A Cu/Polypyrrole-Coated Stainless Steel Mesh Membrane Cathode for Highly Efficient Electrocoagulation-Coupling Anti-Fouling Membrane Filtration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Architecture Preparation of the Cu/PPy-SSM
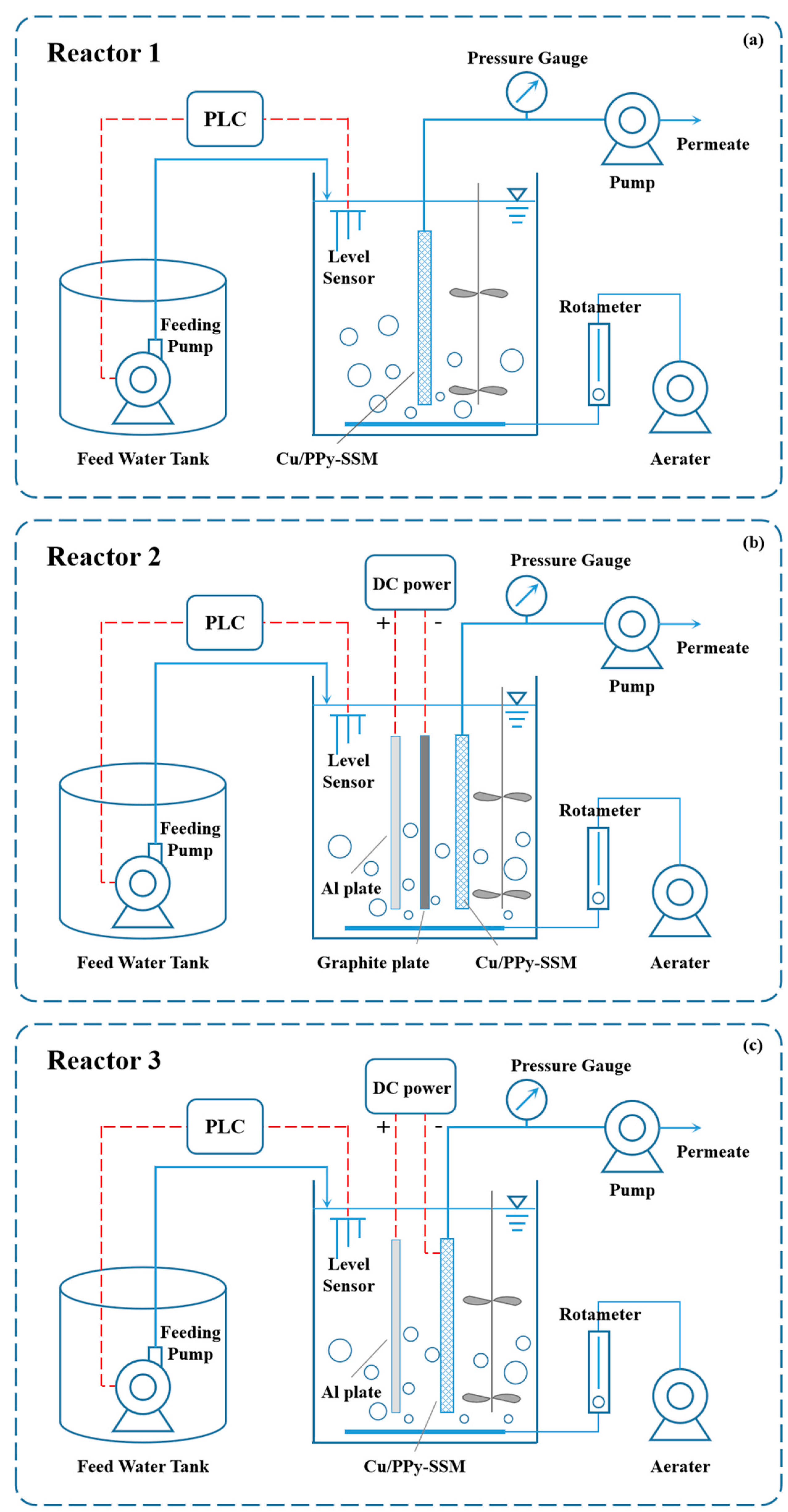
2.2. ECMR Design
2.3. Analytical Methods
2.4. Statistical Analysis and Parameters Optimization
3. Results and Discussions
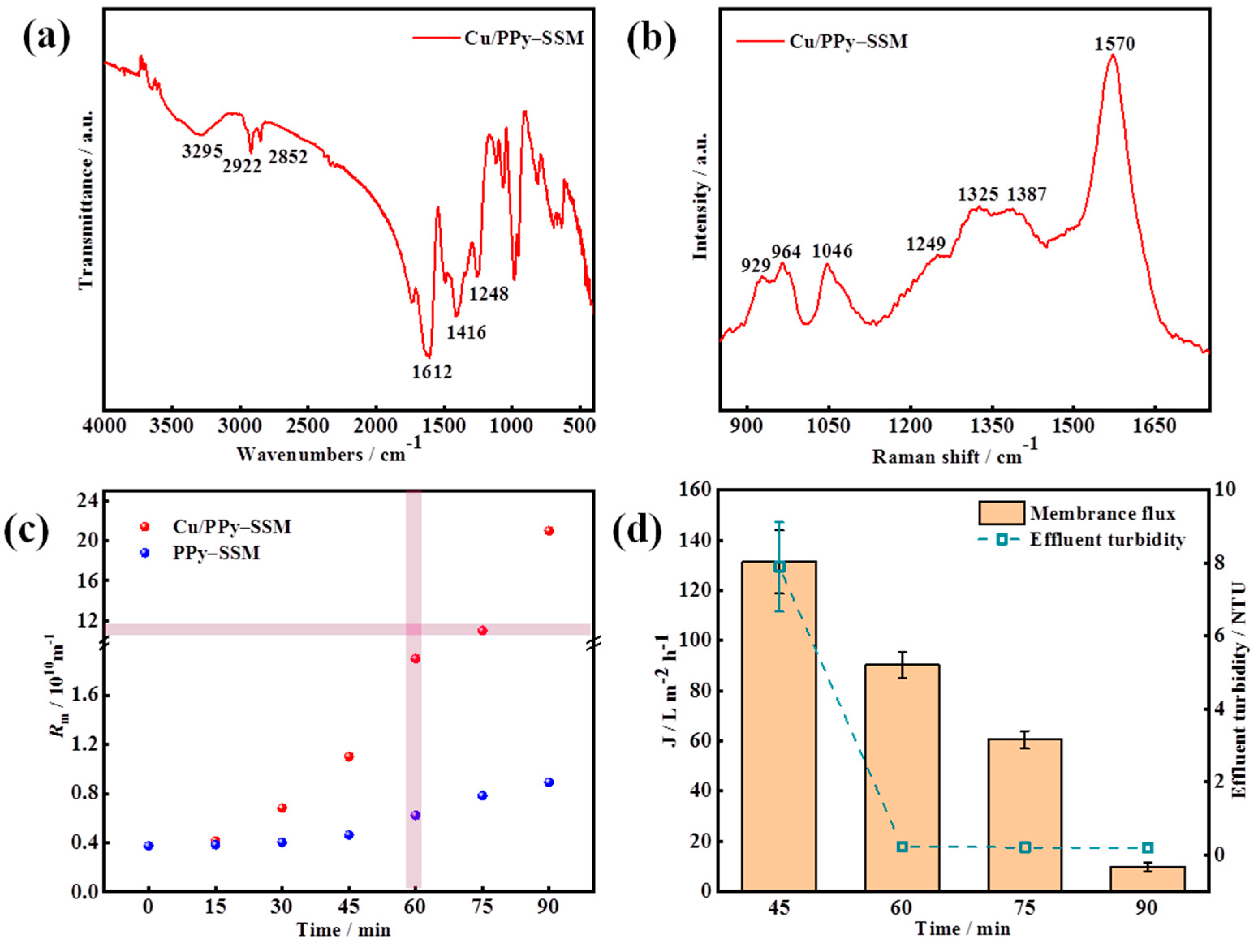
3.1. Characterization
3.1.1. Material Morphology and Microstructure Analysis
3.1.2. Effect of Electrodeposition Time on Membrane Properties
3.1.3. Electrochemical Characterization
3.2. Performance of the ECMR
3.3. Single-Factor Experiments
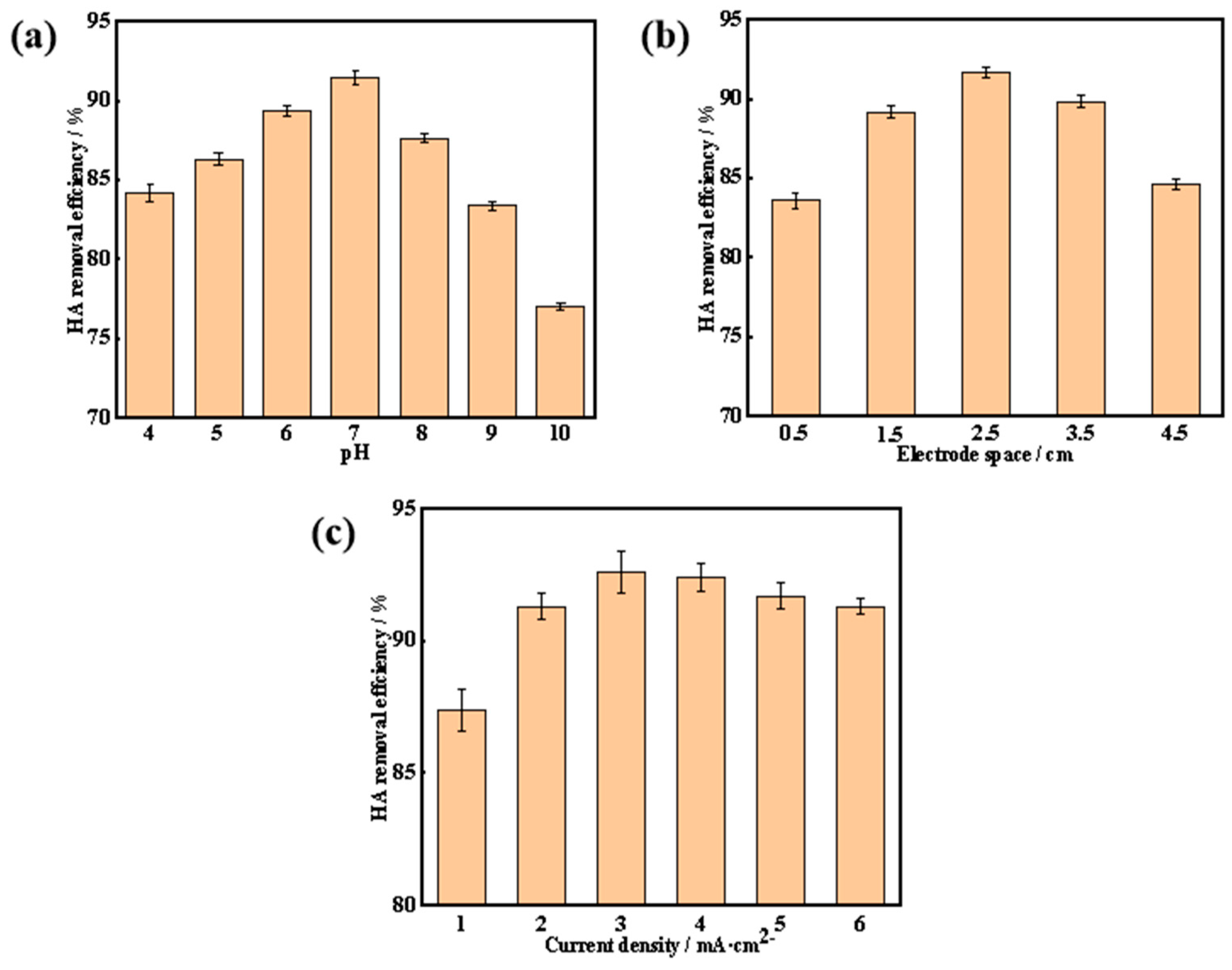
3.3.1. Effect of pH on the HA Removal Efficiency
3.3.2. Effect of Electrode Space on the HA Removal Efficiency
3.3.3. Effect of Current Density on the HA Removal Efficiency
3.4. Optimization of the ECMR Operating Parameters by RSM
3.5. Consistency Analysis between Model and Optimal Operating Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Ankoliya, D.; Mudgal, A.; Sinha, M.K.; Patel, V.; Patel, J. Application of electrocoagulation process for the treatment of dairy wastewater: A mini review. Mater. Today Proc. 2022; in press. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroha, A.K. Electrocoagulation for the treatment of textile industry effluent–a review. J. Environ. Manag. 2013, 128, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Eiband, M.M.S.; de Melo, J.V.; Martínez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef] [Green Version]
- Sahu, O.; Mazumdar, B.; Chaudhari, P. Treatment of wastewater by electrocoagulation: A review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Gao, B.; Yang, F. Cathode membrane fouling reduction and sludge property in membrane bioreactor integrating electrocoagulation and electrostatic repulsion. Sep. Purif. Technol. 2012, 100, 44–50. [Google Scholar] [CrossRef]
- Sardari, K.; Fyfe, P.; Lincicome, D.; Wickramasinghe, S.R. Combined electrocoagulation and membrane distillation for treating high salinity produced waters. J. Membr. Sci. 2018, 564, 82–96. [Google Scholar] [CrossRef]
- Gamage, N.P.; Chellam, S. Aluminum electrocoagulation pretreatment reduces fouling during surface water microfiltration. J. Membr. Sci. 2011, 379, 97–105. [Google Scholar] [CrossRef]
- Hua, L.-C.; Huang, C.; Su, Y.-C.; Chen, P.-C. Effects of electro-coagulation on fouling mitigation and sludge characteristics in a coagulation-assisted membrane bioreactor. J. Membr. Sci. 2015, 495, 29–36. [Google Scholar] [CrossRef]
- Ben-Sasson, M.; Lin, Y.; Adin, A. Electrocoagulation-membrane filtration hybrid system for colloidal fouling mitigation of secondary-effluent. Sep. Purif. Technol. 2011, 82, 63–70. [Google Scholar] [CrossRef]
- Geng, C.; Zhao, F.; Niu, H.; Zhang, J.; Dong, H.; Li, Z.; Chen, H. Enhancing the permeability, anti-biofouling performance and long-term stability of TFC nanofiltration membrane by imidazole-modified carboxylated graphene oxide/polyethersulfone substrate. J. Membr. Sci. 2022, 664, 121099. [Google Scholar] [CrossRef]
- Chen, H.; Xu, C.; Zhao, F.; Geng, C.; Liu, Y.; Zhang, J.; Kang, Q.; Li, Z.J.A.S.S. Designing the anti-biofouling surface of an ultrafiltration membrane with a novel zwitterionic poly (aryl ether oxadiazole) containing benzimidazole. Appl. Surf. Sci. 2023, 609, 155447. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Jaber, L.; Backer, S.N.; Chatla, A.; Kochkodan, V.; Al-Ansari, T.; Shanableh, A.; Atieh, M.A. Oxidized carbide-derived carbon as a novel filler for improved antifouling characteristics and permeate flux of hybrid polyethersulfone ultrafiltration membranes. Chemosphere 2022, 313, 137425. [Google Scholar] [CrossRef]
- Jaber, L.; Almanassra, I.W.; Backer, S.N.; Kochkodan, V.; Shanableh, A.; Atieh, M.A. A Comparative Analysis of the Effect of Carbonaceous Nanoparticles on the Physicochemical Properties of Hybrid Polyethersulfone Ultrafiltration Membranes. Membranes 2022, 12, 1143. [Google Scholar] [CrossRef]
- Tafti, A.D.; Mirzaii, S.M.S.; Andalibi, M.R.; Vossoughi, M. Optimized coupling of an intermittent DC electric field with a membrane bioreactor for enhanced effluent quality and hindered membrane fouling. Sep. Purif. Technol. 2015, 152, 7–13. [Google Scholar] [CrossRef]
- Sun, J.; Hu, C.; Zhao, K.; Li, M.; Qu, J.; Liu, H. Enhanced membrane fouling mitigation by modulating cake layer porosity and hydrophilicity in an electro-coagulation/oxidation membrane reactor (ECOMR). J. Membr. Sci. 2018, 550, 72–79. [Google Scholar] [CrossRef]
- Ma, B.; Yu, W.; Liu, H.; Yao, J.; Qu, J. Effect of iron/aluminum hydrolyzed precipitate layer on ultrafiltration membrane. Desalination 2013, 330, 16–21. [Google Scholar] [CrossRef]
- Ma, B.; Yu, W.; Liu, H.; Qu, J. Comparison of iron (III) and alum salt on ultrafiltration membrane fouling by alginate. Desalination 2014, 354, 153–159. [Google Scholar] [CrossRef]
- Shakeri, A.; Salehi, H.; Rastgar, M. Antifouling electrically conductive membrane for forward osmosis prepared by polyaniline/graphene nanocomposite. J. Water Process Eng. 2019, 32, 100932. [Google Scholar] [CrossRef]
- Ahmed, F.; Lalia, B.S.; Kochkodan, V.; Hilal, N.; Hashaikeh, R. Electrically conductive polymeric membranes for fouling prevention and detection: A review. Desalination 2016, 391, 1–15. [Google Scholar] [CrossRef]
- Xu, L.L.; Xu, Y.; Liu, L.; Wang, K.P.; Patterson, D.A.; Wang, J. Electrically responsive ultrafiltration polyaniline membrane to solve fouling under applied potential. J. Membr. Sci. 2019, 572, 442–452. [Google Scholar] [CrossRef]
- Xu, L.L.; Shahid, S.; Patterson, D.A.; Emanuelsson, E.A.C. Flexible electro-responsive in-situ polymer acid doped polyaniline membranes for permeation enhancement and membrane fouling removal. J. Membr. Sci. 2019, 578, 263–272. [Google Scholar] [CrossRef]
- Wang, K.; Xu, L.; Li, K.; Liu, L.; Zhang, Y.; Wang, J. Development of polyaniline conductive membrane for electrically enhanced membrane fouling mitigation. J. Membr. Sci. 2019, 570, 371–379. [Google Scholar] [CrossRef]
- Ho, K.; Teow, Y.; Mohammad, A.; Ang, W.; Lee, P. Development of graphene oxide (GO)/multi-walled carbon nanotubes (MWCNTs) nanocomposite conductive membranes for electrically enhanced fouling mitigation. J. Membr. Sci. 2018, 552, 189–201. [Google Scholar] [CrossRef]
- Dudchenko, A.V.; Rolf, J.; Russell, K.; Duan, W.; Jassby, D. Organic fouling inhibition on electrically conducting carbon nanotube–polyvinyl alcohol composite ultrafiltration membranes. J. Membr. Sci. 2014, 468, 1–10. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Lau, K.K. Ultrasonic-assisted secondary growth of deca-dodecasil 3 rhombohedral (DD3R) membrane and its process optimization studies in CO2/CH4 separation using response surface methodology. J. Nat. Gas Sci. Eng 2016, 30, 50–63. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Chew, T.L.; Lau, K.K. Optimization of spinning parameters on the fabrication of NH2-MIL-53(Al)/cellulose acetate (CA) hollow fiber mixed matrix membrane for CO2 separation. Sep. Purif. Technol. 2019, 215, 32–43. [Google Scholar] [CrossRef]
- Mubashir, M.; Ashena, R.; Bokhari, A.; Mukhtar, A.; Saqib, S.; Ali, A.; Saidur, R.; Khoo, K.S.; Ng, H.S.; Karimi, F.; et al. Effect of process parameters over carbon-based ZIF-62 nano-rooted membrane for environmental pollutants separation. Chemosphere 2022, 291, 133006. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Zhang, J.; Zhang, X.; Ma, J.; Wu, Z. A novel composite conductive microfiltration membrane and its anti-fouling performance with an external electric field in membrane bioreactors. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Formoso, P.; Pantuso, E.; De Filpo, G.; Nicoletta, F.P. Electro-conductive membranes for permeation enhancement and fouling mitigation: A short review. Membranes 2017, 7, 39. [Google Scholar] [CrossRef]
- Zhang, Q.; Vecitis, C.D. Conductive CNT-PVDF membrane for capacitive organic fouling reduction. J. Membr. Sci. 2014, 459, 143–156. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Li, W.-W.; Sheng, G.-P.; Shi, B.-J.; Yu, H.-Q. In-situ utilization of generated electricity in an electrochemical membrane bioreactor to mitigate membrane fouling. Water Res. 2013, 47, 5794–5800. [Google Scholar] [CrossRef] [PubMed]
- Kharade, P.; Mane, S.; Kulkarni, S.; Joshi, P.; Salunkhe, D. Ground nut seed like hydrophilic polypyrrole based thin film as a supercapacitor electrode. J. Mater. Sci. Mater. Electron. 2016, 27, 3499–3505. [Google Scholar] [CrossRef]
- Fonner, J.M.; Schmidt, C.E.; Ren, P. A combined molecular dynamics and experimental study of doped polypyrrole. Polymer 2010, 51, 4985–4993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, T.; Meng, J.; Lei, J.; Zheng, X.; Wang, Y.; Zhang, J.; Cao, X.; Li, X.; Qiu, X. A novel conductive composite membrane with polypyrrole (PPy) and stainless-steel mesh: Fabrication, performance, and anti-fouling mechanism. J. Membr. Sci. 2021, 621, 118937. [Google Scholar] [CrossRef]
- Yan, Q.; Pan, W.; Zhong, S.; Zhu, R.; Li, G.J.P.i.o.C. Effect of solvents on the preparation and corrosion protection of polypyrrole. Prog. Org. Coat. 2019, 132, 298–304. [Google Scholar] [CrossRef]
- Sun, M.; Yan, L.; Zhang, L.; Song, L.; Guo, J.; Zhang, H. New insights into the rapid formation of initial membrane fouling after in-situ cleaning in a membrane bioreactor. Process Biochem. 2019, 78, 108–113. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, X. Optimization of extraction process of crude polysaccharides from Pomegranate peel by response surface methodology. Carbohydr. Polym. 2013, 92, 1197–1202. [Google Scholar] [CrossRef]
- Liu, M.; Yang, L.; Liu, T.; Tang, Y.; Luo, S.; Liu, C.; Zeng, Y. Fe2P/reduced graphene oxide/Fe2P sandwich-structured nanowall arrays: A high-performance non-noble-metal electrocatalyst for hydrogen evolution. J. Mater. Chem. A 2017, 5, 8608–8615. [Google Scholar] [CrossRef]
- Kourdali, S.; Badis, A.; Saiba, A.; Boucherit, A.; Boutoumi, H. Humic acid removal by electrocoagulation using aluminium sacrificial anode under influencing operational parameters. Desalin. Water Treat. 2013, 52, 5442–5453. [Google Scholar] [CrossRef]
- Harif, T.; Khai, M.; Adin, A. Electrocoagulation versus chemical coagulation: Coagulation/flocculation mechanisms and resulting floc characteristics. Water Res. 2012, 46, 3177–3188. [Google Scholar] [CrossRef]
- Sasson, M.B.; Adin, A. Fouling mechanisms and energy appraisal in microfiltration pretreated by aluminum-based electroflocculation. J. Membr. Sci. 2010, 352, 86–94. [Google Scholar] [CrossRef]
- Bayar, S.; Yıldız, Y.S.; Yılmaz, A.E.; İrdemez, S. The effect of stirring speed and current density on removal efficiency of poultry slaughterhouse wastewater by electrocoagulation method. Desalination 2011, 280, 103–107. [Google Scholar] [CrossRef]
- Gan, C.-Y.; Manaf, N.H.A.; Latiff, A.A. Optimization of alcohol insoluble polysaccharides (AIPS) extraction from the Parkia speciosa pod using response surface methodology (RSM). Carbohydr. Polym. 2010, 79, 825–831. [Google Scholar] [CrossRef]
- Mao, R.; Wang, Y.; Zhang, B.; Xu, W.; Dong, M.; Gao, B. Impact of enhanced coagulation ways on flocs properties and membrane fouling: Increasing dosage and applying new composite coagulant. Desalination 2013, 314, 161–168. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Gomes, J.A.; Das, K.K.; Cocke, D.L. Electrochemical treatment of Orange II dye solution—Use of aluminum sacrificial electrodes and floc characterization. J. Hazard. Mater. 2010, 174, 851–858. [Google Scholar] [CrossRef]






| Factor | Code | Coding Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| pH | A | 6 | 7 | 8 |
| Plate spacing (cm) | B | 1.5 | 2.5 | 3.5 |
| Current density (mA/cm2) | C | 1 | 3 | 5 |
| Number | Coding Level | Removal of HA Rides (%) | ||
|---|---|---|---|---|
| X1 | X2 | X3 | ||
| 1 | 0 | 0 | 0 | 92.67 |
| 2 | 1 | −1 | 0 | 84.05 |
| 3 | 0 | 1 | −1 | 86.81 |
| 4 | −1 | 0 | 1 | 88.31 |
| 5 | 0 | 0 | 0 | 92.22 |
| 6 | −1 | 0 | −1 | 85.64 |
| 7 | 0 | 0 | 0 | 92.27 |
| 8 | 0 | 0 | 0 | 92.89 |
| 9 | 0 | −1 | −1 | 85.76 |
| 10 | −1 | −1 | 0 | 87.15 |
| 11 | 1 | 0 | 1 | 85.31 |
| 12 | 0 | −1 | 1 | 90.25 |
| 13 | 1 | 1 | 0 | 84.23 |
| 14 | 1 | 0 | −1 | 82.24 |
| 15 | 0 | 0 | 0 | 92.71 |
| 16 | −1 | 1 | 0 | 87.71 |
| 17 | 0 | 1 | 1 | 90.45 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Probability > f |
|---|---|---|---|---|---|
| Model | 195.77 | 9 | 21.75 | 139.25 | <0.0001 |
| X1 | 21.06 | 1 | 21.06 | 134.82 | <0.0001 |
| X2 | 0.50 | 1 | 0.50 | 3.17 | 0.1183 |
| X3 | 24.05 | 1 | 24.05 | 153.94 | <0.0001 |
| X1X2 | 0.036 | 1 | 0.036 | 0.23 | 0.6454 |
| X1X3 | 0.040 | 1 | 0.040 | 0.26 | 0.6284 |
| X2X3 | 0.18 | 1 | 0.18 | 1.16 | 0.3179 |
| X12 | 99.24 | 1 | 99.24 | 635.28 | <0.0001 |
| X22 | 15.40 | 1 | 15.40 | 98.57 | <0.0001 |
| X32 | 22.71 | 1 | 22.71 | 145.36 | <0.0001 |
| Residual | 1.09 | 7 | 0.16 | ||
| Lack of Fit | 0.75 | 3 | 0.25 | 2.92 | 0.1638 |
| Pure Error | 0.34 | 4 | 0.086 | ||
| Corrected Total | 196.86 | 16 |
| Item | c.v.% | R-Squared | Adjusted R-Squared | Predetermined R-Squared | Adequate Precision |
|---|---|---|---|---|---|
| Value | 0.45 | 0.9944 | 0.9873 | 0.9363 | 35.078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hao, Z.; Han, J.; Sun, Y.; He, M.; Yao, Y.; Yang, F.; Liu, M.; Zhang, H. A Cu/Polypyrrole-Coated Stainless Steel Mesh Membrane Cathode for Highly Efficient Electrocoagulation-Coupling Anti-Fouling Membrane Filtration. Sustainability 2023, 15, 1107. https://doi.org/10.3390/su15021107
Li Y, Hao Z, Han J, Sun Y, He M, Yao Y, Yang F, Liu M, Zhang H. A Cu/Polypyrrole-Coated Stainless Steel Mesh Membrane Cathode for Highly Efficient Electrocoagulation-Coupling Anti-Fouling Membrane Filtration. Sustainability. 2023; 15(2):1107. https://doi.org/10.3390/su15021107
Chicago/Turabian StyleLi, Yuna, Zixin Hao, Jinglong Han, Yueyang Sun, Mengyao He, Yuang Yao, Fuhao Yang, Meijun Liu, and Haifeng Zhang. 2023. "A Cu/Polypyrrole-Coated Stainless Steel Mesh Membrane Cathode for Highly Efficient Electrocoagulation-Coupling Anti-Fouling Membrane Filtration" Sustainability 15, no. 2: 1107. https://doi.org/10.3390/su15021107





