | 5210 B, Standard methods, APHA 2017 [33] |
| TS | 2540 B, Standard methods, APHA 2017 [33] |
| VS | 2540 E, Standard methods, APHA 2017 [33] |
| SS | 2540 D, Standard methods, APHA 2017 [33] |
| VSS | 2540 E, Standard methods, APHA 2017 [33] |
| VFA | Titration method [34] |
| Protein (PN) | Folin-phenol reagent method [35] |
| Polysaccharides (PS) | Sulphuric acid-UV method [36] |
| Factor | Range and Levels (Coded) | |||||
|---|---|---|---|---|---|---|
| −1.682 | −1 | 0 | +1 | +1.682 | ||
| H2O2 concentration (mg/g-TS) | (A) | 50 | 110.81 | 200 | 289.19 | 350 |
| Current density (mA/cm2) | (B) | 10 | 16.08 | 25 | 33.92 | 40 |
| Reaction time (min) | (C) | 15 | 36.28 | 67.5 | 98.71 | 120 |
| Run No. | H2O2 Conc. mg/g-TS (A) | Current Density mA/cm2 (B) | Reaction Time min (C) | sCOD mg/L | EPS mg/L | VSS Removal % |
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 1499 | 562 | 16.58 |
| 2 | +1 | +1 | −1 | 3110 | 1005 | 24.78 |
| 3 | +1 | +1 | +1 | 5305 | 1448 | 66.2 |
| 4 | +1 | −1 | +1 | 3644 | 1066 | 29.56 |
| 5 | 0 | 0 | 0 | 2769 | 724 | 39.06 |
| 6 | 0 | 0 | 0 | 3444 | 807 | 30.2 |
| 7 | 0 | 0 | 0 | 3279 | 789 | 29.5 |
| 8 | 0 | 0 | 0 | 3588 | 851 | 33.96 |
| 9 | 0 | 0 | −1.682 | 1355 | 440 | 22.46 |
| 10 | 0 | 0 | 0 | 3570 | 750 | 37.24 |
| 11 | −1 | +1 | +1 | 3405 | 634 | 52.02 |
| 12 | 0 | +1.682 | 0 | 4199 | 1128 | 38.26 |
| 13 | −1 | −1 | +1 | 1905 | 598 | 28.32 |
| 14 | 0 | 0 | 0 | 3780 | 859 | 38.04 |
| 15 | +1 | −1 | −1 | 1809 | 208 | 17.76 |
| 16 | 0 | 0 | +1.682 | 4095 | 893 | 55.92 |
| 17 | 0 | −1.682 | 0 | 2004 | 406 | 22.16 |
| 18 | +1.682 | 0 | 0 | 3219 | 851 | 29.92 |
| 19 | −1.682 | 0 | 0 | 1494 | 471 | 15.9 |
| 20 | −1 | +1 | −1 | 2085 | 965 | 13.18 |
| Sources | Sum of Square | Degree of Freedom | Mean Squares | F-Values | p-Values Prob > F |
|---|---|---|---|---|---|
| sCOD concentration a | |||||
| Model | 2.105 × 107 | 9 | 2.339 × 106 | 28.77 | <0.0001 (significant) |
| Residual | 8.128 × 105 | 10 | 81,282.05 | - | - |
| Lack of fit | 1.896 × 105 | 5 | 37,917.69 | 0.3042 | 0.8913 (not significant) |
| Pure error | 6.232 × 105 | 5 | 1.246 × 105 | - | - |
| sEPS concentration b | |||||
| Model | 1.469 × 106 | 6 | 2.448 × 105 | 40.62 | <0.0001 (significant) |
| Residual | 78,340.96 | 13 | 6026.23 | - | - |
| Lack of fit | 63,879.63 | 8 | 7984.95 | 2.76 | 0.1392 (not significant) |
| Pure error | 14,461.33 | 5 | 2892.27 | - | |
| VSS removal efficiency c | |||||
| Model | 3474.66 | 9 | 386.07 | 25.41 | <0.0001 (significant) |
| Residual | 151.94 | 10 | 15.19 | - | - |
| Lack of fit | 67.50 | 5 | 13.50 | 0.7993 | 0.5941 (not significant) |
| Pure error | 84.45 | 5 | 16.89 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, M.I.; Farooqi, I.H.; Basheer, F.; Rameez, H.; Isa, M.H. Pretreatment of Slaughterhouse Effluent Treatment Plant Sludge Using Electro-Fenton Process for Anaerobic Digestion. Sustainability 2023, 15, 3159. https://doi.org/10.3390/su15043159
Siddiqui MI, Farooqi IH, Basheer F, Rameez H, Isa MH. Pretreatment of Slaughterhouse Effluent Treatment Plant Sludge Using Electro-Fenton Process for Anaerobic Digestion. Sustainability. 2023; 15(4):3159. https://doi.org/10.3390/su15043159
Chicago/Turabian StyleSiddiqui, Mohd Imran, Izharul Haq Farooqi, Farrukh Basheer, Hasan Rameez, and Mohamed Hasnain Isa. 2023. "Pretreatment of Slaughterhouse Effluent Treatment Plant Sludge Using Electro-Fenton Process for Anaerobic Digestion" Sustainability 15, no. 4: 3159. https://doi.org/10.3390/su15043159






