A Smallholders’ Mariculture Device for Rearing Seafood: Environmentally Friendly and Providing Improved Quality
Abstract
:1. Introduction
2. Materials and Methods
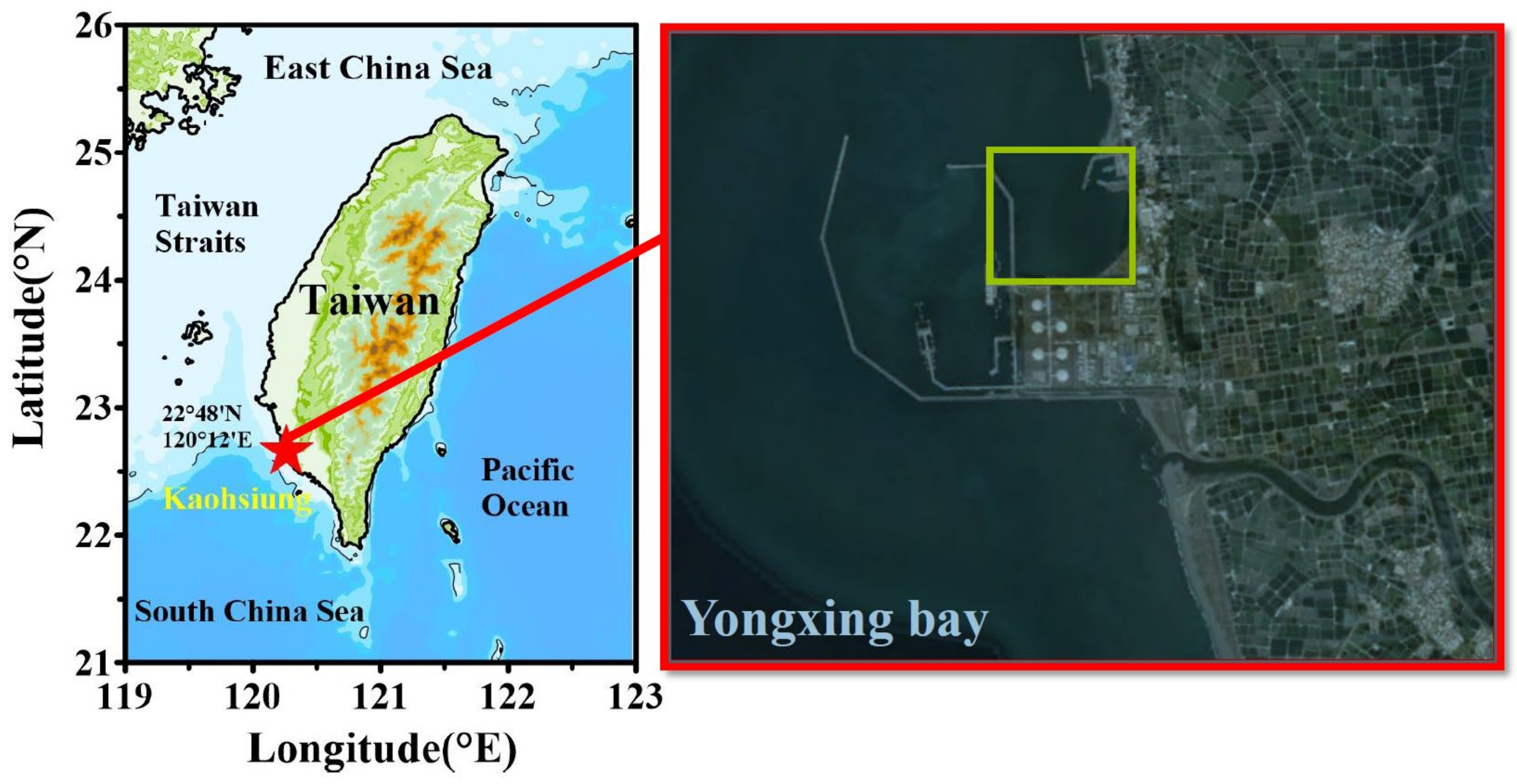
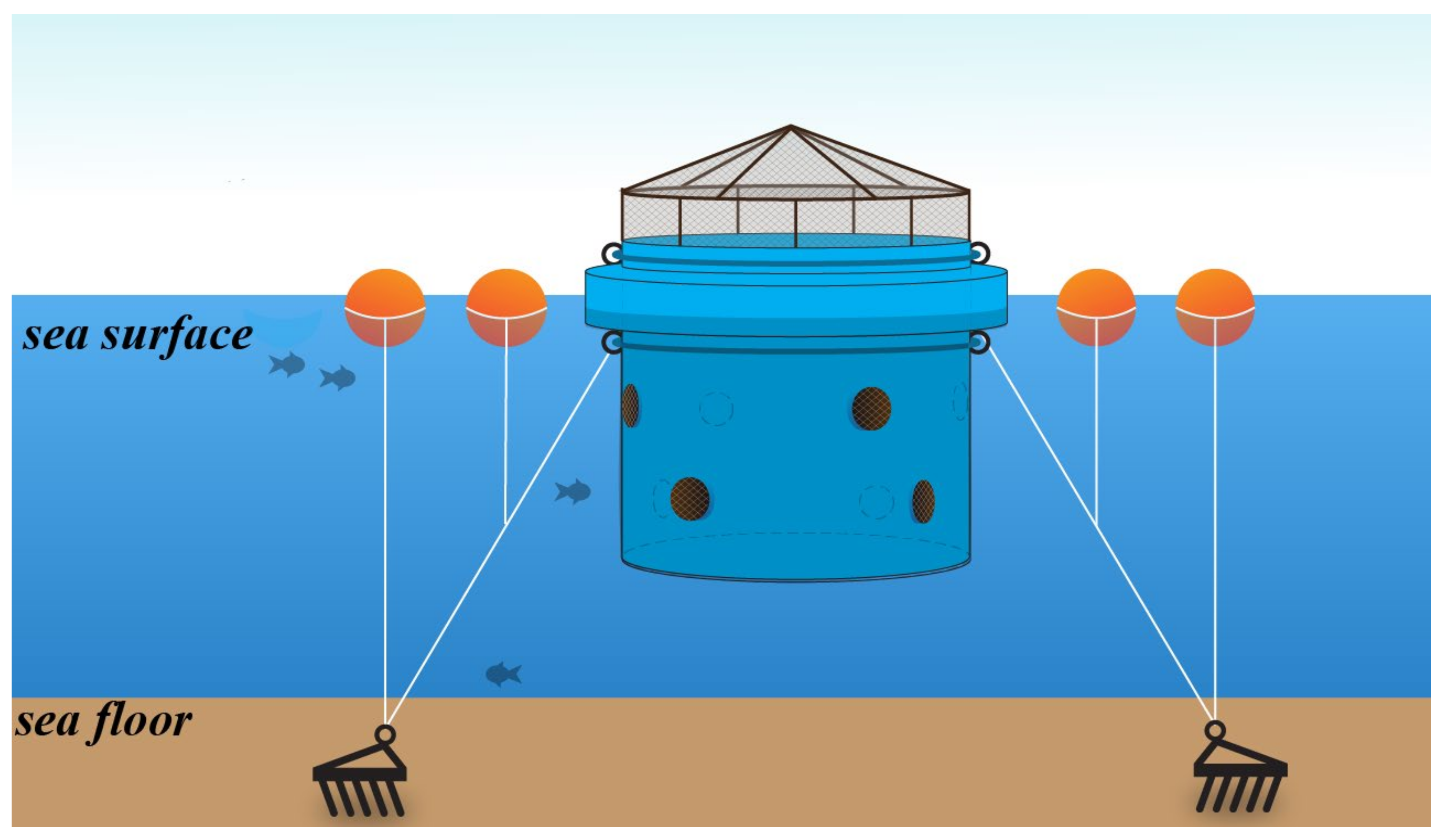
2.1. Proposed Mobile Cage Tank System and Experiments
2.2. Surrounding Hydrographic Conditions
2.3. Free Amino Acids
2.4. Blind Taste Test
2.5. Statistical Analysis
3. Results
3.1. Hydrographic Conditions in Tanks
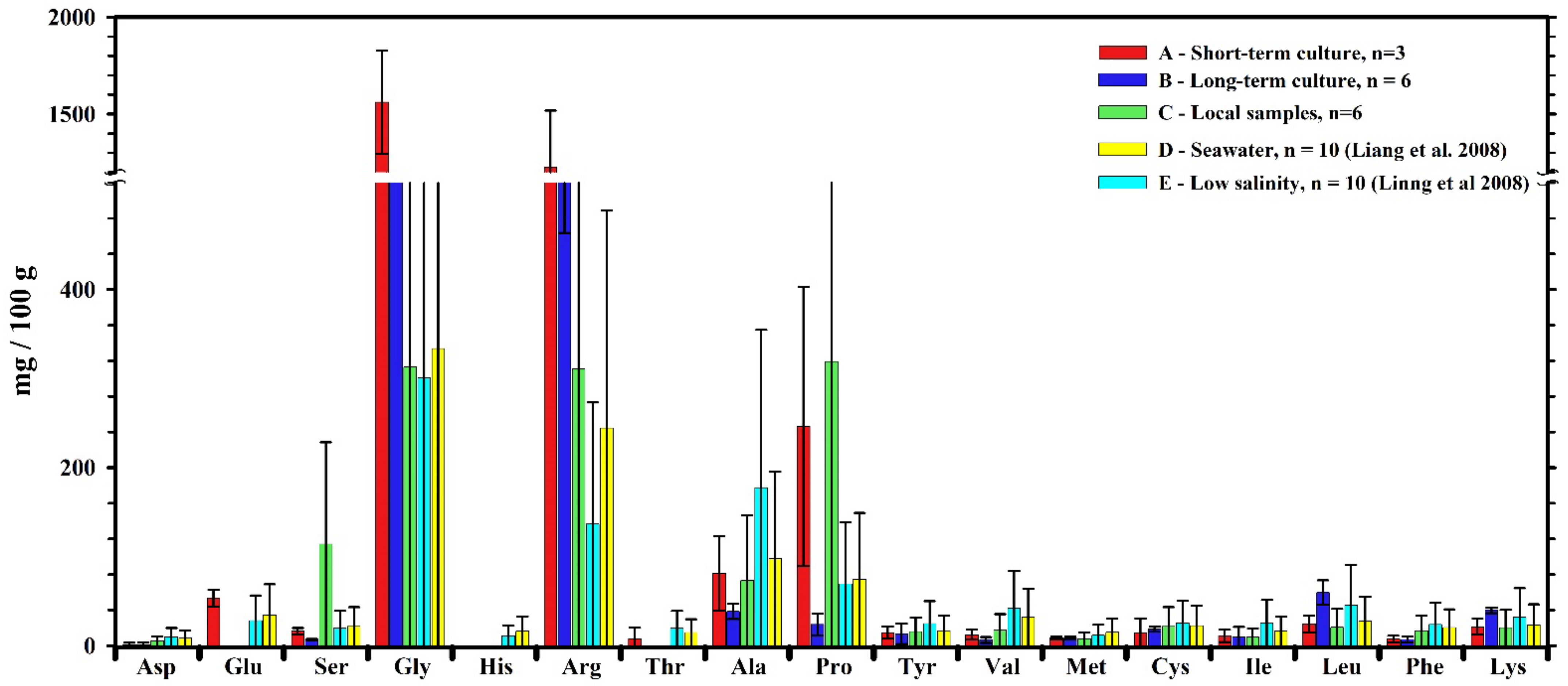
3.2. FAA Concentrations in White Shrimp (Penaeus vannamei)
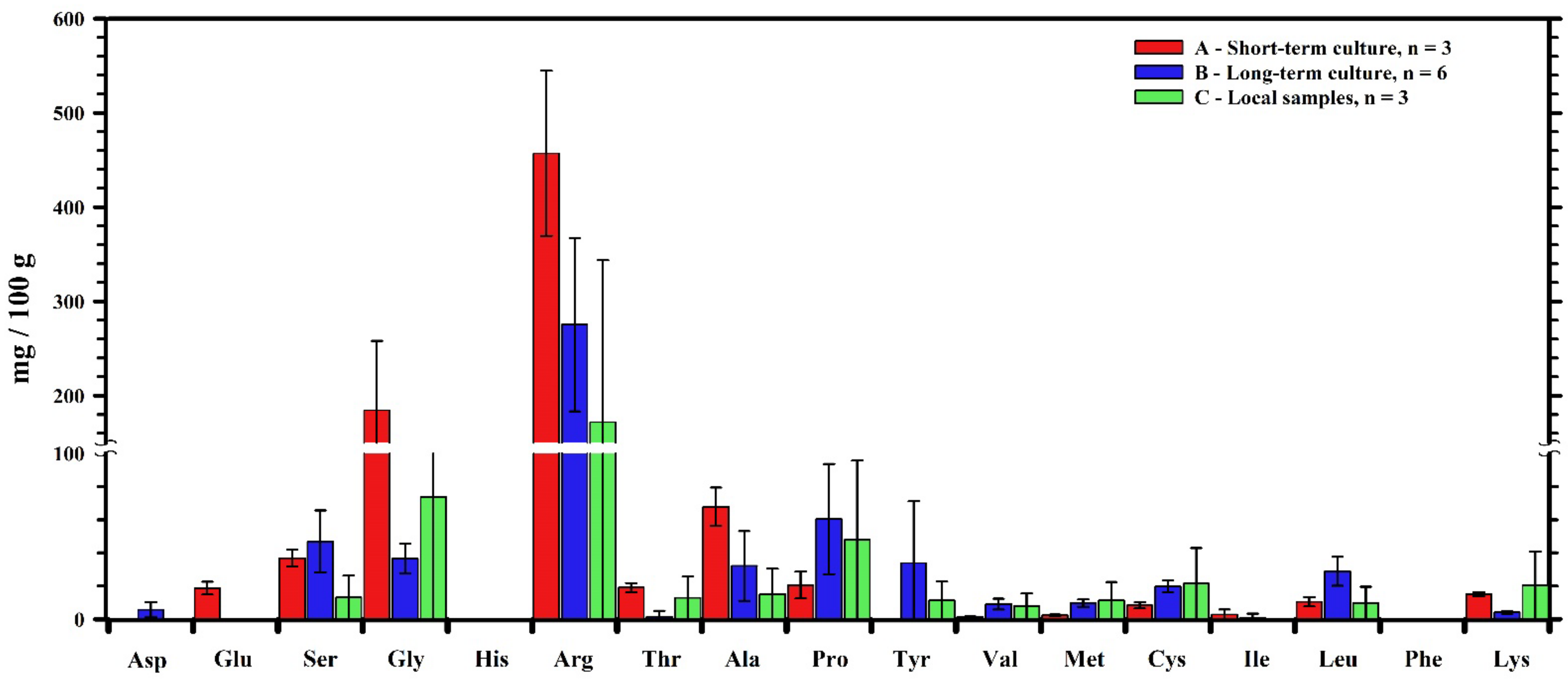
3.3. FAAs Concentration in Groupers (Epinephelus lanceolatus)
3.4. Blind Sensory Test: Groupers
4. Discussion
4.1. Operating the SEFLU-TANK in the Marine Environment
4.2. Improvements in the Quality of Fish and Shrimp
4.3. Implications of Short-Term Cultivation
4.4. Public Policy and Industrial Transformation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019; Onlined & Revised edition; United Nations: New York, NY, USA, 2019; Available online: https://www.un.org/development/desa/pd/news/world-population-prospects-2019-0 (accessed on 21 November 2022).
- Beddington, S.J. The future of food and farming. Int. J. Agric. Manag. 2011, 1, 2–6. [Google Scholar]
- Farber, S.C.; Costanza, R.; Wilson, M.A. Economic and ecological concepts for valuing ecosystem services. Ecol. Econ. 2002, 41, 375–392. [Google Scholar] [CrossRef]
- De Groot, R.S.; Fisher, B.; Christie, M.; Aronson, J.; Braat, L.; Haines-Young, R.; Gowdy, J.; Maltby, E.; Neuville, A.; Polasky, S. Integrating the ecological and economic dimensions in biodiversity and ecosystem service valuation. In The Economics of Ecosystems and Biodiversity (TEEB): Ecological and Economic Foundations; Earthscan/Routledge: London, UK, 2010; pp. 9–40. [Google Scholar]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Garcia, S.M.; de Leiva Moreno, I. Global overview of marine fisheries. In Responsible Fisheries in the Marine Ecosystem; Fishery Resouces Division, FAO: Rome, Italy, 2003; p. 10. [Google Scholar]
- Benoit, H.P.; Swain, D.P. Impacts of environmental change and direct and indirect harvesting effects on the dynamics of a marine fish community. Can. J. Fish. Aquat. Sci. 2008, 65, 2088–2104. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Yan, F.; Yu, H.; Su, F.; Lyne, V.; Cui, Y.; Kang, L.; Wu, W. Global fisheries responses to culture, policy and COVID-19 from 2017 to 2020. Remote Sens. 2021, 13, 4507. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; Subasinghe, R.P.; Josupeit, H.; Cai, J.; Zhou, X. The role of crustacean fisheries and aquaculture in global food security: Past, present and future. J. Invertebr. Pathol. 2012, 110, 158–165. [Google Scholar] [CrossRef]
- Tacon, A.G.; Metian, M. Fish matters: Importance of aquatic foods in human nutrition and global food supply. Rev. Fish. Sci. 2013, 21, 22–38. [Google Scholar] [CrossRef]
- Troell, M.; Naylor, R.L.; Metian, M.; Beveridge, M.; Tyedmers, P.H.; Folke, C.; Arrow, K.J.; Barrett, S.; Crépin, A.-S.; Ehrlich, P.R.; et al. Does aquaculture add resilience to the global food system. Proc. Natl. Acad. Sci. USA 2014, 111, 13257–13263. [Google Scholar] [CrossRef] [Green Version]
- Pradeepkiran, J.A. Aquaculture role in global food security with nutritional value: A review. Transl. Anim. Sci. 2019, 3, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Fisheries Agency. Fisheries Statistical Yearbook Taiwan 2020, Kinmen and Matsu Area; Fisheries Agency, Council of Agriculture: Kaohsiung, Taiwan, 2021. [Google Scholar]
- Diaz, R.J.; Nestlerode, J.; Diaz, M.L. A Global Perspective on the Effects of Eutrophication and Hypoxia on Aquatic Biota and Water Quality; US Environmental Protection Agency: Washington, DC, USA, 2004. [Google Scholar]
- Kaiser, D.; Unger, D.; Qiu, G. Particulate organic matter dynamics in coastal systems of the northern Beibu Gulf. Cont. Shelf Res. 2014, 82, 99–118. [Google Scholar] [CrossRef]
- Wang, J.; Beusen, A.H.; Liu, X.; Bouwman, A.F. Aquaculture production is a large, spatially concentrated source of nutrients in Chinese freshwater and coastal seas. Environ. Sci. Technol. 2019, 54, 1464–1474. [Google Scholar] [CrossRef]
- Jang, C.-S.; Liou, Y.-T.; Liang, C.-P. Probabilistically determining roles of groundwater use in aquacultural fishponds. J. Hydrol. 2010, 388, 491–500. [Google Scholar] [CrossRef]
- Sun, P.L.; Yang, C.-C.; Lin, T.-W. How to amend land subsidence treatment policies to solve coastal subsidence problems in Taiwan. Reg. Environ. Chang. 2011, 11, 679–691. [Google Scholar] [CrossRef]
- Jang, C.-S.; Chen, C.-F.; Liang, C.-P.; Chen, J.-S. Combining groundwater quality analysis and a numerical flow simulation for spatially establishing utilization strategies for groundwater and surface water in the Pingtung Plain. J. Hydrol. 2016, 533, 541–556. [Google Scholar] [CrossRef]
- Yu, J.-H.; Chang, F.-Y.; Tseng, C.-H.; Wu, C.-H. Construction of Internet of Things System in Coastal Aquaculture Environment. In Proceedings of the 2019 International Conference on Intelligent Computing and Its Emerging Applications (ICEA), Tainan, Taiwan, 30 August–1 September 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 97–100. [Google Scholar]
- Weerakkody, W.S.; Ling, K.H.; Hsieh, H.-H.; Abedneko, V.G.; Shyu, J.-F.; Lee, T.-M.; Shih, Y.-Y.; Ranatunga, R.; Santschi, P.H.; Hung, C.-C. Carbon capture by macroalgae Sarcodia suae using aquaculture wastewater and solar energy for cooling in subtropical regions. Sci. Total Environ. 2023, 855, 158850. [Google Scholar] [CrossRef]
- Xue, C.H. Comparison and analysis of sensory components between cultured and wild Penaeus chinensis. J. Qingdao Ocean Univ. 1991, 20, 343–347. [Google Scholar]
- Chen, Q. Effect of different salinity culture of flesh content and nutrients of Penaeus vannamei. Mar. Sci. 2001, 21, 16–18. [Google Scholar]
- Lovell, R.T. New off-flavors in pond-cultured channel catfish. Aquaculture 1983, 30, 329–334. [Google Scholar] [CrossRef]
- Maga, J.A. Musty/earthy aromas. Food Rev. Int. 1987, 3, 269–284. [Google Scholar] [CrossRef]
- Wu, J.-T.; Hsu, Y.-M. Relation of algae to earthy odors of fish in Taiwan. Bot. Bull. Acad. Sin. 1988, 29, 183–188. [Google Scholar]
- Shahidi, F.; Cadwallader, K.R. Flavor and Lipid Chemistry of Seafoods: An Overview; ACS Publications: Washington, DC, USA, 1997. [Google Scholar]
- Hsieh, H.-H.; Chuang, M.-H.; Shih, Y.-Y.; Weerakkody, W.S.; Huang, W.-J.; Hung, C.-C.; Muller, F.L.L.; Ranatunga, R.R.M.K.P.; Wijethunga, D.S. Eutrophication and Hypoxia in Tropical Negombo Lagoon, Sri Lanka. Front. Mar. Sci. 2021, 1216, 678832. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture. Contributing to Food Security and Nutrition for All. 2016. 204p. Available online: https://www.fao.org/3/i5555e/i5555e.pdf (accessed on 21 November 2022).
- Yan, Q.; Cheng, T.; Song, J.; Zhou, J.; Hung, C.-C.; Cai, Z. Internal nutrient loading is a potential source of eutrophication in Shenzhen Bay, China. Ecol. Indic. 2021, 127, 107736. [Google Scholar] [CrossRef]
- Chen, C.-C.; Gong, G.-C.; Chiang, K.-P.; Shiah, F.-K.; Chung, C.-C.; Hung, C.-C. Scaling effects of a eutrophic river plume on organic carbon consumption. Limnol. Oceanogr. 2021, 66, 1867–1881. [Google Scholar] [CrossRef]
- Ever Spring Marine Aquaculture Company, LTD. Available online: https://www.seafarm.com.tw/Seafarm.com.tw (accessed on 10 October 2022).
- Lan, H.-Y.; Afero, F.; Huang, C.-T.; Chen, B.-Y.; Huang, P.-L.; Hou, Y.-L. Investment Feasibility Analysis of Large Submersible Cage Culture in Taiwan: A Case Study of Snubnose Pompano (Trachinotus anak) and Cobia (Rachycentron canadum). Fishes 2022, 7, 151. [Google Scholar] [CrossRef]
- De Silva, S.S.; Phillips, M.J. A review of cage aquaculture: Asia (excluding China). FAO Fish. Tech. Pap. 2007, 498, 21. [Google Scholar]
- Tacon, A.G.; Halwart, M. Cage aquaculture: A global overview. FAO Fish. Tech. Pap. 2007, 498, 3. [Google Scholar]
- Shainee, M.; Ellingsen, H.; Leira, B.J.; Fredheim, A. Design theory in offshore fish cage designing. Aquaculture 2013, 392, 134–141. [Google Scholar] [CrossRef]
- Pai, S.-C.; Yang, C.-C.; Riley, J.P. Formation kinetics of the pink azo dye in the determination of nitrite in natural waters. Anal. Chim. Acta 1990, 232, 345–349. [Google Scholar] [CrossRef]
- Chen, J.-C.; Chen, S.-F. Effects of nitrite on growth and molting of Penaeus monodon juveniles. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1992, 101, 453–458. [Google Scholar] [CrossRef]
- Li, Z.S.; Ma, S.; Shan, H.W.; Wang, T.; Xiao, W. Responses of hemocyanin and energy metabolism to acute nitrite stress in juveniles of the shrimp Litopenaeus vannamei. Ecotoxicol. Environ. Saf. 2019, 186, 109753. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Nitrite implications and its management strategies in aquaculture: A review. Rev. Aquac. 2020, 12, 878–908. [Google Scholar] [CrossRef]
- Liang, M.; Wang, S.; Wang, J.; Chang, Q.; Mai, K. Comparison of flavor components in shrimp Litopenaeus vannamei cultured in sea water and low salinity water. Fish. Sci. 2008, 74, 1173–1179. [Google Scholar] [CrossRef]
- Holmes, P.R.; Catherine, W.Y. Red Tides in Hong Kong Waters. Asian Mar. Biol. 1985, 1, 1–10. [Google Scholar]
- Wong, C.K.; Wong, C.K. HPLC pigment analysis of marine phytoplankton during a red tide occurrence in Tolo Harbour, Hong Kong. Chemosphere 2003, 52, 1633–1640. [Google Scholar] [CrossRef]
- Wang, J.; Bouwman, A.F.; Liu, X.; Beusen, A.H.; Van Dingenen, R.; Dentener, F.; Yao, Y.; Glibert, P.M.; Ran, X.; Yao, Q.; et al. Harmful algal blooms in Chinese coastal waters will persist due to perturbed nutrient ratios. Environ. Sci. Technol. Lett. 2021, 8, 276–284. [Google Scholar] [CrossRef]
- Chien, A.C. First Pineapples, Now Fish: To Pressure Taiwan, China Flexes Economic Muscle-Taiwan’s Lucrative Grouper Industry is Bracing for Heavy Losses after China’s Recent Ban on Imports of the Fish from the Island, Business. The New York Times, 22 June 2022. Available online: https://www.nytimes.com/2022/06/22/business/china-taiwan-grouper-ban.html?_ga=2.228487581.1078857677.1668658017-1926554262.1668658017(accessed on 10 October 2022).
- Lai, Y.-C.; Cheng, H.-T.; Yang, S.-M.; Kao, E. Taiwan to Improve Grouper Industry Management after China Ban. Focus Taiwan, 2022. Available online: https://focustaiwan.tw/cross-strait/202206220020(accessed on 12 November 2022).
- Wang, S.; Ou, J.; Chang, J.; Liu, K. Characteristics of the Trash Fish Generated by Bottom Trawling in Surrounding Waters of Guei-Shan Island, Northeastern Taiwan. J.-Fish. Soc. Taiwan 2007, 34, 379. [Google Scholar]
- Clover, C. The End of the Line: How Overfishing Is Changing the World and What We Eat; University of California Press: Berkeley, CA, USA, 2008. [Google Scholar]
- Allsopp, M.; Page, R.; Johnston, P.; Santillo, D. (Eds.) State of the World’s Oceans; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- FA (Fisheries Agency). Fisheries Statistical Yearbook 2008 Taiwan, Kinmen and Matsu Area; Fisheries Agency, Council of Agriculture, Executive Yuan: Kaohsiung, Taiwan, 2008. [Google Scholar]
- Chen, C.L. Factors influencing participation of ‘top-down but voluntary fishery management—Empirical evidence from Taiwan. Mar. Policy 2010, 34, 150–155. [Google Scholar] [CrossRef]
- Chen, J.-L.; Hsu, K.; Chuang, C.-T. How do fishery resources enhance the development of coastal fishing communities: Lessons learned from a community-based sea farming project in Taiwan. Ocean. Coast. Manag. 2020, 184, 105015. [Google Scholar] [CrossRef]
- Chen, J.-L.; Chuang, C.-T.; Jan, R.-Q.; Liu, L.-C.; Jan, M.-S. There is a need to supply a good quality aquatics source for recreational fishing a case study in Penghu, Taiwan. Ocean. Coast. Manag. 2013, 85, 58–64. [Google Scholar] [CrossRef]
- Huang, W.-C. A Study on Spatial Redevelopment and Management Strategy in the Fishing Ports (RW9602-1745); Council of Agriculture, Executive Yuan: Taipei, Taiwan, 1996. Available online: https://www.grb.gov.tw/search/planDetail?id=1258215 (accessed on 10 October 2022).
- Hsu, T.-M. The Rise and Decline of Taiwan Fishing Ports and Ethical Reflection (RW10106-3956); Executive Yuan: Taipei, Taiwan, 2013. Available online: https://www.grb.gov.tw/search/planDetail?id=2314525 (accessed on 10 October 2022).
- Yang, C.-M. Reuse of Idle or Discarded Fishing Ports: Differential Analysis of the Perspectives of Different Stakeholders; (RW10605-8240); Council of Agriculture: Taipei, Taiwan, 2016. Available online: https://www.grb.gov.tw/search/planDetail?id=11577118 (accessed on 10 October 2022).
- Chen, C.-L.; Chang, Y.-C. A transition beyond traditional fisheries: Taiwan’s experience with developing fishing tourism. Mar. Policy 2017, 79, 84–91. [Google Scholar] [CrossRef]
- Hung, C.-C.; Chung, C.-C.; Gong, G.-C.; Jan, S.; Tsai, Y.; Chen, K.-S.; Chou, W.C.; Lee, M.-A.; Chang, Y.; Chen, M.-H.; et al. Nutrient supply in the southern East China Sea after typhoon Morakot. J. Mar. Res. 2013, 71, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Huguenin, J.E. The design, operations and economics of cage culture systems. Aquac. Eng. 1997, 16, 167–203. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, J. 2008. The Status of Mariculture in Northern China. In FAO/NACA Regional Workshop on the Future of Mariculture: A Regional Approach for Responsible Development in the AsiaPacific Region; Lovatelli, A., Phillips, M.J., Arthur, J.R., Yamamoto, K., Eds.; FAO: Rome, Italy, 2008; pp. 271–284. Available online: http://marineagronomy.org/sites/default/files/Lovatelli%20et%20al.%202006.%20Future%20of%20mariculture__Asia%20Pacific.pdf#page=299 (accessed on 21 November 2022).
- Xu, Z.; Qin, H. Fluid-structure interactions of cage-based aquaculture: From structures to organisms. Ocean Eng. 2020, 217, 107961. [Google Scholar] [CrossRef]
- Chai, H.J. A technique of off-odor removal for seafood products. In Proceedings of the Best Practices in Agri-Food Innovations in Asia and the Pacific and 14th General Assembly Meeting of APAARI, Taichung, Taiwan, 1–3 November 2016; Asia-Pacific Association of Agricultural Research Institutions: Bangkok, Thailand, 2016. [Google Scholar]
- Liu, Y.; Huang, Y.; Wang, Z.; Cai, S.; Zhu, B.; Dong, X. Recent advances in fishy odour in aquatic fish products, from formation to control. Int. J. Food Sci. Technol. 2021, 56, 4959–4969. [Google Scholar] [CrossRef]
- Food and Agriculture: Key to Achieving the 2030 Agenda for Sustainable Development. Available online: http://www.fao.org/policy-support/tools-and-publications/resources-details/en/c/422261/ (accessed on 12 November 2022).
- Hsieh, H.-H.; Weerathunga, V.; Weerakkody, W.S.; Huang, W.-J.; Muller, F.L.; Benfield, M.C.; Hung, C.-C. The effects of low pH on the taste and amino acid composition of tiger shrimp. Sci. Rep. 2021, 11, 21180. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, M.; Jin, Y.; Deng, S.; Tao, N.; Qiu, W. Simultaneous Detection and Analysis of Free Amino Acids and Glutathione in Different Shrimp. Foods 2022, 11, 2599. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, H.; Shi, H.; Xue, C.; Wang, Q.; Yu, F.; Xue, Y.; Wang, Y.; Li, Z. The flavor profile changes of Pacific oysters (Crassostrea gigas) in response to salinity during depuration. Food Chem. X 2022, 16, 100485. [Google Scholar] [CrossRef]
- Bi, S.; Chen, L.; Sun, Z.; Wen, Y.; Xue, Q.; Xue, C.; Li, Z.; Sun, C.; Wei, Z.; Liu, H. Investigating influence of aquaculture seawater with different salinities on non-volatile taste-active compounds in Pacific oyster (Crassostrea gigas). J. Food Meas. Charact. 2021, 15, 2078–2087. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, M.; Zheng, Y.; Xu, C.-H.; Tao, N.P.; Wu, X.; Wang, X. Brackish water improves the taste quality in meat of adult male Eriocheir sinensis during the postharvest temporary rearing. Food Chem. 2021, 343, 128409. [Google Scholar] [CrossRef]
- Chen, K.; Li, E.; Gan, L.; Wang, X.; Xu, C.; Lin, H.; Qin, J.G.; Chen, L. Growth and lipid metabolism of the pacific white shrimp Litopenaeus vannamei at different salinities. J. Shellfish. Res. 2014, 33, 825–832. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Nitrogen Biogeochemistry of Aquaculture Pond Sediments; Louisiana State University and Agricultural & Mechanical College: Baton Rouge, LA, USA, 1995. [Google Scholar]
- Mazik, P.M.; Hinman, M.L.; Winkelmann, D.A.; Klaine, S.J.; Simco, B.A.; Parker, N.C. Influence of nitrate and chloride concentrations on survival and hematological profiles of striped bass. Trans. Am. Fish. Soc. 1991, 120, 247–254. [Google Scholar] [CrossRef]
- Refaey, M.M.; Li, D.; Tian, X.; Zhang, Z.; Zhang, X.; Li, L.; Tang, R. High stocking density alters growth performance, blood biochemistry, intestinal histology, and muscle quality of channel catfish Ictalurus punctatus. Aquaculture 2018, 492, 73–81. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, D.; Mei, J.; Xie, J.; Qiu, W. A Preliminary Study on the Effects of Nitrite Exposure on Hematological Parameters, Oxidative Stress, and Immune-Related Responses in Pearl Gentian Grouper. Fishes 2022, 7, 235. [Google Scholar] [CrossRef]
- Nabhitabhata, J.; Prempiyawat, R.; Klaokliang, K. Estimation on optimum stocking density of grouper, Epinephelus tauvina (Forskal) in cages on basis of dissolved oxygen budget: Pang-Rat River. In Proceedings of the Seminar on Fisheries 1988, Bangkok, Thailand, 21–23 September 1988. [Google Scholar]
| Parameters | Surrounding Water | Tank A | Tank B | Average |
|---|---|---|---|---|
| Temperature (°C) | 23.52 ± 1.78 | 23.18 ± 2.24 | 23.72 ± 1.72 | 23.45 ± 2.01 |
| Salinity(psu) | 31.31 ± 1.84 | 31.24 ± 1.93 | 31.30 ± 2.02 | 31.27 ± 1.96 |
| DO (ppm) | 7.06 ± 0.23 | 7.14 ± 0.30 | 7.11 ± 0.26 | 7.12 ± 0.28 |
| pH | 8.12 ± 0.11 | 7.91 ± 0.20 | 8.09 ± 0.18 | 8.00 ± 0.21 |
| NO2− (μM) | 0.04 ± 0.08 | 0.08 ± 0.08 | 0.04 ± 0.08 | 0.06 ± 0.08 |
| Unit (mg/100 g) | A | B | C | D | E | |
|---|---|---|---|---|---|---|
| Aspartic acid | Asp | 1.2 ± 2.1 | 1.1 ± 2.6 | 5.3 ± 5.9 | 8.5 ± 1.1 | 9.9 ± 2.1 |
| Glutamate | Glu | 53.5 ± 9.5 | 0 ± 0 | 0 ± 0 | 34.5 ± 1.7 | 28.2 ± 1.8 |
| Serine | Ser | 16.3 ± 3.6 | 7 ± 0.6 | 114.2 ± 33.9 | 21.6 ± 1.5 | 19.6 ± 2.4 |
| Glycine | Gly | 1561.8 ± 266.4 | 675.6 ± 37.7 | 313.1 ± 49 | 333.7 ± 2.5 | 301 ± 1.5 |
| Histidine | His | 0 ± 0 | 0 ± 0 | 0 ± 0 | 16.5 ± 0.6 | 11.2 ± 0.9 |
| Arginine | Arg | 1225 ± 292.2 | 567.8 ± 104.1 | 311 ± 63 | 244.5 ± 8.2 | 136.6 ± 2.3 |
| Threonine | Thr | 7.4 ± 12.8 | 0 ± 0 | 0 ± 0 | 14.8 ± 0.8 | 19.5 ± 1.1 |
| Alanine | Ala | 81.2 ± 42 | 38.4 ± 8.4 | 73.3 ± 16.4 | 97.8 ± 2.3 | 177.4 ± 3.1 |
| Proline | Pro | 246.3 ± 156.7 | 23.9 ± 12.4 | 319 ± 24.4 | 74.6 ± 1.2 | 69.4 ± 1 |
| Tyrosine | Tyr | 14.7 ± 6.8 | 13.3 ± 11.5 | 15.8 ± 5.7 | 16.8 ± 0.8 | 24.9 ± 1 |
| Valine | Val | 12.4 ± 5.6 | 6.4 ± 3.3 | 17.6 ± 5 | 32 ± 0.8 | 41.9 ± 0.7 |
| Methionine | Met | 8.7 ± 1.4 | 8.5 ± 1.9 | 7.5 ± 1.6 | 15.1 ± 3.1 | 11.8 ± 2.1 |
| Cysteine | Cys | 14.8 ± 15.9 | 18.6 ± 3 | 21.6 ± 5.1 | 22.4 ± 1.4 | 25.3 ± 2.1 |
| Isoleucine | Ile | 11.1 ± 7 | 9.8 ± 11.2 | 9.7 ± 5.8 | 16.4 ± 1.2 | 25.7 ± 2 |
| Leucine | Leu | 24.2 ± 9.3 | 59.6 ± 13.8 | 20.6 ± 11.2 | 27.4 ± 0.7 | 45.4 ± 1.2 |
| Phenylalanine | Phe | 7.3 ± 3.8 | 6.9 ± 3.4 | 16.8 ± 17.6 | 20.5 ± 2.1 | 23.9 ± 1.1 |
| Lysine | Lys | 21.3 ± 9.1 | 39.6 ± 3.2 | 20.1 ± 17.8 | 23.2 ± 1.9 | 32.3 ± 2.2 |
| Total | 3307.3 ± 704.4 | 1476.3 ± 123.2 | 1265.6 ± 182.2 | 1020.2 ± 10.9 | 1003.9 ± 11.6 | |
| mg/100 g | A | B | C | |
|---|---|---|---|---|
| Aspartic acid | Asp | 0 ± 0 | 5.7 ± 4.6 | 0 ± 0 |
| Glutamate | Glu | 18.7 ± 3.9 | 0 ± 0 | 0 ± 0 |
| Serine | Ser | 36.8 ± 5.1 | 47 ± 18.6 | 13.1 ± 22.7 |
| Glycine | Gly | 184.4 ± 73.6 | 36.6 ± 9 | 73.8 ± 26.6 |
| Histidine | His | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Arginine | Arg | 457.1 ± 87.7 | 275.4 ± 92.1 | 171.9 ± 35.1 |
| Threonine | Thr | 19 ± 2.8 | 1.5 ± 3.6 | 12.9 ± 2.3 |
| Alanine | Ala | 67.8 ± 11.5 | 32.1 ± 21 | 15.2 ± 3.2 |
| Proline | Pro | 20.6 ± 8.1 | 60.4 ± 33.2 | 47.8 ± 6.8 |
| Tyrosine | Tyr | 0 ± 0 | 33.8 ± 37.4 | 11.4 ± 0.7 |
| Valine | Val | 1.4 ± 0.4 | 9.1 ± 3.3 | 7.8 ± 1.4 |
| Methionine | Met | 2.4 ± 0.4 | 9.7 ± 2.3 | 11.3 ± 0.6 |
| Cysteine | Cys | 8.4 ± 1.9 | 19.7 ± 3.5 | 21.5 ± 1.8 |
| Isoleucine | Ile | 2.8 ± 3.1 | 1 ± 2.4 | 0 ± 0 |
| Leucine | Leu | 10.5 ± 2.8 | 28.8 ± 8.7 | 9.8 ± 1.7 |
| Phenylalanine | Phe | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Lysine | Lys | 15.1 ± 1.1 | 4.2 ± 0.8 | 20.4 ± 4.9 |
| Total | 845.1 ± 141.2 | 564.9 ± 122 | 416.8 ± 59.5 | |
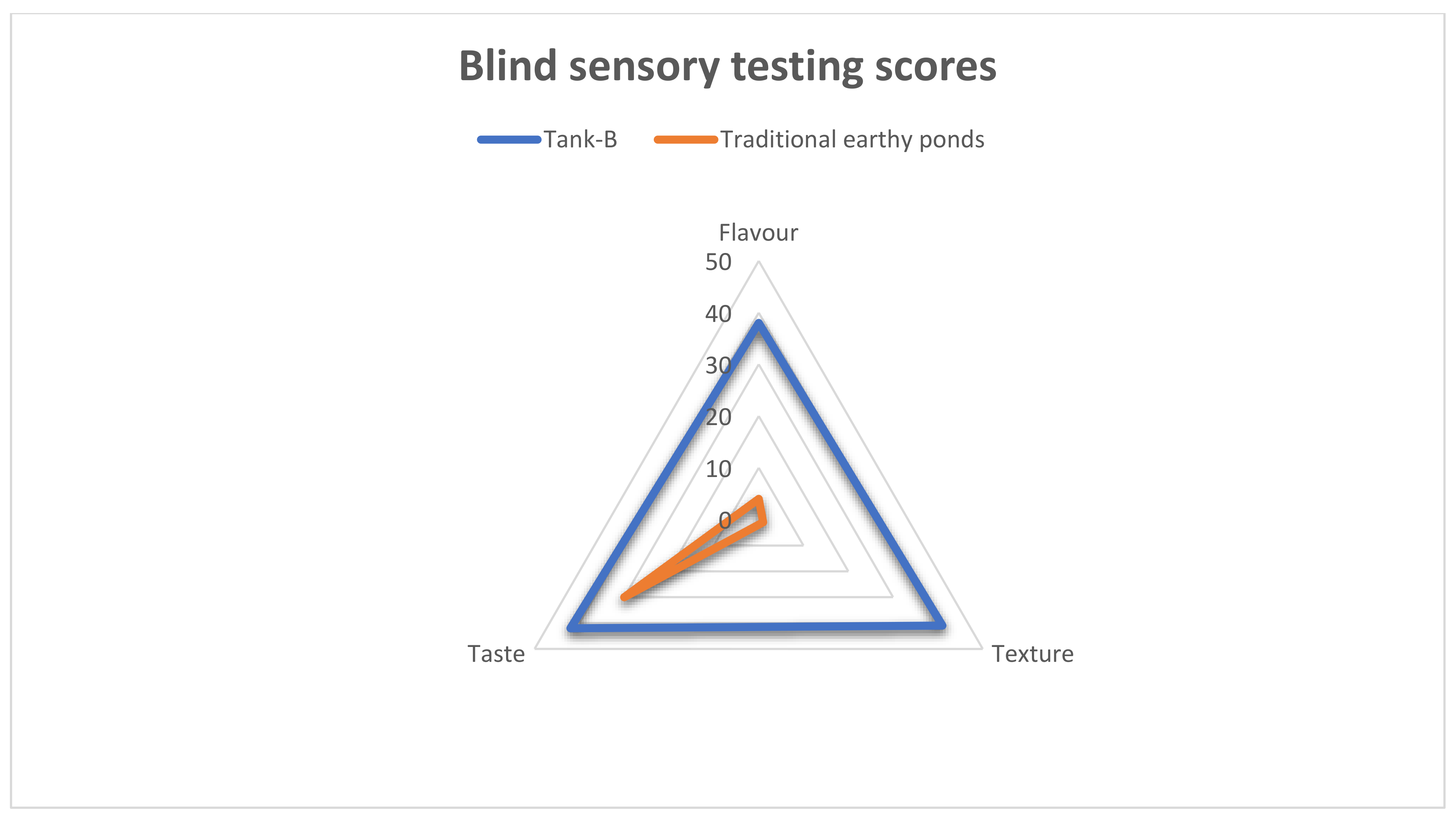
| n = 42 | Scores (Percentage of Agreement) | ||
|---|---|---|---|
| Tank-B | Traditional Earth Pond | ||
| Flavor | Umami/Sweetness | 38 (90.5%) | 4 (9.5%) |
| Texture | Springy/Firm | 41 (97.6%) | 1 (2.4%) |
| Taste | Without Earthy-odor | 42 (100%) | 30 (71.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-Y.; Chen, C.-L.; Shih, Y.-Y.; Hsieh, H.-H.; Huang, W.-J.; Santschi, P.H.; Hung, C.-C. A Smallholders’ Mariculture Device for Rearing Seafood: Environmentally Friendly and Providing Improved Quality. Sustainability 2023, 15, 862. https://doi.org/10.3390/su15010862
Lin T-Y, Chen C-L, Shih Y-Y, Hsieh H-H, Huang W-J, Santschi PH, Hung C-C. A Smallholders’ Mariculture Device for Rearing Seafood: Environmentally Friendly and Providing Improved Quality. Sustainability. 2023; 15(1):862. https://doi.org/10.3390/su15010862
Chicago/Turabian StyleLin, Tsang-Yuh, Chung-Ling Chen, Yung-Yen Shih, Hsueh-Han Hsieh, Wei-Ji Huang, Peter H. Santschi, and Chin-Chang Hung. 2023. "A Smallholders’ Mariculture Device for Rearing Seafood: Environmentally Friendly and Providing Improved Quality" Sustainability 15, no. 1: 862. https://doi.org/10.3390/su15010862





